Translate this page into:
Comparative assessment of various extraction approaches for the isolation of essential oil from polygonum minus using ionic liquids
⁎Corresponding authors. habibullah_kust@yahoo.com (Habib Ullah), cecili@utp.edu.my (Cecilia Devi Wilfred)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The recent trend of green consumerism in the modern society has triggered the use of plant-derived chemicals in the food, cosmetics, and fragrance industries. Ionic liquids emerged as an effective solvent for extraction of bioactive compounds from numerous plant resources. The objective of this research was to study the ionic liquid-assisted extraction of essential oil from polygonum minus and to compare the impact of various processing methods such as; microwave, ultrasonic, reflux and mechanical stirring on the yield of essential oil. Ionic liquids with various anions (NTf2, Cl, and Ac) and cations (AMIM, BMIM, and HMIM) were investigated. The performance of these ionic liquids was also compared with the organic solvent such as toluene, pentane, and hexane. The results indicated the cationic and anionic components exhibit remarkable impact on the extraction efficiency of the respective ionic liquids. In addition different processing parameter including extraction time, concentration and solid-liquid ratio were also optimized for all processing techniques employed. The use of Clevenger apparatus in combination with the ionic liquids based various processing techniques was also explored. The combination of Clevenger apparatus with ionic liquid based microwave-assisted extraction (ILMAE) technique showed the highest extraction efficiency of essential oil under the optimized condition of 21 min and 60 °C using [AMIM] Ac. We believe the present work would open for a new pathway for ILs based green extraction of a natural product from renewable resources.
Keywords
Microwave
Ultrasonic
Ionic liquids
Clevenger apparatus
Essential oil
1 Introduction
The essential oil (EO) is a volatile component of all the aromatic plants and shown the important application in different areas such as food and cool drink industries for flavor. It is also used in perfume, soaps, cosmetics, textile and paint industry. It has numerous biological activities such as antibacterial, antifungal, anticancer and antioxidant. Among the polygonum minus which have been investigated for the treatment of digestion, food stuff, traditionally used for body aches, digestive disorders, dandruff and perfume. The other species such as Polygonum hydropiper is one of the most numerous genuses in the family Polygonaceae which is abundant in Asia. This plant were used for the treatment of inflammation, rheumatoid arthritis, headache, colic pain, fever, chill, joint pain, edema and infectious diseases (Muhammad et al., 2013; Thakker et al., 2016; Chang et al., 2016; Ayaz et al., 2015; Ahmad et al., 2016). Numerous components naturally present in the EO which is characterized as terpenoids, alkane, alcohol, aldehyde, ketone, ester and acid with stable components (waxes and paraffin) (Verzera et al., 2004). In industries, the EO were extracted from the plant fruit and used as a co-product in the formation of juices (Matsuda et al., 2014). There are various techniques used for extraction of EO at the industrial scale such as distillation, reflux, and soxhlet. However, the high energy used in these methods degrade the oxygenated compounds (Stuart et al., 2001). Membrane and supercritical fluid extraction methods were also employed. A high quality of EO is obtained from membrane separation but these techniques tend to clog and have high maintenance costs (Arce et al., 2006; Brose et al., 1995; Zanousi et al., 2016). Recently, ionic liquids emerged as a potential solvent and show a specific advantage in the field of separation due to non-volatile, non-flammable, low vapor presser, and have high thermal stability. ILs used for extraction of EO instead of conventional solvents due to its toxic, and volatile nature (Benvenuti et al., 2001; Arce et al., 2007). Currently, ultrasonic and microwave- assisted extraction (UAE and MAE) methods have been recognized as the best technique for the extraction (Eskilsson and Björklund, 2000; Sun et al., 2013). These techniques reduced the extraction duration, the amount of solvent and enhance the yield of the chemical constituents. (Proestos and Komaitis, 2008; Careri et al., 2007). Liu et al. (2016) extracted the EO from Eucalyptus globulus leaves using Brönsted acidic ionic liquid combined with microwave and obtained a yield of EO about 8.76%. Jiao et al. (2013a) extracted EO using ionic liquids via microwave-assisted hydrodistillation (MILP–MHD) technique from Dryopteris fragrans. MILP–MHD gave a yield (0.91%) of EO with a shorter extraction time of 14.2 min. However, less attention is paid to extract the EO from P.minus using various techniques and ionic liquids.
The objectives of this study are to compare ionic liquids extraction efficiency with organic solvent using different extraction methods. ILs was employed for extraction of EO from P.minus on the basis of different cations and anions. The performance of different ionic liquids contains various cations and anions were investigated. Various experimental parameters such as time, solid/liquid ratio and concentration were optimized to get maximum yield of EO from P.minus.
2 Experimental
2.1 Material and chemical
The P.minus were purchased from the local market of Malaysia and identified by Professor Dr. Abdul Latiff Mohamad (plant taxonomist) Department of Biological Science University of Sains Malaysia. A voucher specimen (# 211) has been deposit at the herbarium of the Department biological Science University of sains. The material was washed with distilled water three times to remove dust and impurities, and was dry for twelve days at 45 °C in the oven. After that, it was grounded in powder form at 60–80 mesh size. Ionic liquids, 1-butyl-3-methylidazolum bis (trifluoromethyl sulfonyl) [BMIM]NTf2 (490092), 1-butyl-3-methylimidazolium chloride [BMIM] Cl(490079), 1-hexyl-3-methylimidazolium acetate [HMIM][Ac](491031), hexane and toluene were purchased from Merck Malaysia. 1-allyl-3-methylimidazolium acetate [AMIM] Ac (51053), 1-butyl-3-methylimidazolium acetate [BMIM] Ac (39952), and pentane (158941) were purchased from sigma Aldrich Malaysia.
2.2 Extraction procedures
For extraction of EO, four different techniques were employed. GC–MS were used for analysis.
