Comparative effect of organic and inorganic selenium supplementation on selenium status in camel
*Corresponding author at: CIRAD-ES, Campus International de Baillarguet, TA C/112 A, UMR SELMET, 34398 Montpellier, France. Tel.: +966 538925629; fax: +33 467593795 faye@cirad.fr (B. Faye)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Available online 31 October 2013

Abstract
Selenium deficiency is widely described in livestock from the Arabian Peninsula, notably in the camel, and selenium supplementation is based on cattle or horse requirements, usually with sodium selenite product. In order to test the effect of organic Se supplementation vs inorganic Se, 24 pregnant camels were subjected to 3 treatment groups starting one month before delivery (control without Se, non-organic bolus, organic Se). Blood, milk and feces samples were collected from one month before delivery to 3 months of lactation. At delivery, the organic group had a significant higher Se concentration (P < 0.01) in serum (8.21 ± 1.38 μg/100 mL) and in colostrum (7.27 ± 2.89 μg/100 mL) than in inorganic group (3.90 ± 0.68 and 3.72 ± 0.71, respectively) and than in control group (5.45 ± 2.38 and 2.70 ± 0.66, respectively). In calf serum, the Se concentration was significantly higher (P < 0.001) in the two supplemented groups (6.32 ± 2.81 and 5.99 ± 3.31 μg/100 mL in organic and inorganic groups, respectively) than in control (3.42 ± 1.41 μg/100 mL). The Se in mother serum decreased after parturition but was highly correlated to Se serum in calf and to Se fecal excretion. Se in milk was lower than in colostrum in all groups (P < 0.01). Treatments had no significant effect on somatic cell count. This study revealed that organic supplementation in camel appeared more efficient.
Keywords
Organic selenium
Non-organic selenium
Camel
Milk
Supplementation
Somatic cell count
1 Introduction
To maintain camel health and efficient production, it requires that essential dietary nutrients be provided in appropriate amounts. Nutritional importance of selenium in camels was reported (Faye and Seboussi, 2009). The selenium imbalance is a common feature in Saudi Arabia both in human (Al-Saleh et al., 2006) and in livestock leading to sudden death in young animals by heart failure, white muscle disease and low reproductive performance in adult males and females (El-Neweehy et al., 2001). In spite of a lack of epidemiological data in camel, the selenium deficiency appears widely observed in the Arabian Peninsula (El Khouly et al., 2001; Faye and Seboussi, 2009). In Saudi Arabia, a previous study has shown the beneficial impact of diet enriched with barley, naturally rich in selenium, for improving selenium status in camel (Althamna et al., 2011). Yet, most of the Saudi camel farmers in Al-Jouf area did not distribute oral selenium supplementation to their animals, but rather used non-organic selenium solution by injection in pregnant or new-born camels based on formula set up for cattle and small ruminants. However, the effect of such unique injection at the end of pregnancy is quite insufficient for a significant improvement of the selenium status of the new-born, especially on the level of selenium in milk (Faye et al., 2013).
In all species, including man, the selenium status in serum or plasma is closely depending on the selenium intake and absorption in digestive tract (Haldimann et al., 1998). Elsewhere, it has been shown in recent studies that camel response on serum selenium concentration was very sensitive to oral selenium supplementation compared to other species (Faye and Seboussi, 2009; Seboussi et al., 2009). Especially, a comparative study involving camels and cows having comparable weight and fed with the same diet and supplemented orally with 2 mg of selenium per day under selenite form has shown that serum selenium was 2-fold in cow while it was 10-fold in camel after supplementation (Bengoumi et al., 1998). However, organic selenium which is now the main form of selenium supplementation for livestock in many countries has never been tested in camel contrary to cattle (Guyot et al., 2007), sheep (Davis et al., 2008), goat (Kachuee et al., 2013), pig (Horky et al., 2013), horse (Jancikova et al., 2013) or rats (Sochor et al., 2012), and its effect on selenium in serum and milk around the parturition was never assessed in camel.
The objective of this study was to compare the effect of supplemented selenium source provided to pregnant and lactating camels on measures of selenium status in the dam around parturition and newborn calf.
2 Material and methods
2.1 Location and animals
This study was carried out in the camel farm of Al-Jouf “Camel & Range Research Center” located in north-west Saudi Arabia, 950 km from Riyadh. Average annual temperature was 20 °C, ranging from 12 °C to 27 °C, and average annual rainfall was 55 mm. The herd was composed of camels of four ecotypes (Malhah, Wadhah, Hamrah and Safrah) but belonging to a very close genotype (Abdallah and Faye, 2012; Almathen et al., 2012). The weight of the animals selected for the experiment was on average 615 ± 101 kg. Animals were weighed in the morning after milking at the beginning of the trial then every month before watering and feeding with a platform scale Mettler Toledo©, 3000 kg capacity. Individual milk yield was recorded during milking. They were multiparous with parity between 2 and 9. Camels were kept in-door throughout the year and housed in pens. The diet normally distributed to the camel herd was composed of alfalfa hay (ad-libitum) as forage, then barley grain (3 kg/day/animal), salt (NaCl) in grains, and wheat bran (1 kg/day/animal) as mixed ratio in a separate manger. Thus, all camels received the same basal diet. The selenium concentration (in mg/kg DM) was 0.11 in barley, 0.12 in Alfalfa, and 0.15 in wheat bran. There was no detectable Se in water. As the calving season occurred between December and February, all the camels were approximately at the same stage of reproductive cycle. After delivery, the camel calves were in permanence with their dam for three weeks and not milked except for milk sampling. Three weeks after delivery, the camels were milked twice a day at 6:30 and 16:30 by a milking machine (Kurstsan Milking Machine, type SSM, Istanbul, Turkey). The milk quantity recorded daily did not include the part drunk by camel calves. After milking, dams were suckled by their calves for striping. The calves stayed with their mother for 45 min after each milking and then were separated.
2.2 Experimental procedure
Twenty-four female camels at late pregnancy stage at the beginning of the experiment (approximately last month) were assigned randomly in 3 groups of eight each. Parity, live weight, birth weight of the calf and pregnancy length, reported in Table 1, were not significantly different (ANOVA test) between groups. The camels were in good health throughout the experiment.
| Group | Parity | Liveweight | Birthweight | Gest. length |
|---|---|---|---|---|
| Control (n = 8) | 3.6 ± 2.8 (2–9) | 517 ± 64 (426–624) | 39 ± 2.0 (36–42) | 389 ± 11 (375–408) |
| Se-Inorg (n = 8) | 3.8 ± 2.2 (3–8) | 657 ± 61 (582–766) | 39 ± 3.0 (36–44) | 372 ± 6 (362–392 |
| Se-Org (n = 8) | 6.6 ± 2.1 (5–8) | 669 ± 49 (602–748) | 39 ± 4.8 (35–47) | 384 ± 11 (370–402) |
The selenium supplementation was given to these animals according to the following procedure:
-
Group Se-Inorg: eight camels were supplemented with long acting selenium pellets (Pharmplex. Nottingham, UK) introduced by esophageal tube in the rumen at the beginning of the experiment approximately one month before parturition. The dose was one bolus for all the experiments. The bolus was composed of 3 g of elemental selenium per 30 g sustained release pellet. Selenium ions are continually available in the rumen from the dissolving elemental boluses and are available for rumen microbes to convert into selenomethionine and selenocystine which can be absorbed by the animal. The release of selenium was over 12 months, i.e. daily offer of 8 mg Se/animal.