2.2.1 Microwave assisted extraction
The extraction of EO was performed by Anton Paar (synthos 3000) microwave apparatus and multimode microwave (Em-s2088w). In a typical experiment, plant material was mixed with organic solvents (toluene, pentane and hexane) in the microwave vessel. Temperature, time and power were control by the external sensor. The mixture was heated at 60 °C for 60 min with continued stirring with and without Clevenger apparatus (the various cations and anions are shown in Fig. 1).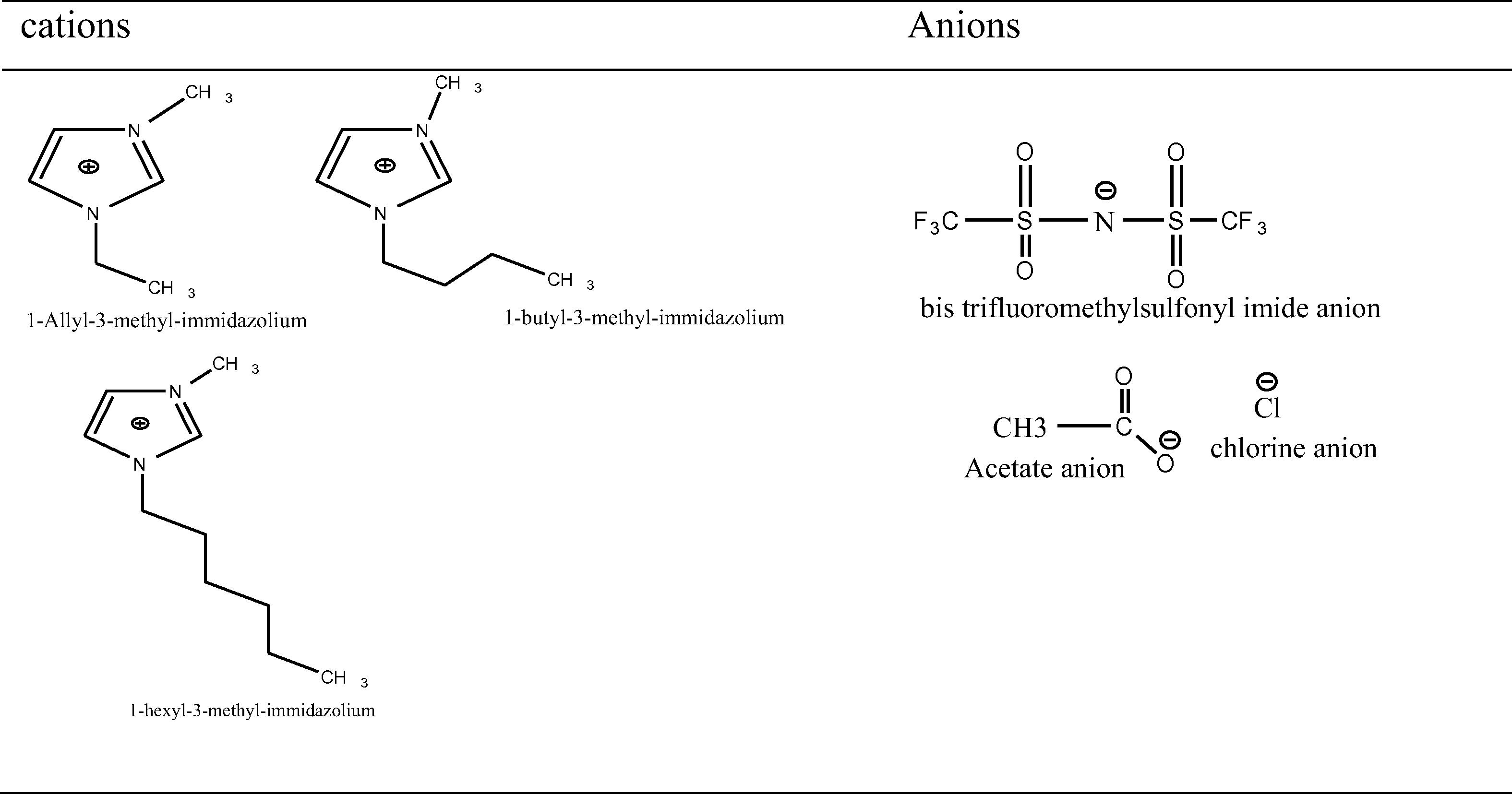
cations and anions.
In ILs based microwave assisted method plant material was mixed with aqueous ionic liquids with different concentrations, solid-liquid ratio and heated at 60 °C, 5 min with and without Clevenger apparatus. The reaction time increases 6, 7 and 8 min. The resulting residues were filtered with nylon membrane filter paper (0.02 mm). The EO was stored at 4 °C for further analysis. The extraction procedure is shown in Fig. 2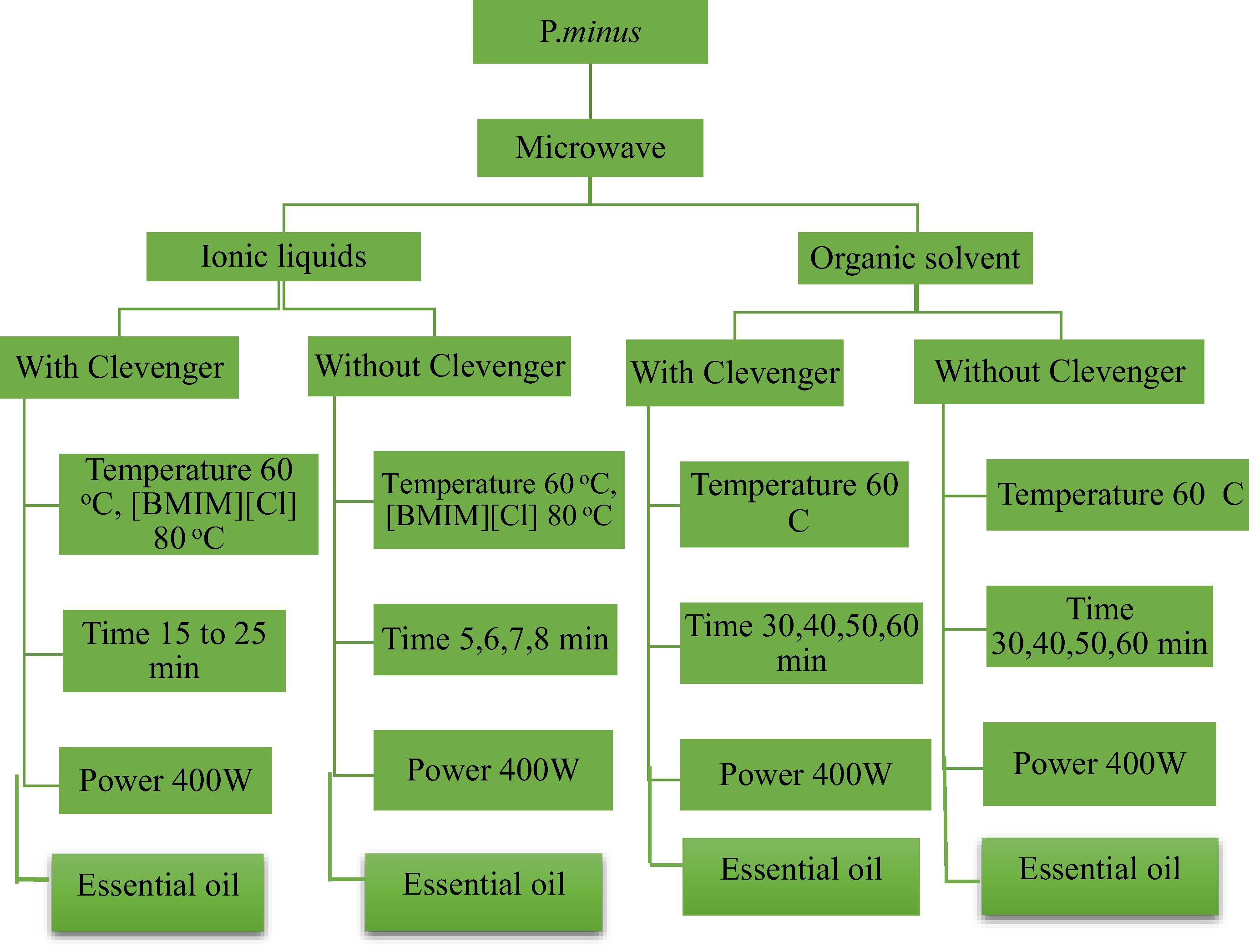
The flow chart for microwave-assisted extraction based on ionic liquids, organic solvent with and without Clevenger apparatus.
2.2.2 Ultrasonic assisted-extraction
Ultrasonic-assisted extraction of EO was performed using Sonic Vibra cell (T910 DH). Plant material was mixed with solvents (toluene, hexane and pentane) in the flask. The extraction was also performed with and without Clevenger apparatus.
In case of ILs, the plant material was mixed with aqueous ILs with a different concentration in the flasks. The extraction was conducting by using the same set of conditions with and without Clevenger apparatus as shown in Fig. 3.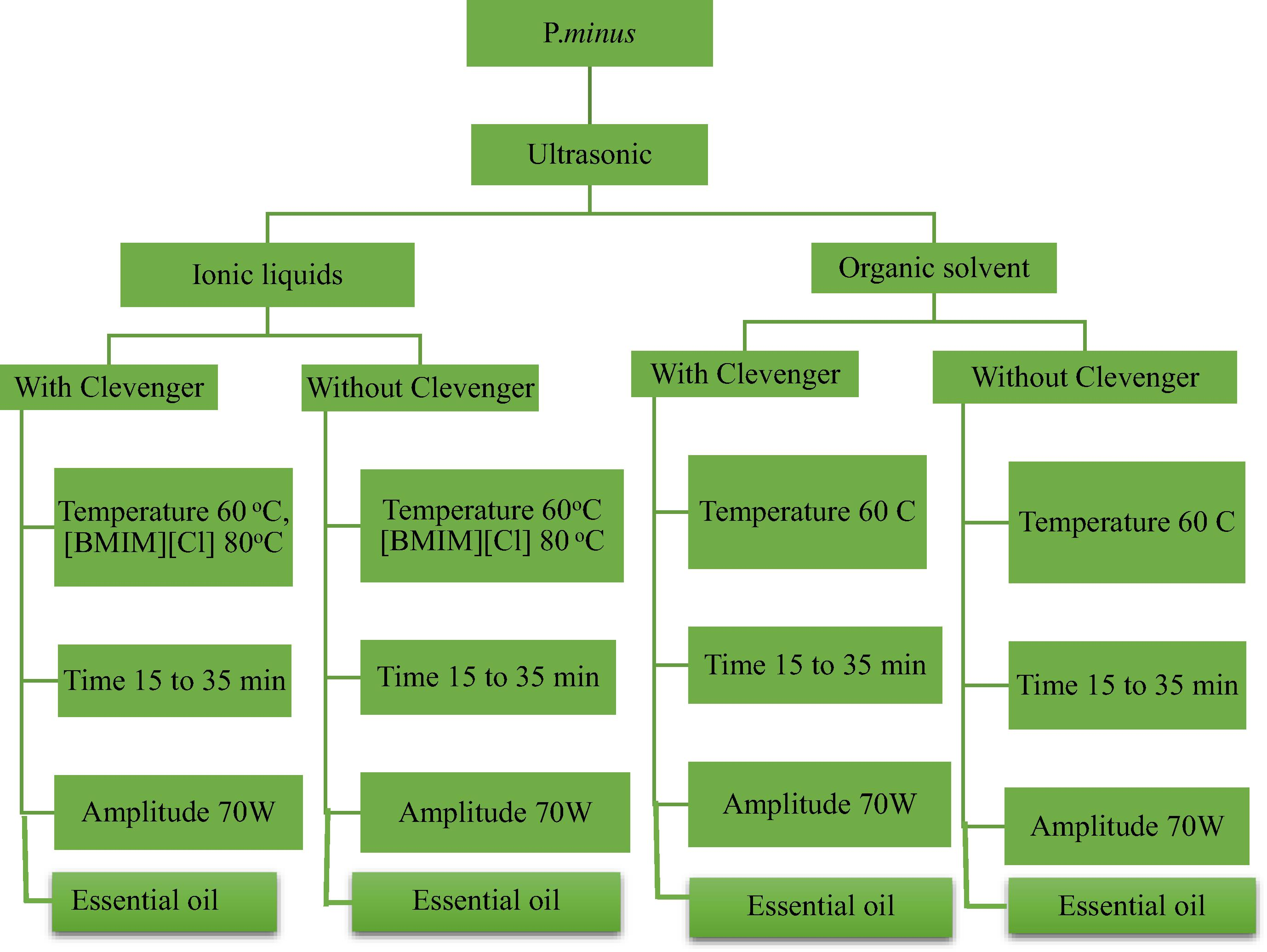
The flow chart for ultrasonic-assisted extraction based on ionic liquids, organic solvent with and without Clevenger apparatus.