-
Group Se-Org: eight camels supplemented with organic selenium 3000 mg/kg (alkosel®3000, Lallemand Animal Nutrition, Canada). This product is an inactivated whole cell yeast (Saccharomyces cerevisiae) produced by growing yeast in the presence of measured amounts of selenium salts. Live yeast cells absorb selenium and biochemically transform it into selenoproteins (selenomethionine). The quantity distributed to each camel was 1.2 g of organic compound per day for 75 days. This quantity corresponded to a daily offer of 3.6 mg Se/animal. The Se organic was in powder form and given daily by putting the powder in a date.
-
Group control: eight camels receiving basal diet without Se supplementation.
Elsewhere, all camels received vitamin E (15 IU/kg DM) in the diet.
2.3 Sampling procedure
The whole-length of the experiment was 90 days, one month before calving + 60 days after calving. Blood samples were collected monthly from mammary vein i.e. at d-30 (one month before parturition), d1 (day of calving), d30, d60, d75 of the mother and from jugular vein at d1 and d30 of the calf in vacutainer tubes without anticoagulant. The first blood sampling in camel calf was done just after delivery before colostrum suckling:
-
-
The blood samples were centrifuged at 3000g for 20 min and serums were stored at −80 °C until analysis.
-
-
Milk samples were collected every 2 weeks, i.e., d1 (colostrum), d15, d30, d45, d60, d75 and d90. The raw milk samples were stored in a plastic flask (100 mL) at −80 °C until analyses. Colostrum was collected before nursing the calf. As it was before the starting the use of milking machine, the colostrum and milk samples at d15 were collected by hand in the morning. Other milk samples were collected during the morning milking in the dairy pot of the milking machine. Feces samples were collected monthly directly from the rectum at d1, d30, and d60. Fecal samples were dried at 65 °C, ground and stored in a dry place.
-
-
Feed samples were collected at the beginning of the experiment: 500 g of each component of the diet was collected, dried and ground and stored in a dry place.
-
-
No water sample was achieved during the trial, but former analysis a year before was available, the source of water being the same.
2.4 Laboratory analysis
Selenium was determined in serum, milk and feces. In blood and milk samples, selenium was determined with Hybrid Vapor Generator (HVG-1, Shimadzu, Japan) according to the procedure described in detail by Seboussi et al. (2009). The serum and milk Se data are reported as μg/100 mL. The selenium concentration in feed is generally expressed in ppm or mg/kg.
Selenium content in the different components of the diet, in feces (reported as mg/kg DM) and in water was determined at IDAC laboratory (KSA) by Inductively Coupled argon Plasma – Atomic Emission Spectrometer (ICP AES), Varian Vista MPX – CCD Simultaneous. Quantification of selenium was performed by the standard addition method, using an 11 point standard curve. AccuTrace™ Reference Standard solutions used were Quality Control Standard #1AccuStandard® and Laboratory Performance Check Standard AccuStandard®. Vitamin E was determined in the feed only by High-Performance Liquid Chromatography (HPLC) system at IDAC laboratory.
The somatic cell counts- SCC (cells/ml) in the milk samples were determined using NucleoCounter SCC-100 (coulter electronic – ChemometecA/s, Denmark). Chemical component of milk percentage of fat, total protein, lactose and ash was determined by automatic milk analyzer device (lactoscan MCC) calibrated for camel milk.
2.5 Statistical analysis
The mean selenium and standard deviation were calculated for each group. The variance analysis (repeated measures ANOVA) for time series was applied to evaluate the difference between control and treated groups all along the experiment by taking into account the time effect, the treatment effect and the interaction between repetition and treatment (control, Se-Inorg and Se-Org). Pearson correlation was determined to assess the relationships between the selenium concentrations in the different substrates. As the SCC values are generally log-normal (Shook, 1982), means and S.D were calculated after a logarithm transformation of the raw data. However, the values were expressed in cells/ml to be more explicit and the means were geometric means.
The software XLSTAT (Addinsoft©) was used for the data analysis (module “data modelisation”, tool “ANOVA for repeated measures” using estimation method based on least square).
3 Results
As the feed intake was approximately 6 kg D.M of alfalfa and that the totality of barley and wheat bran was ingested (no refusals), the selenium intake provided by the basal diet according to selenium content in the different components of the feeds was approximately 1.20 mg per day (i.e. 0.12 mg/kg for 10 kg DM intake approximately). According to the mean weight (Life Weight-LW: 615 kg) of the animals, the selenium intake due to the diet corresponded to 2.4 μg/kg LW (with a range of 1.66–2.92 μg/kg LW). There was no significant difference between the groups regarding mean parity, breed, mean body weight at calving, gestation length and calf weight at delivery.
3.1 Selenium in serum
On average, all groups included, the serum selenium concentration in dam was 7.83 ± 3.74 μg/100 mL with significant higher values at d-30 (11.56 ± 1.84 μg/100 mL) followed by a fall up to 5.85 ± 2.38 at d1 then 5.59 ± 2.67 at d30, 7.60 ± 3.89 at d60 and 6.75 ± 2.9 μg/100 mL at d75 (P < 0.001). There was no difference at d-30 between the 3 groups, then the group Se-org was significantly higher at delivery (d1) and d30 (P < 0.05). At d60, serum selenium concentration in the control group was significantly lower than the supplemented groups (P < 0.01). At d75, the 3 groups were different (P < 0.001) with a higher value for the group Se-org, intermediate value for the group Se-Inorg and a still lower value in the control group (Fig. 1).
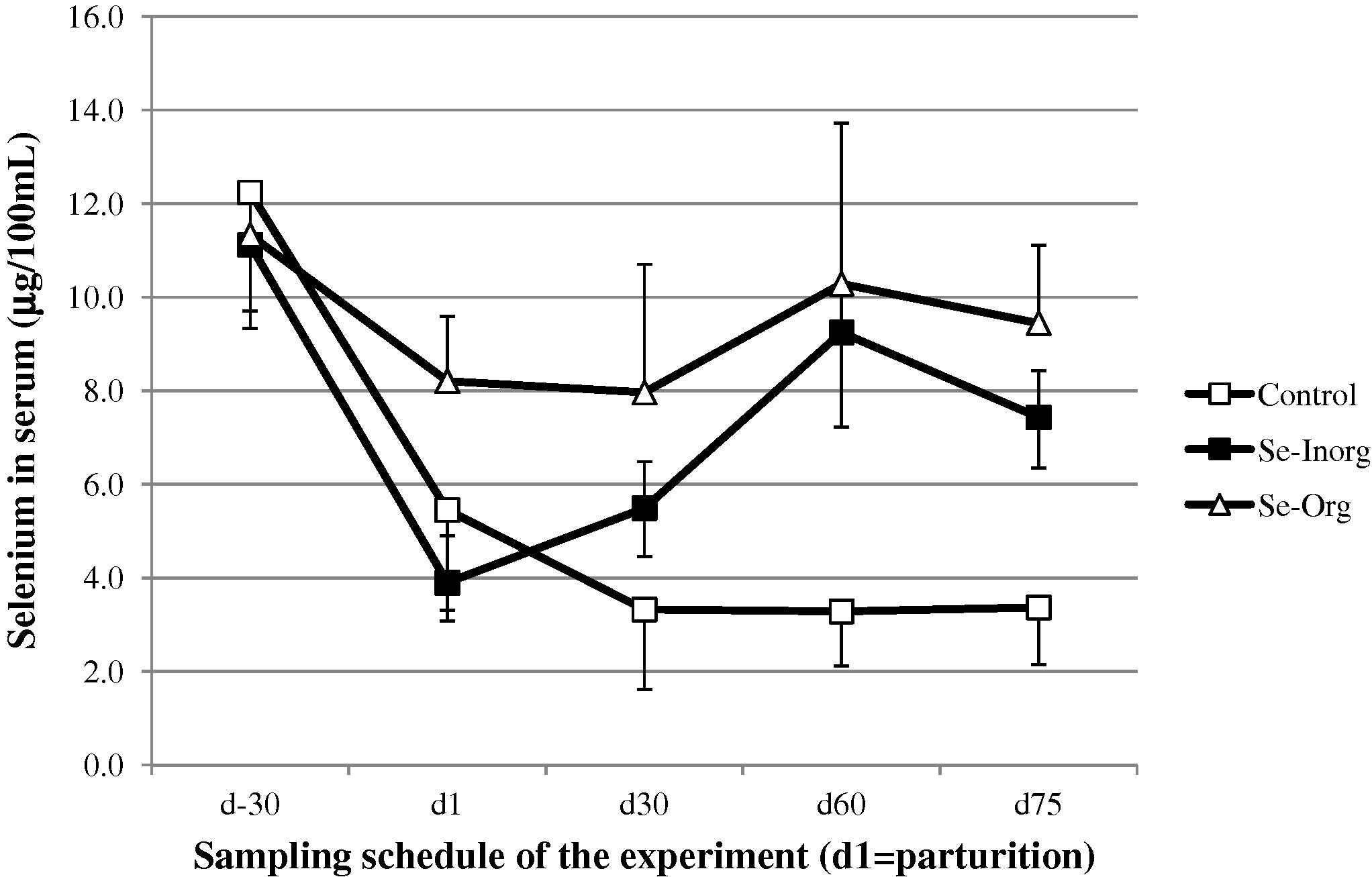
- Change (mean and SD) in selenium concentration in camel dam serum around the parturition (□ control group without supplementation, ■ group receiving inorganic selenium under bolus form, △ group receiving organic selenium).