2.2.3 Extraction of essential oil via mechanical stirring
In this experiment, (Model: Ika RW20 digital) apparatus were used for extraction. Plant material is mixed with 40 mL of solvents (like toluene, hexane and pentane) and stirs for one hour at room temperature. The resulting residue is filtered. In the case of ionic liquids, the different temperature was used as shown in Fig. 4.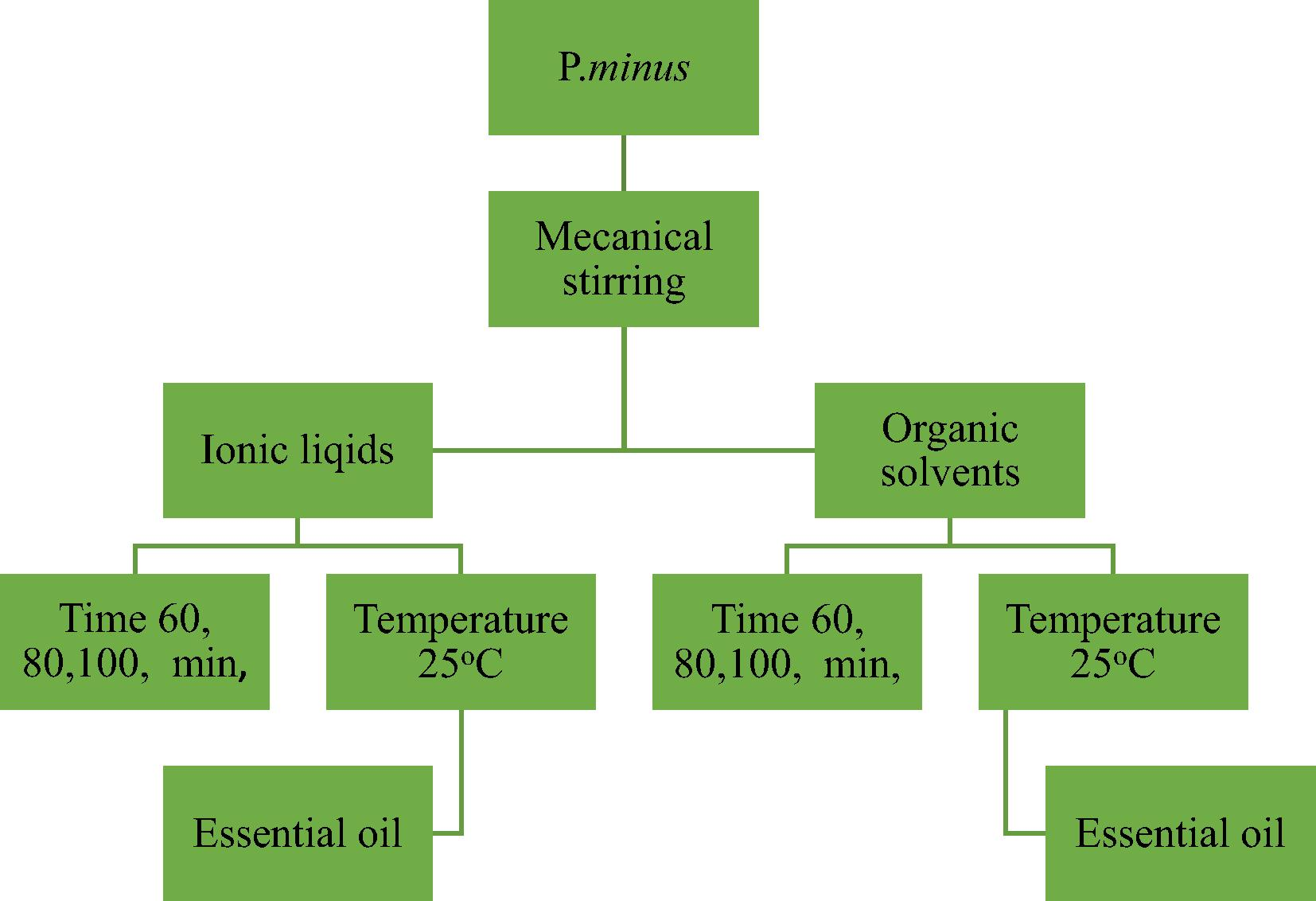
The flow chart for mechanical stirring extraction based on ionic liquids, organic solvent with and without Clevenger apparatus.
2.2.4 Extraction of essential oil via reflux method
The Reflux apparatus fitted with a round bottom flask and the suction pump was utilized in this scheme. Plant material was mixed with 40 mL of solvent (ionic liquids, toluene, hexane and pentane) in the reaction flask and heated at 60 °C for one hour. The temperature was constant at 60 °C for different time periods of 70, 80 and 90 min with and without Clevenger apparatus are shown in Fig. 5.
The flow chart for Reflux extraction based on ionic liquids, organic solvent with and without Clevenger apparatus.
2.3 Characterization
2.3.1 GCMS analysis
Gas chromatography-mass spectrometry (GCMS) spectra were recorded using GC–MS spectrometer (Agilent Technologies) with HP-5MS non-polar column (30 m length × 0.25 mm diameter × 0.25 μM thickness). Injection temperature: 220 °C; detection temperature: 280 °C; Column temperature, 50 °C, 3 min; 20 °C/min −100 °C, 3 min; 30 °C/min–250 °C, 3 min. Flow rate: 1.3 ml/min. Injection volume: 1 μl. Injection method split ratio 1:100. 70 eV, electron ionization mass spectra were acquired over the mass range 35–400 amu. Type of detector: quadruple. Mass spectrometry: scan mode. TIC, Total ion chromatogram. Retention indices of chemical constituents of EO were determined using the standard method of Kováts Indices to support the identification of the chemical constituents.
2.4 Compounds identification