The mean selenium concentration in calf serum at parturition (all groups included) was not significantly different than in the mother at d1 (5.13 ± 2.56 vs 5.85 ± 2.38 μg/100 mL), and significantly higher (P < 0.01) from d30 (8.71 ± 2.97), d60 (10.56 ± 2.2) and d75 (10.57 ± 1.93 μg/100 mL). Selenium concentration in calf serum was significantly lower (P < 0.001) in the control group up to d60 (Fig. 2).
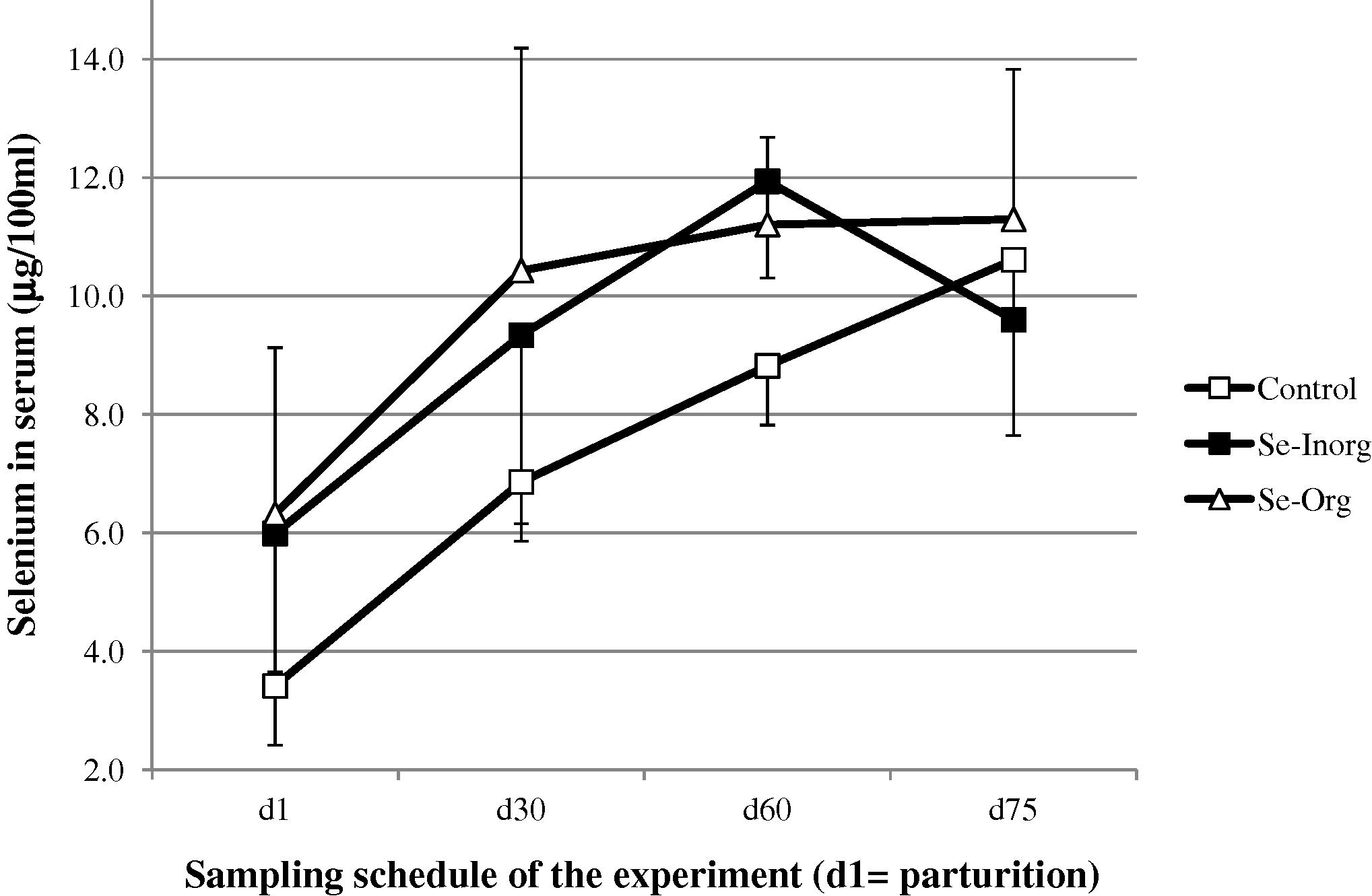
- Change (mean and SD) in selenium concentration in camel calf serum after parturition (□ control group without supplementation, ■ group receiving inorganic selenium under bolus form, △ group receiving organic selenium).
3.2 Selenium in milk
On average, all groups included, the higher milk selenium concentration (P < 0.001) was observed in colostrum (4.62 ± 2.68 μg/100 mL) and tends to decrease gradually in milk: 1.87 ± 0.72 at d30, 1.58 ± 0.55 at d45, 1.39 ± 0.35 at d60, 1.25 ± 0.36 at d75, then 1.20 ± 0.30 at d90. The highest value was observed in colostrum of Se-Org group (7.27 ± 2.89 μg/100 mL). The difference between Se-Org group and the two other groups was significant at d1 (P < 0.001) and d30 (P < 0.05). From d45, no difference was observed (Fig. 3). There was no significant difference in the milk composition (%) between the control, Se-Inorg and Se-Org groups, for total protein (2.6 ± 0.06, 2.9 ± 0.10 and 2.9 ± 0.2, respectively), fat (3.6 ± 0.5, 3.3 ± 0.3 and 3.1 ± 0.6, respectively), lactose (3.7 ± 0.09, 4.2 ± 0.14 and 4.1 ± 0.27, respectively) and ash (0.65 ± 0.02, 0.75 ± 0.02 and 0.73 ± 0.05, respectively).