The various compounds in the EO were identified on computer matching with libraries (NIST98, Mass finder library) built up from pure substances (Du et al., 2010; McLafferty, 1989) combined with the evaluation of GC–MS retention index on the nonpolar column. Retention index was calculated with the help of alkane series on the non-polar column (HP-5) with Kováts retention index formula.
3 Results and discussion
3.1 Effect of various processing parameters on the extraction of essential oil from P.minus
3.1.1 Anions and cations
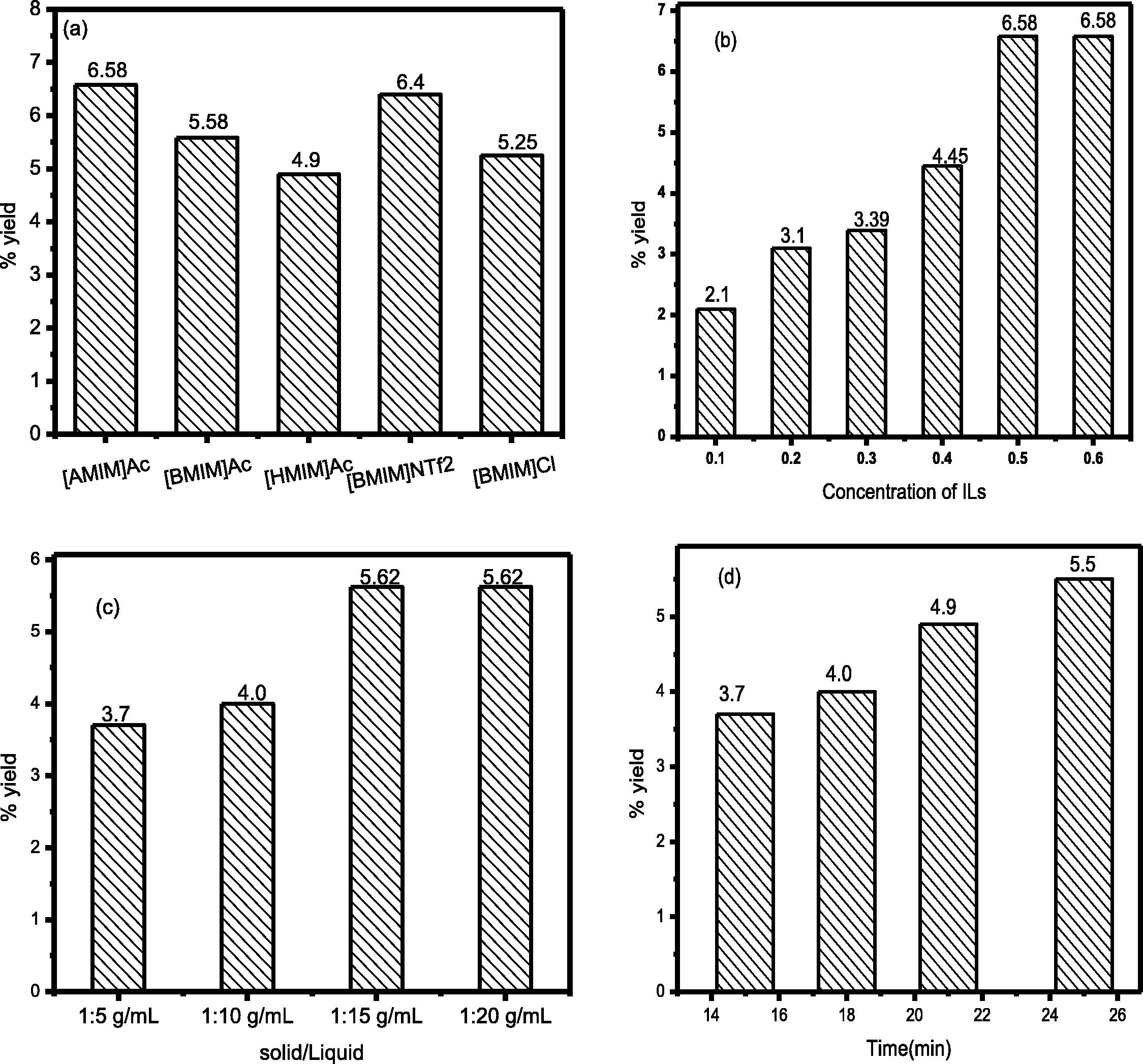
In order to study the effect of various anions and cations with Clevenger apparatus are shown in the Fig. 6A. Among various anions, Ac and cation [AMIM] gave best results (9.61%) with corresponding extraction efficiency due to low viscosity and high hydrogen bond basicity. By increasing the alkyl chain length from [BMIM] to [HMIM], the viscosity and hydrophobicity increase (Bi et al., 2011; Bica et al., 2011), which could result in decreased interaction between EO and ionic liquids (Pinkert et al., 2009). When the alkyl chain length increases the viscosity of ILs increased and requires a higher temperature for extraction. The extraction efficiency of [BMIM] Ac is higher (9.58%) than [HMIM] Ac (9.56%) of low viscosity (Zhao et al., 2016; Fukaya et al., 2008). Among the various anions [Ac] have higher extraction efficiency as compared to Cl and NTf2. It is because Ac anion with [BMIM] cation has a low viscosity as compared to Cl and NTf2 anions. Without Clevenger apparatus, the extraction efficiency decreased because the volatile compounds released due to an open system. The cation [AMIM] and the anion Ac without Clevenger gave the highest extraction (6.58%) efficiency compared to [BMIM] (5.45%) and [HMIM] (4.90%) as shown in the Fig. 5a. Among the various anions Ac have higher (6.58%) extraction efficiency compared to Cl (5.25%) and [NTf2] (6.40%) with cation [BMIM] 6a.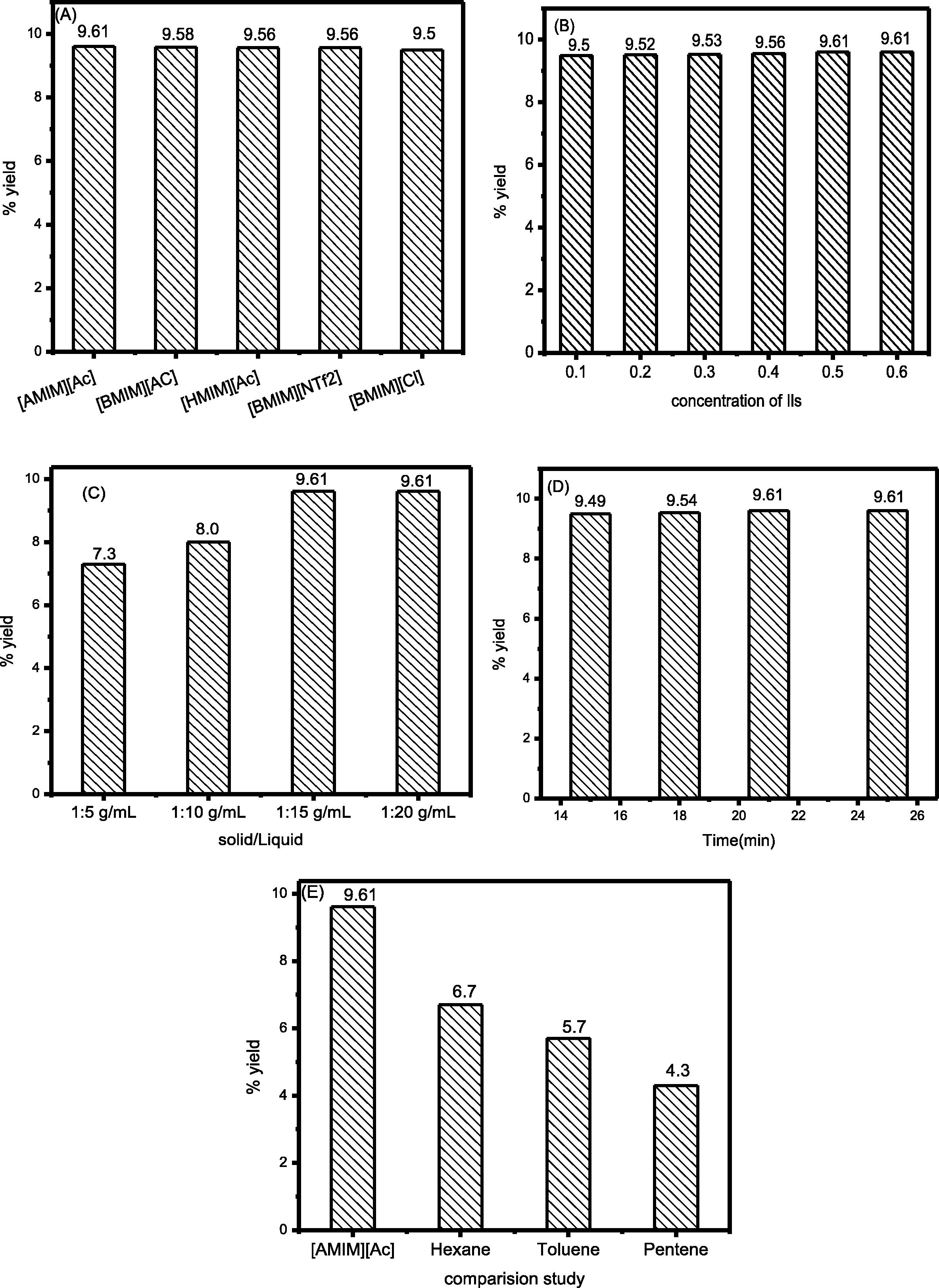
Effect of ILs with Clevenger apparatus (A), concentration (B), the solid-liquid ratio (C), time (D) and comparison study of ILs and an organic solvent (E). Effect of ILs without Clevenger apparatus (a), concentration (b), the solid-liquid ratio (c), time (d).
Effect of ILs with Clevenger apparatus (A), concentration (B), the solid-liquid ratio (C), time (D) and comparison study of ILs and an organic solvent (E). Effect of ILs without Clevenger apparatus (a), concentration (b), the solid-liquid ratio (c), time (d).
3.1.2 Concentration
In the Fig. 6B showed the various concentrations of aqueous ILs (0.10–0.60 mol/L) were used for the extraction of the EO. The high yield of EO (9.61%) was obtained when the concentration was 0.50 mol/L with Clevenger apparatus. Further increasing the concentration did not show any improvement in the extraction efficiency (Huddleston et al., 2001; Fan et al., 2012). Various concentrations of aqueous ILs (0.10 to 0.60 mol/L) were also used without Clevenger apparatus. The highest yield of EO (6.58%) was obtained when the concentrations were 0.50 mol/L without Clevenger apparatus shown Fig. 6b.
3.1.3 Effect of IL dose
To study the effect of IL dose on EO extraction efficiency various dose of IL ranging from 5 to 20 mL were used in keeping other condition same with and without Clevenger apparatus are shown in the Fig. 6C. The result demonstrates that the yield of EO gradually increases from 5 to 15 mL.
The (9.61% yield) of EO was obtained when the IL dose was 15 mL. However, from 15 to 20 mL no further increased in the EO yield was observed. This indicates that 15 mL is the optimum amount for extraction of EO (Jin et al., 2011). So we choose 15 mL doses for extraction of EO from plant biomass. The increase in the EO yield with the increase of IL amount is assigned to decrease in viscosity of IL and increase the active site. Higher yield of EO from plant biomass is due to efficient dispersion and diffusion of IL inside the pores of plant sample (Jiao et al., 2013b). The high extraction yield was obtained without Clevenger apparatus obtained (5.62%) as shown in the Fig. 6c. The yield decrease without Clevenger apparatus because of open system and volatile components do not accumulate.
3.1.4 Extraction time
The reaction time plays a vital role in the extraction of EO from plant biomass. Fig. 6D shows the effect of reaction time on the yield of EO from the plant while keeping the other conditions same. The extraction was carried out at various times from 15 min to 25 min. EO extraction increases when the time increased from 15 min to 21 min with Clevenger apparatus. After 21 min there was no further increase in the EO extraction was observed (Fig. 6D). Overall the optimum extraction of EO was obtained in ILMAE with Clevenger apparatus (9.61%) (Wei et al., 2013).
Without Clevenger apparatus, the extraction was carried out at various times from 15 to 25 min. The extraction efficiency decreased without Clevenger apparatus. The high extraction efficiency was obtained (5.50%) at 25 min as shown in the Fig. 6d.
3.2 Comparison study for different extraction methods using ILs and organic solvent
Generally, the conventional methods for extraction of bioactive compounds are maceration and Soxhlet extraction (Ho et al., 2007; Bowen-Forbes et al., 2009). However, long reaction time and a large amount of solvent are required for these processes. These drawbacks can be avoided by using alternative extraction methods and solvents, such as ultrasound (UAE), microwave-assisted extraction (MAE). In these two apparatus, MAE is best and has high extraction proficiency of EO. The yield of the compounds achieved by these methods has been compared with conventional techniques such as mechanical stirring and heat reflux method. As indicated in Fig. 6E the best extraction yield (9.61%) was achieved with ILMAE with Clevenger apparatus. Similarly, an extraction yield (about 9.58%) was also achieved in the case of ILUAE with Clevenger apparatus. The extraction of EO from P.minus is influenced by the solubility of compounds in the particular solvent used for the extraction process. The effect of different solvent on essential oil extraction has been evaluated and the results are shown in Fig. 6E. The best result (9.61%) was obtained with a solid/liquid ratio of 1:15.
3.3 Chemical composition of essential oil