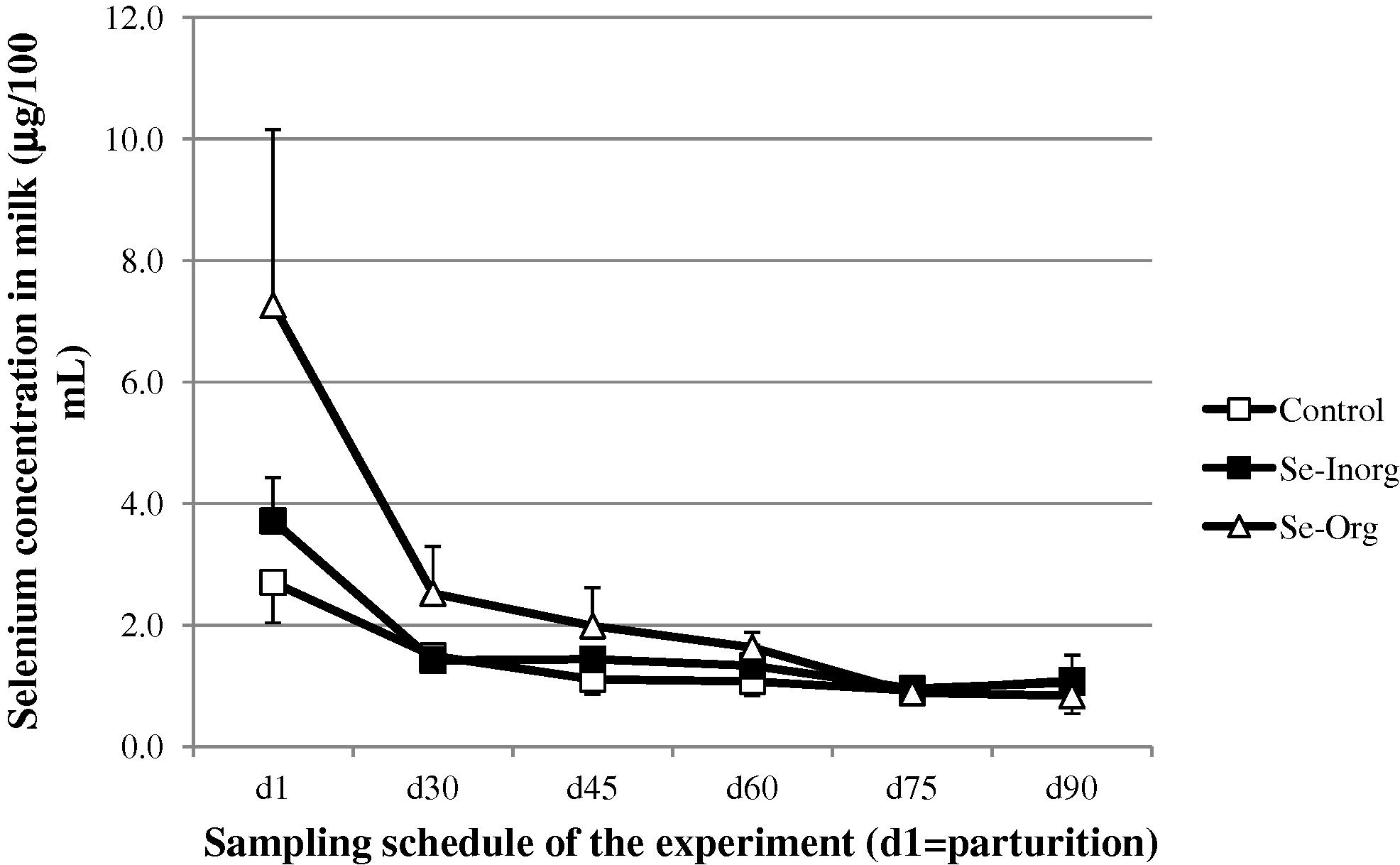
- Change (mean and SD) in selenium concentration in camel milk after parturition (□ control group without supplementation, ■ group receiving inorganic selenium under bolus form, △ group receiving organic selenium).
3.3 Selenium in feces
In feces, all groups included, the selenium concentration increased from 119.4 ± 38.2 μg/kg at d1 to 121.1 ± 83.6 at d30 and 141.6 ± 40.3 at d60 but this variation was not significant. There was no difference between the 3 groups in selenium fecal excretion at d1 and d60, but at d30, Se-Org group had higher fecal concentration than Se-Inorg and control (P < 0.005). Fecal Se in Se-Inorg group was not significantly higher than control (Table 2).
| Group | d1 | d30 | d60 |
|---|---|---|---|
| Control (n = 8) | 118.1 ± 39.9a | 56.9 ± 31.6a | 158.6 ± 35.8a |
| Se-Inorg (n = 8) | 118.4 ± 47.2a | 128.4 ± 96.1a | 144.8 ± 43.1a |
| Se-Org (n = 8) | 121.9 ± 36.7a | 177.8 ± 70.4b | 121.5 ± 40.6a |
a,bMeans in a column with common superscripts do not differ.
3.4 Somatic cell count
Considering all the experiments and all milk samples, SCC values varied from 10,000 (limit of detection of the SCC counter) to 416,000 cells/mL. Only the value reported at d30 is significantly higher than at d90 (P < 0.05). On average all groups included, SCC changed irregularly from 50,430 cells/mL in colostrum to 61,820 cells/mL in milk after 2 months of lactation with important fluctuations (Fig. 4). However no significant difference was observed throughout the experiment between the 3 groups of camels: 33,310 ± 50,757 cells/mL in the control group vs 45,844 ± 44,141 cells/mL in the Se-Inorg group and 50,443 ± 48,693 cells/mL in the Se-Org group.
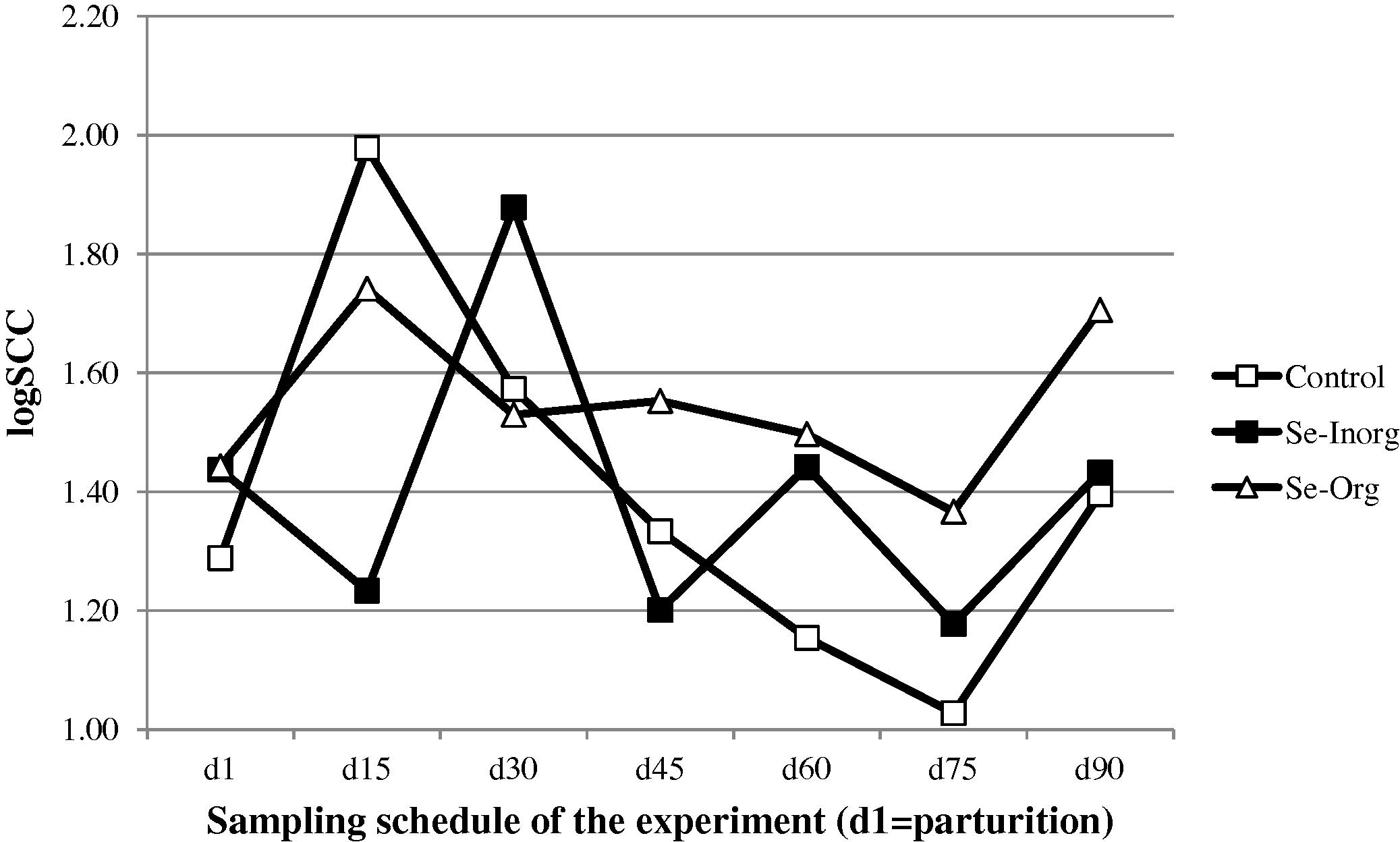
- Change in somatic cell count (in log) in camel milk after parturition (□ control group without supplementation, ■ group receiving inorganic selenium under bolus form, △ group receiving organic selenium).
3.5 Interactions
There was a positive significant correlation between selenium in mother’s serum and calf’s serum (P < 0.01). The selenium concentration in feces was correlated (P < 0.01) to the selenium concentration in serum of the mother (Table 3). Elsewhere, the relationship between selenium in milk and serum selenium of the calf was negative (P < 0.05). No correlation was found with SCC in milk (Table 3).
| Variables | Serum-dam | Serum-calf | Milk | SCC | Feces |
|---|---|---|---|---|---|
| Serum-dam | 1 | 0.380b | 0.151 | 0.156 | 0.461b |
| Serum-calf | 0.380b | 1 | −0.287a | 0.041 | 0.210 |
| Milk | 0.151 | −0.287a | 1 | 0.046 | −0.037 |
| SCC | 0.156 | 0.041 | 0.046 | 1 | −0.094 |
| Feces | 0.461b | 0.210 | −0.037 | −0.094 | 1 |
However, by considering values for each date of sampling, all groups included, the correlation between Se-serum in mother and Se-milk was significantly positive at d1 (r = .589, P < 0.05), d30 (r = .671, P < 0.01), d60 (r = .0.573, P < 0.05) and d75 (r = .668, P < 0.01). Regarding the relationship between Se-serum in calf and Se-milk, the correlation was only significantly positive in colostrum (r = .654, P < 0.01). No correlation was observed with milk (d30, d60 and d75).
4 Discussion
The diet was globally poor in selenium (0.12 mg/kg) and did not provide enough quantity for a lactating animal which is considered to be 0.5–1 mg/kg/day for camel (Faye and Seboussi, 2009). According to the mean live weight of the camels in our experiment, the diet provided 1.6–2.9 μg/kg LW which is lower than that recommended (10–20 μg/kg LW) for camel (Faye and Seboussi, 2009). Besides, some cases of heart failure in camel calves were observed previously in the farm. The vitamin E content in the diet corresponded to the requirements for dairy cattle. In most of the studies on camel, there was no correlation between selenium and vitamin E status in plasma concentration (Seboussi et al., 2008; Seboussi et al., 2009). However, a high level of Se selenium seemed to depress the vitamin E level in plasma (Faye and Seboussi, 2010) as it was observed in horse affected by selenosis (Crain, 2007).
The mean concentration of whole blood/serum selenium reported in the literature for camel was around 10 μg/100 mL, value considered as sufficient for the maintenance of suitable metabolic functions (Faye and Seboussi, 2009). The values reported in our study at the beginning of the experiment (11.56 ± 1.78 μg/100 mL) were quite comparable to the observations of Hamliri et al. (1990) in Morocco on whole blood, Barri and Al-Sultan (2007) in Saudi Arabia on serum, Seboussi et al. (2008) in Emirates on serum, and Liu et al. (1994) in China on whole blood of Bactrian camel. However, lower values were reported on whole blood in Sudan (Abdel Rahim, 2005), and on serum in Morocco again (Bengoumi et al., 1998) and in Iran (Nazifi et al., 2009; Pourjafar et al., 2012). In fact, a high variability was observed generally with a range between 1.2 and 20 μg/100 mL. In a recent paper, Abdelrahman et al. (2013) reported a significant difference between two camel breeds in Saudi Arabia with a higher value for Malhah breed (14.7 μg/100 mL on average) than for Wadhah breed (7.3 μg/100 mL). In our experiment, the groups were heterogeneous regarding the breed and no difference was observed at the beginning of the trial (d-30): 12.3 for Hamrah, 11.4 for Wadhah, 10.4 for Malhah, and 8.2 μg/100 mL for Safrah. Moreover, in most of the publications, the selenium status of the diet is not known and the analytical procedures are not described. Globally, selenium status in camel is poorly documented.
No age effect was reported (Faye et al., 2008). At reverse, the physiological status could be responsible for important variation in serum selenium concentrations. The fall in serum Se observed at parturition in our study was not reported in the experiment achieved in Emirates on pregnant and lactating camels (Seboussi et al., 2009). However, a significant decrease of gluthatione-peroxidase (GSH-Px), metallo-enzyme generally highly correlated to selenium, was decreased after parturition by these authors in camel and in sheep by White et al. (1989). Such post-partum decrease probably linked to lactation stress was reported for serum Se in sheep (Travnicek et al., 2007). One reason of the decrease in Se in the mother serum after delivery, independently of Se form received by the animals could be attributed to the active placental transfer of Se and to the milk selenium excretion. Indeed, the quantity of selenium was higher in colostrum emphasizing the efficiency of maternal transfer through the milk (Faye et al., 2011) just after the parturition.
The camels receiving organic Se presented the highest Se values both in serum and in colostrum compared to camels receiving non-organic Se. Similar results were observed in cattle (Gunter et al., 2013), in goat (Kachuee et al., 2013) and sheep (Davis et al., 2006) emphasizing a better efficiency of organic-selenium supplementation on blood and milk status of the supplemented animals. It is interesting to note that the ratio Se-Org/control at d1 was similar in serum-dam (1.51), in serum calf (1.85) and quite higher in milk (2.69). The organic form of Se is regarded as a more efficient form for the transfer from the mother to her calf, notably by colostrum (Guyot et al., 2007). The better transfer of Se under seleno-methionine form through the milk is due to its efficient incorporation in milk proteins (Anan et al., 2009).
The ratio of Se-Org group/Se-Inorg group at d1 was also similar in mother Se-serum (2.10) and in milk (1.96) but the ratio in camel calf (1.06) did not reflect the difference observed in mother and in milk contrary to the observation of Seboussi et al. (2009) who stated a similar proportion in serum dam, serum calf and milk between the group receiving sodium selenite and the control group. Yet, Weiss et al. (1984) suggested that selenium concentration in blood serum of the dam near parturition can be used as an indicator of serum selenium status of the neonatal calf. The significant correlation between Se serum in mother and in calf was already reported by Seboussi et al. (2009).