The EO was analyzed by GC–MS and the list of 31 compounds identified from retention index using Kovats retention index formula from ILMAE shown in Table 1 and.
No
Compounds
Retention Indices a
Relative amount b
ILMAE % content
Rt
1
1-Hexanol
867
1.61
32.82
2
α-Pinene
931
1.16
34.48
3
Undecane
1100
2.46
34.81
4
Nonanal
1105
1.87
35.84
5
1-Nonanol
1173
1.02
35.89
6
Decanal
1209
2.01
35.97
7
1-Decanol
1274
16.29
36.31
8
Isobornyl acetate
1285
15.13
37.57
9
Undecanal
1308
1.01
38.11
10
α-Cubebene
1349
3.72
38.61
11
Xanthorrhizol
1368
2.53
38.67
12
(-)-α-Panasinsene
1381
1.74
38.74
13
Dodecanal
1388
1.36
38.85
14
(E)-Caryophyllene
1412
43.29
39.65
15
Trans-α-Bergamotene
1415
1.13
39.87
16
Farnesene
1452
0.06
40.18
17
α-Caryophyllene
1455
0.57
40.28
18
Alloaromadendrene
1458
0.71
40.30
19
β-Himachalene
1474
0.58
40.58
20
α-Selinene
1483
0.51
41.12
21
Valencene
1489
0.63
41.17
22
Phylloquinone
1490
3.78
41.34
23
Tetradecanal
1495
1.50
41.51
24
α-Curcumene
1510
2.56
41.94
25
δ-Cadinine
1522
1.08
42.73
26
Nerolidol
1531
5.40
43.15
27
α- Bisabolol
1683
0.70
43.18
28
Farnesol
1544
0.90
43.30
29
Decanoic acid
1562
0.70
44.14
30
β-Caryophyllene oxide
1565
1.02
44.76
31
cis-Lanceol
1765
2.02
41.49
The compounds dodecanal (43.29%) was found the major component of the essential oil followed by 1-decanol (15.13%), Isobornyl acetate (15.13%), n-decanoic acid (5.72%). Additionally, Alkane, terpenoids, and alcohol were found. The number of components extracted by ILMAE, ILUAE, reflux and mechanical stirring methods was 31, 16, 13 and 11 components respectively as shown in the Fig. 7a–d. The yield of each principal component depends on the specific extraction techniques used. The greater proportion of terpenoids, alkane, and alcohol were present in the EO obtained from ILMAE may be due to the reduction of hydrolytic and thermal effects (Fischer et al., 1988). Additionally, sesquiterpenes which have a higher dipolar moment could be interconnected more strongly with the microwave radiation, hence they can be extracted completely and more easily than other terpene hydrocarbons which have lesser dipolar moments (Bousbia et al., 2009). Therefore, this novel technique could offer a potential of producing high-quality EO that might be more important and can be used in various fields.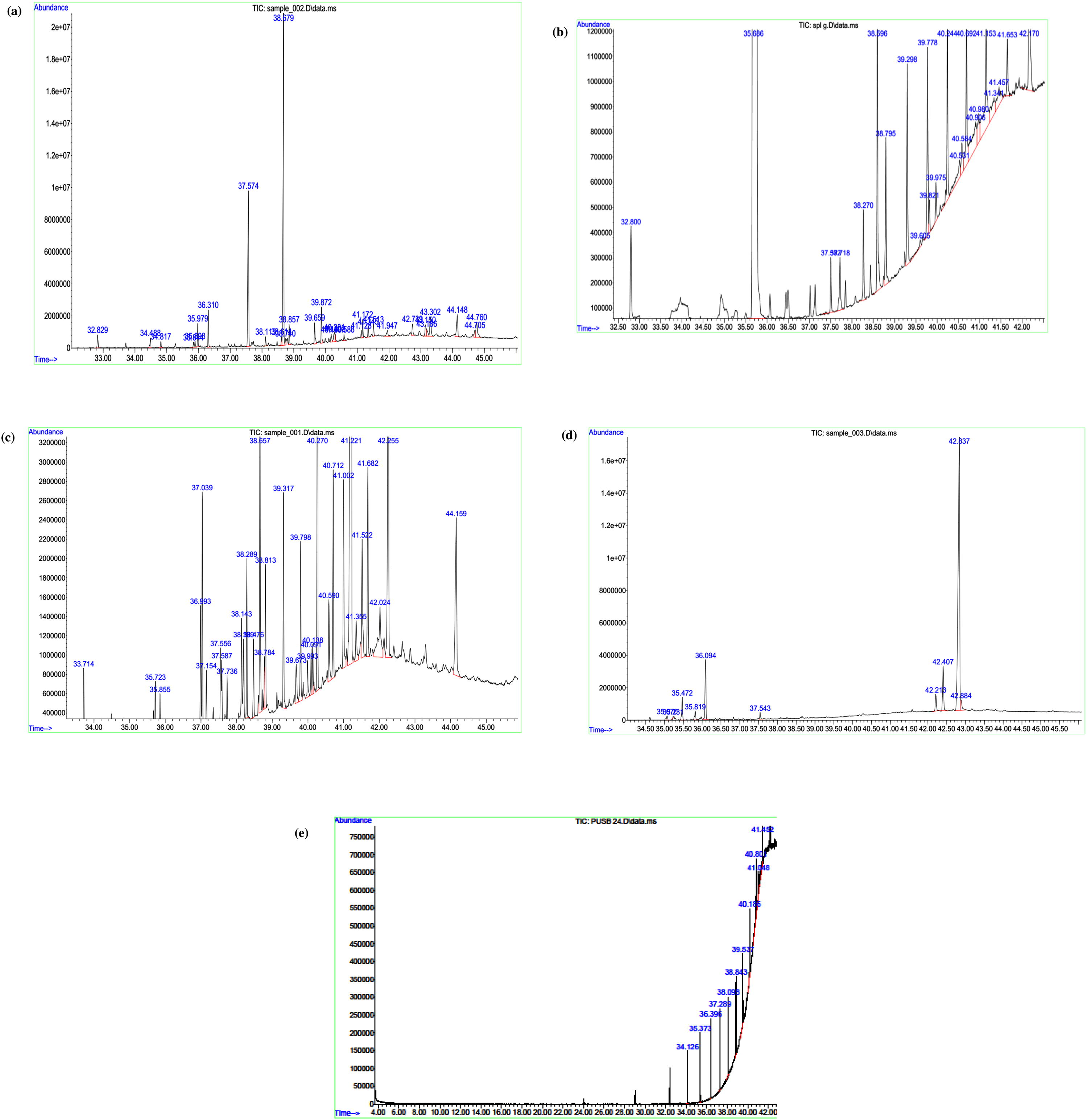
GC–MS spectra of essential oil with Clevenger apparatuses are, ILMAE (7a), ILUAE (7b), reflux (7c), mechanical stirring (7d).
For extraction without Clevenger apparatus, the compounds iso-Caryophyllene (3.02%) was found the major constituent of the essential oil followed by Tetradecanal (2.35%), Decanoic acid (2.02%). Additionally, sesquiterpenes hydrocarbons and alcohol were determined to be the two major groups presented in the essential oil. Dodecanoic acid, drimenin and tetradecanal were the principal components of the other extracted methods. As shown in Table 2, essential oils obtained by ILMAE, ILUAE and heat reflux method were similar in their composition. In ILMAE methods, extracted 11 compounds but in ILUAE 9 compounds and heat reflux method extracted 5 compounds as shown in Tables 2–4 in Fig. 8a,b,c.
No
Compounds
aRetention Indices
bRA
ILMAE% content
Rt
1
Nerolidol
1531
1.02
32.80
2
Decanoic acid
1562
2.02
35.68
3
Dodecanoic acid
1565
1.27
35.70
4
trans-α-(Z)-Bergamotol
1606
2.00
38.27
5
Tetradecanal
1614
2.35
38.59
6
Alloaromadendrene oxide-(1)
1458
1.09
38.79
7
trans- Longipinocarveol
1634
0.80
39.29
8
Neoisolongifolene, 8-bromo-
1639
1.57
39.77
9
iso-Caryophyllene
1737
3.02
40.24
10
Drimenol
1770
0.69
40.69
11
Drimenin
1941
1.14
42.17
No
Compounds
Retention Indices a
Relative amount b
ILUAE% content
Rt
1
undecane
1100
2.46
34.12
2
Nonanal
1105
1.87
35.37
3
1-Decanol
1274
16.29
36.31
4
Isobornyl acetate
1285
15.13
37.26
5
Undecanal
1308
1.01
38.09
6
Dodecanal
1388
1.36
38.85
7
(E)-Caryophyllene
1412
43.29
39.65
8
β-Himachalene
1474
0.58
40.80
9
Tetradecanal
1495
1.50
41.45
No
Compounds
Retention Indices a
Relative amount b
Heat reflux% content
Rt
1
α-Caryophyllene
1455
0.57
40.48
2
Alloaromadendrene
1458
0.71
40.87
3
α-Selinene
1483
0.51
41.08
4
Tetradecanal
1495
1.50
41.44
5
α-Curcumene
1510
2.56
41.73
GC–MS spectra of essential oil without Clevenger apparatus, ILMAE (8a), ILUAE (8b), reflux (8c).