Regarding the concentration of selenium in camel milk (on average 2.08 ± 1.76 μg/100 mL), it appeared quite lower than in the previous study of Seboussi et al. (2009) which was on average 8.64 μg/100 mL in the camel without supplementation. Few references were available on selenium content in camel milk. Al-Awadi and Srikumar (2001) reported low values (1.39 μg/100 mL) but without mentioning the lactation stage. In cow milk, the selenium concentration could vary from 1.94 to 5.37 μg/100 mL with Se dietary selenium between 0.15 and 0.40 ppm (Juniper et al., 2006). In the review by Alaejos and Romero (1995), Se concentration in milk was regarded as decreasing in order from human to sheep, then goat, then cow. According to our results, Se in camel milk was comparable to ewe milk (Davis et al., 2008), but more analyses are necessary in different Se supplementation contexts to establish convenient references for camel milk.
The organic Se supplementation appears more efficient than the non-organic form to increase the selenium content in colostrum and in milk up to one month. Similar observation was done by Gunter et al. (2013) in cattle. Ortman and Pehrson (1999) reported that Se concentrations in milk were increased by more than 190% in cows fed with seleno-yeast compared to cows fed with sodium selenite or selenite. This proportion is quite comparable to our findings which suggest a more efficient incorporation of Se in milk, especially in proteins (Alaejos and Romero, 1995).
In all groups, and in spite of the maintenance of Se supplementation, the selenium concentration in milk decreased regularly up to a mean value of 1.0 μg/100 mL approximately, according to a similar trend observed by Seboussi et al. (2009) as it is shown in comparison (Fig. 5). Similar findings were reported by Salman et al. (2013) in dairy cattle. After 7 days post-partum, the Se content in milk decreased by 60%, 42% and 35% in the control, inorganic and organic groups, respectively (Salman et al., 2013). In our trial, the decrease after 30 days was more important in organic group (65%) compared to inorganic (61%) and control (45%). Other authors reported higher Se content in colostrum compared to normal milk in dairy cattle (Weiss and Hogan, 2005). Salman et al. (2013) reported 3.0, 2.4 and 2.5 times greater Se concentration in cow colostrum, in Se-yeast, sodium selenite and control groups, respectively. Those values are comparable to our results: 2.9, 2.6 and 1.8, respectively. In ewe, Davis et al. (2006) reported higher Se concentration in colostrum compared to milk at 28 days and 56 days post-partum, whatever the level of Se supplementation (given under selenite form). However, this trend is not described in all references. For example, in goat, Petrera et al. (2009) did not describe such a trend in Se concentration in milk after delivery. Selenium in milk being mainly associated to the protein fraction (Debski et al., 1987), the decrease in Se concentration in milk might be linked to the change in total protein content after parturition as it is suggested by the data of Musaad et al. (2013) regarding the milk composition of the camels from our experiment (Fig. 6). The casein fraction of milk contains the most important part (60%) of selenium in cow (Debski et al., 1987), but this proportion could change with the species. Camel milk contains less casein than cow milk, but other fractions could play an important role, notably the whey proteins. In colostrum, the most abundant fraction from whey protein is the immunoglobulins. Data on Se in camel colostrum are lacking, but it is stated that selenium could stimulate immuno-protection of the new-born (Rock et al., 2001).
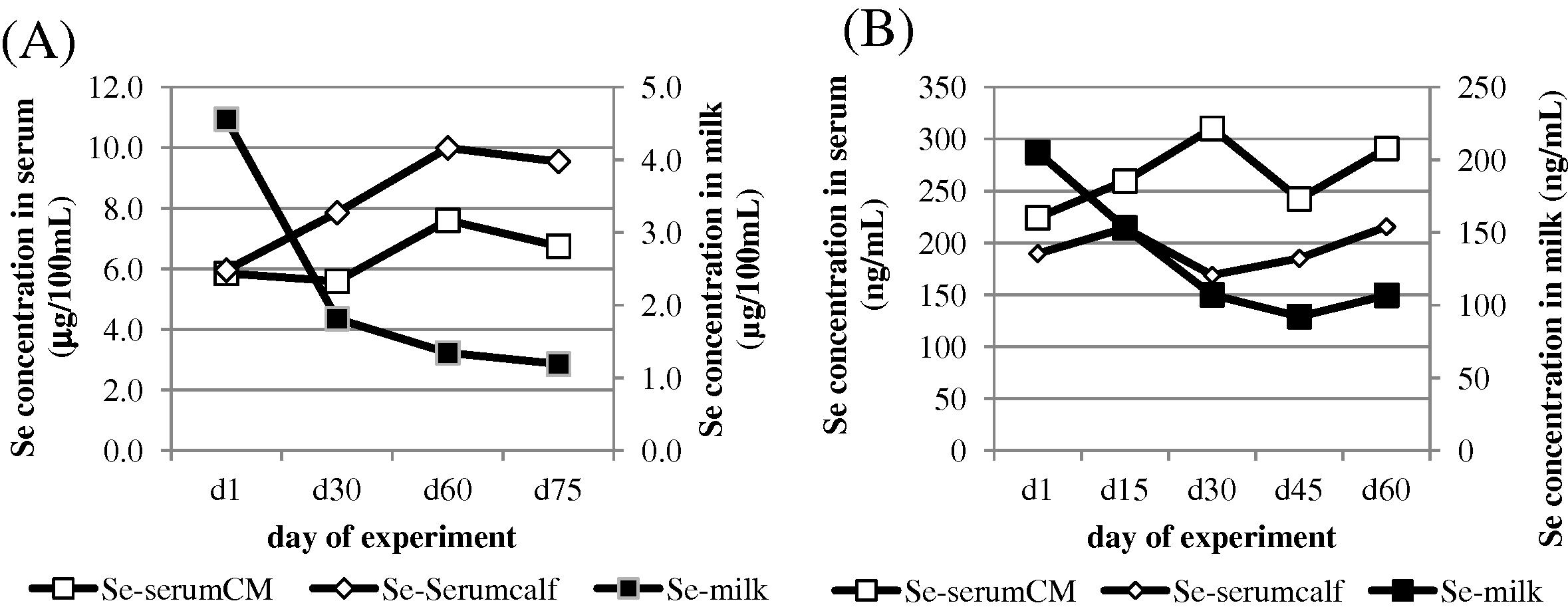
-
Post-partum changes in mean selenium concentration of mother (□) and calf serum (◊) and in milk ■ according to present results (A) and revisited data of Seboussi et al. (2009) (B).
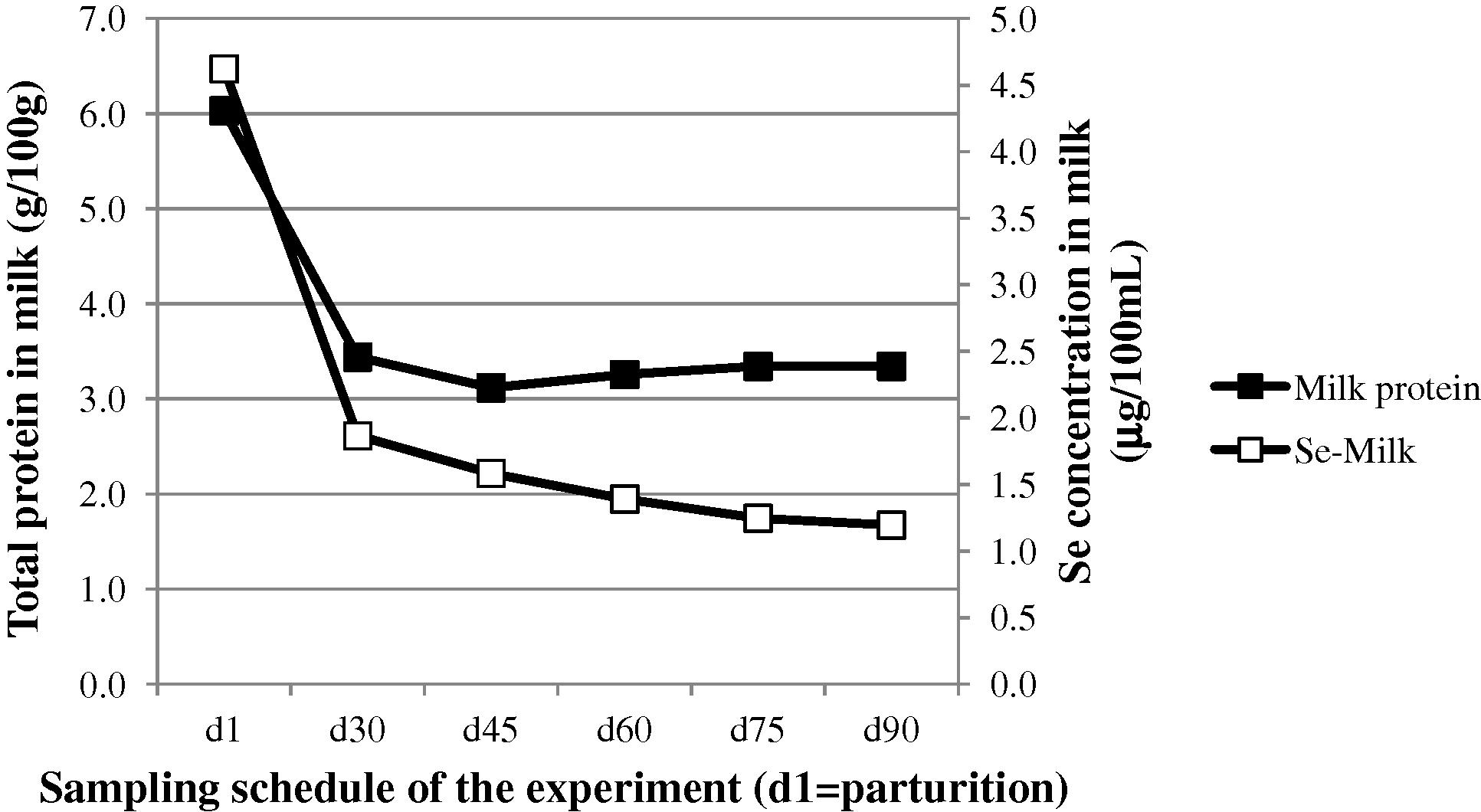
-
Post-partum changes in total milk protein ■ and milk selenium (□) concentrations in the camels from our experiment (data on milk protein from Musaad et al. (2013)).
In the conditions of our trial, the Se-Org supplementation appeared to have a more efficient milk transfer than for non-organic. However, the negative correlation between selenium in milk and Se-serum in calf was surprising. It was not observed in the experiment of Seboussi et al. (2009). Moreover, this negative correlation was observed within each group. However, this negative correlation could be explained by the priority given to the maintenance of a suitable metabolism function in mother organism after the colostrum phase which allows an important supply of selenium for the new-born calf. In the same date, the correlation remained significantly positive for colostrum. Guyot et al. (2007), reported higher concentrations in Se-serum in calf suggesting that placental transfer of Se during pregnancy might be more efficient than through milk. Moreover, supplementation with inorganic Se could have a substantial effect when animals are fed with low Se diet, but the impact would be less with higher Se diet (Salman et al., 2013). In consequence, the amplitude of the increase in milk-Se might change according to the Se content in diet.
The Se status is also regulated by the fecal excretion which represents 55–75% of the excreted Se according to the level of supplementation in camel (Faye and Seboussi, 2009). As well as in dairy cattle (Juniper et al., 2006), and in camel (Seboussi et al., 2008), fecal Se excretion increased when animals were supplemented, but in our study, this increase was reported at d30. No difference was observed after 2 months post-partum in spite of the difference observed in serum. However, in milk also, there was no difference in Se excretion between groups after 2 months. The Se-fecal concentration in our study (56.9–177.8 g/kg according to the groups and dates) was lower than values reported by Seboussi et al. (2008) (562–603 g/kg according to level of supplementation in sodium selenite in non-pregnant, non lactating camels) and in 2009 (239–941 g/kg according to the level of supplementation in sodium selenite in lactating camels). In these two previous studies, the Se fecal concentration was measured in feces collected on animals supplemented with higher level of selenium in the diet and for a longer time. However, as for these previous studies and contrary to Juniper et al. (2006) in cattle, no linear effect was clearly observed between Se excretion and dietary selenium. The higher excretion in organic supplemented camels compared to inorganic supplemented ones, at least at d30, would suggest that the Se requirement was better reached with organic Se.
In camel, somatic cell count determination is not commonly used, but as for other species, could be used as indirect diagnostic tool for detecting uninfected and infected quarters (Saleh and Faye, 2011). Elsewhere, beneficial effect of better Se status after Se supplementation was described in cow, even if inconsistent results were reported (Hemingway, 1999). After intramammary inoculation with a strain of Staphyloccocus aureus in dairy cow, SCC was lower throughout the 7-day period post-inoculation in supplemented cows (with inorganic Se) compared to non-supplemented animals (Kruze et al., 2007). In our study, the variability within group was too important to observe any difference although the SCC values were in the normal range for a camel with only 3.5% of samples above 200,000 cells/mL and one sample above 400,000 cells/mL. In fact, SCC is a multifactorial response and the udder infection status is the predominant factor for explaining the observed variability rather than the Se status of the animal (Kruze et al., 2007; Nagy et al., 2013).
5 Conclusion
The more efficient effect of supplementation with organic selenium compared to non-organic was confirmed in camel as it was stated by several authors in other ruminating animals. Such supplementation in animals living in highly-deficient areas as the Arabian Peninsula, where the economical importance of camel is great, must be encouraged. In particular, organic selenium allows a better protection of the new-born camel by a higher Se transfer into milk just after parturition and by a better bioavailability of Se. As the young camels are often affected by sudden death syndrome linked to selenium deficiency, a convenient supplementation of the mother at the end of pregnancy and at the beginning of lactation has to be promoted in the camel farms. Moreover, as the Se supply by milk decreases regularly after delivery, the supplementation must be renewed in young camels after weaning, especially in deficient areas.
Acknowledgements
This study has been achieved within FAO camel project UTF/SAU/021/SAU with the support of Camel and Range Research Center (CRRC). The authors thank Mr Sallal Issa Al-Mutairi, former head of the CRRC for his encouragement and support. We thank also the staff of the camel farm (Mrs Hassan and Surish) for the monitoring of the camel feeding and milking. Thanks also to FAO team leader at Riyadh, Dr A. Oihabi who supported this project.
References
- Phenotypic classification of Saudi Arabian camel (Camelus dromedarius) by their body measurements. Emir. J. Food Agric.. 2012;24(3):272-280.
- [Google Scholar]
- The relationship between whole blood selenium (Se) concentration and the activity of the seleno-enzyme, glutathione peroxidase (GSH-Px E.C.I.11.1.9) in camel (Camelus dromedarius) J. Arid Environ.. 2005;62:359-362.
- [Google Scholar]
- Selenium and iodine status of two camel breeds (Camelus dromedaries) raised under semi-intensive system in Saudi Arabia. Ital. J. Anim. Sci.. 2013;12(e14):83-87.
- [Google Scholar]
- Trace elements and their distribution in protein fractions of camel milk in comparison to other commonly consumed milks. J. Dairy Res.. 2001;68:463-469.
- [Google Scholar]
- Almathen, F., Mwaracharo, J., Hanotte, O., 2012. Genetic diversity and relationships of indigenous Saudi Arabia camel Camelus dromedarius populations. In: E.H. Johnson et al. (Eds.), Proc 3rd ISOCARD Conference, 29th January-1st February, 2012, Mascate (Sultanate of Oman), pp 40–41.
- Selenium and copper status of camels in Al-Jouf area (Saudi Arabia) Trop. Anim. Health Prod.. 2011;45:1039-1046.
- [Google Scholar]
- Effects of chemical species of selenium on maternal transfer during pregnancy and lactation. Life Sci.. 2009;84:888-893.
- [Google Scholar]
- Studies on selenium and vitamin E status of young Megaheem dromedary camels at Al-Ahsa province. J. Camel Pract. Res.. 2007;14:51-53.
- [Google Scholar]
- Comparative effect of selenium concentration and erythrocyte gluthatione peroxidase activity in cattle and camels. Anim. Sci.. 1998;67:461-466.
- [Google Scholar]
- Crain, S.R., 2007. Drought Related Nutrition Problems Threaten Midwest Horses. http//www.freshare.net/article/drought_related_nutrition_problems_threaten_midwest_horses/.
- Effects of selenium levels in ewe diets on selenium in milk and the plasma and tissue selenium concentrations of lambs. Small Rumin. Res.. 2006;65:14-23.
- [Google Scholar]
- Comparative effects of various dietary levels of Se as sodium selenite or Se yeast on blood, wool, and tissue Se concentrations of Wether sheep. Small Rumin. Res.. 2008;74:149-158.
- [Google Scholar]
- Selenium content and distribution of human, cow and goat milk. J. Nut.. 1987;117:1091-1097.
- [Google Scholar]
- Myocardial dystrophy in camel calves in the United Arab Emirates (field cases) Emir. J. Food Agric.. 2001;13:11-17.
- [Google Scholar]
- Some studies on nutritional muscular dystrophy in Qassim region in Saudi Arabia. Effect of administration of vitamin E-selenium preparation to pregnant ewes on serum muscle-specific enzymes in their lambs. Small Rumin. Res.. 2001;41:87-89.
- [Google Scholar]
- Faye, B., Seboussi, R. and Askar, M., 2008. Trace elements and heavy metals status in Arabian camel. In: Faye, B., Sinyavskiy, Y. (Eds.), Proc. of. Intern. Workshop, « Impact of pollution on animal products”. Almaty (Kazakhstan), 27–30 September 2007, pp. 97–106.
- Variability of vitamin E concentration in camel plasma. J. Camel Pract. Res.. 2010;16(2):157-161.
- [Google Scholar]
- Maternal transfer of selenium by blood and milk in camels. J. Camelid Sci.. 2011;4:30-39.
- [Google Scholar]
- Effect of Selenium injection in pregnant camels to the trace element status of their new-born and milk. Emir. J. Food Agric.. 2013;26 (accepted)
- [Google Scholar]
- Effects of supplementary selenium source on the blood parameters in beef cows and their nursing calves. Biol. Trace Elem. Res. 2013
- [CrossRef] [Google Scholar]
- Comparative responses to sodium selenite and organic selenium supplements in Belgian Blue cows and calves. Livest. Sci.. 2007;111:259-263.
- [Google Scholar]
- Determination of selenium in the serum of healthy swiss adults and correlation to dietary intake. J. Trace Elem. Med Biol.. 1998;10:31-45.
- [Google Scholar]
- The relationship between the concentration of selenium in the blood and the activity of glutathione peroxidase in the erythrocytes of the dromedary camel (Camelus dromedarius) Vet. Res. Commun.. 1990;14:27-30.
- [Google Scholar]
- The influences of dietary selenium and vitamin E intakes on milk somatic cell counts and mastitis in cows. Vet. Res. Commun.. 1999;23:481-499.
- [Google Scholar]
- Effect of different doses of organically bound selenium on antioxidant status and levels of metal ions in postpartum sows. Int. J. Electrochem. Sci.. 2013;8:6162-6179.
- [Google Scholar]
- The effect of feed additive containing vitamins and trace elements on the elements profile and growth of skin derivatives in horses. Ann. Anim. Sci.. 2013;12:381-391.
- [Google Scholar]
- Selenium supplementation of lactating dairy cows, effect on selenium concentration in blood milk, urine and faeces. J. Dairy Sci.. 2006;89:3544-3551.
- [Google Scholar]
- The effect of dietary organic and inorganic selenium supplementation on serum Se, Cu, Fe and Zn status during the late pregnancy in Merghoz goats and their kids. Small Rumin. Res.. 2013;110:20-27.
- [Google Scholar]
- Somatic cell count in milk of selenium-supplemented dairy cows after an intramammary challenge with Staphyloccos aureus. J. Vet. Med.. 2007;A54:478-483.
- [Google Scholar]
- Studies on the relationship between sway disease of Bactrian camels and copper status in Gansu Province. Vet. Res. Commun.. 1994;18:251-260.
- [Google Scholar]
- Seasonal and physiological variation of gross composition of camel milk in Saudi Arabia. Emir. J. Food Agric.. 2013;25(8):618-624.
- [Google Scholar]
- Microbiological quality and somatic cell count in bulk milk of dromedary camels (Camelus dromedarius): descriptive statistics, correlations, and factors of variation. J. Dairy Sci.. 2013;96:5625-5640.
- [Google Scholar]
- The relationship between serum level of thyroid hormones, trace elements and antioxidant enzymes in dromedary camel (Camelus dromedarius) Trop. Anim. Health Prod.. 2009;41:129-134.
- [Google Scholar]
- Effect of selenate as a feed supplement to dairy cows in comparison to selenite and selenium yeast. J. Anim. Sci.. 1999;77:3365-3370.
- [Google Scholar]
- Effect of sodium selen ite or Se-Yeast supplementation on selenium status and milk characteristics in dairy goats. Small Rumin. Res.. 2009;82:130-138.
- [Google Scholar]
- Reference selenium, copper, zinc and vitamins A, E and C serum values in dromedary camels. Online J. Vet. Res.. 2012;16(5):251-256.
- [Google Scholar]
- Effect of prenatal source of selenium on passive immunity and thermometabolism of newborn lambs. Small Rumin. Res.. 2001;40(2):129-138.
- [Google Scholar]
- Detection of subclinical mastitis in dromedary camels (Camelus dromedarius) using somatic cell counts, california mastitis test and udder pathogen. Emir. J. Food Agric.. 2011;23(1):48-58.
- [Google Scholar]
- Colostrum and milk selenium, antioxidative capacity and immune status of dairy cows fed sodium selenite or selenium yeast. Arch. Anim. Nutr.. 2013;67(1):48-61.
- [Google Scholar]
- Effect of different selenium supplementation levels on selenium status in camel. Biol. Trace Elem. Res.. 2008;123:124-138.
- [Google Scholar]
- Effect of selenium supplementation on blood status and milk, urine and fecal excretion in pregnant and lactating camel. Biol. Trace Elem. Res.. 2009;128:45-57.
- [Google Scholar]
- Relationship between lactation measures of somatic cell concentration and milk yield. J. Dairy Sci.. 1982;65:419-425.
- [Google Scholar]
- Effect of selenium in organic and inorganic form on liver, kidney, brain and muscle of Wistar rats. Cent. Eur. J. Chem.. 2012;10(5):1442-1451.
- [Google Scholar]
- Selenium content in the blood serum and urine of ewes receiving selenium-enriched unicellular alga chlorella. Vet. Med.. 2007;52:42-48.
- [Google Scholar]
- Maternal transfer and retention of supplemental selenium in neonatal calves. J. Dairy Sci.. 1984;67:416-420.
- [Google Scholar]
- Effect of selenium source on selenium status, neutrophil function, and response to intramammary endotoxin challenge of dairy cows. J. Dairy Sci.. 2005;88:4366-4374.
- [Google Scholar]
- Effects of copper and molybdenum supplements on the copper and selenium status of pregnant ewes and lambs. J. Anim. Sci.. 1989;67:803-809.
- [Google Scholar]