4 Conclusion
In the present study, ILMAE was designed and applied for the isolation of essential oil from P.minus. The optimum conditions for microwave-assisted ionic liquid extraction were studied. Under the optimized conditions, satisfactory extraction efficiency of the essential oil was obtained. Relative to other methods (ultrasonic assisted extraction, heat reflux); the proposed approach provides higher extraction efficiency. The extraction of essential oil can therefore be readily and efficiently achieved by ILMAE. Moreover, with unique characteristics of ILs, the proposed ILMAE approach in this study has environmental protection, convenient, efficient characteristics and high practical valve technique in sample preparation and analysis. Based on higher extraction yield of essential oil, much shorter time rather higher contents of biomolecules, it is strongly believed that this extraction approach has a great potential for isolation of essential oil from aromatic plants.
Acknowledgement
The author acknowledges the facilities at Center of Research in Ionic Liquids (CORIL) Universiti Teknologi PETRONAS.
References
- Chemical composition, antioxidant and anticholinesterase potentials of essential oil of Rumex hastatus D. Don collected from the North West of Pakistan. BMC Complementary Altern. Med.. 2016;16:29.
- [Google Scholar]
- Essential oil terpenless by extraction using organic solvents or ionic liquids. AIChE J.. 2006;52:2089-2097.
- [Google Scholar]
- Citrus essential oil terpenless by extraction using 1-ethyl-3-methylimidazolium ethylsulfate ionic liquid: Effect of the temperature. Chem. Eng. J.. 2007;133:213-218.
- [Google Scholar]
- Comparative chemical profiling, cholinesterase inhibitions and anti-radicals properties of essential oils from Polygonum hydropiper L: A Preliminary anti-Alzheimer’s study. Lipids Health Dis.. 2015;14:141.
- [Google Scholar]
- Supercritical deterpenation of lemon essential oil, experimental data and simulation of the semicontinuous extraction process. J. Supercritical Fluids. 2001;20:29-44.
- [Google Scholar]
- Ultrasonication-assisted extraction and preconcentration of medicinal products from herb by ionic liquids. Talanta. 2011;85:701-706.
- [Google Scholar]
- Ionic liquids and fragrances–direct isolation of orange essential oil. Green Chem.. 2011;13:1997-1999.
- [Google Scholar]
- Comparison of two isolation methods for essential oil from rosemary leaves: Hydrodistillation and microwave hydrodiffusion and gravity. Food Chem.. 2009;114:355-362.
- [Google Scholar]
- Ursolic acid analogues: non-phenolic functional food components in Jamaican raspberry fruits. Food Chem.. 2009;116:633-637.
- [Google Scholar]
- Fractionation of citrus oils using a membrane-based extraction process. Biotechnol. Prog.. 1995;11:214-220.
- [Google Scholar]
- Optimization of a rapid microwave assisted extraction method for the liquid chromatography–electrospray-tandem mass spectrometry determination of isoflavonoid aglycones in soybeans. J. Chromatogr. A. 2007;1152:274-279.
- [Google Scholar]
- Iranian foeniculum vulgare essential oil and alcoholic extracts: chemical composition, antimicrobial, antioxidant and application in olive oil preservation. J. Essential Oil Bearing Plants. 2016;19:1920-1931.
- [Google Scholar]
- A rapid method for simultaneous determination of 14 phenolic compounds in Radix Puerariae using microwave-assisted extraction and ultra high performance liquid chromatography coupled with diode array detection and time-of-flight mass spectrometry. J. Chromatogr. A. 2010;1217:705-714.
- [Google Scholar]
- Analytical-scale microwave-assisted extraction. J. Chromatogr. A. 2000;902:227-250.
- [Google Scholar]
- Optimization of ionic liquid based ultrasonic assisted extraction of puerarin from Radix Puerariae Lobatae by response surface methodology. Food Chem.. 2012;135:2299-2306.
- [Google Scholar]
- Original composition of marjoram flavor and its changes during processing. J. Agric. Food Chem.. 1988;36:996-1003.
- [Google Scholar]
- Cellulose dissolution with polar ionic liquids under mild conditions: required factors for anions. Green Chem.. 2008;10:44-46.
- [Google Scholar]
- Oleanane-type triterpenes from the flowers, pith, leaves, and fruit of Tetrapanax papyriferus. Phytochemistry. 2007;68:631-635.
- [Google Scholar]
- Characterization and comparison of hydrophilic and hydrophobic room temperature ionic liquids incorporating the imidazolium cation. Green Chem.. 2001;3:156-164.
- [Google Scholar]
- Microwave-assisted ionic liquids pretreatment followed by hydro-distillation for the efficient extraction of essential oil from Dryopteris fragrans and evaluation of its antioxidant efficacy in sunflower oil storage. J. Food Eng.. 2013;117:477-485.
- [Google Scholar]
- Microwave-assisted ionic liquids treatment followed by hydro-distillation for the efficient isolation of essential oil from Fructus forsythiae seed. Sep. Purif. Technol.. 2013;107:228-237.
- [Google Scholar]
- Microwave assisted ionic liquid pretreatment of medicinal plants for fast solvent extraction of active ingredients. Sep. Purif. Technol.. 2011;83:45-49.
- [Google Scholar]
- Microwave-assisted method for simultaneous hydrolysis and extraction in obtaining ellagic acid, gallic acid and essential oil from Eucalyptus globulus leaves using Brönsted acidic ionic liquid [HO 3 S (CH 2) 4 mim] HSO 4. Ind. Crops Prod.. 2016;81:152-161.
- [Google Scholar]
- Liquid-liquid equilibria for extraction of citrus essential oil using ionic liquids. J. Solution Chem.. 2014;43:1561-1573.
- [Google Scholar]
- Mclafferty, F.W.S., 1989. The Wiley/NBS registry of mass spectral data.
- Investigations of novel nitrile-based ionic liquids as pre-treatment solvent for extraction of lignin from bamboo biomass. J. Ind. Eng. Chem.. 2013;19:207-214.
- [Google Scholar]
- Ionic liquids and their interaction with cellulose. Chem. Rev.. 2009;109:6712-6728.
- [Google Scholar]
- Application of microwave-assisted extraction to the fast extraction of plant phenolic compounds. LWT-Food Sci. Technol.. 2008;41:652-659.
- [Google Scholar]
- Deterpenation of Brazilian orange peel oil by vacuum distillation. J. Am. Oil. Chem. Soc.. 2001;78:1041-1044.
- [Google Scholar]
- Aqueous ionic liquid based ultrasonic assisted extraction of four acetophenones from the Chinese medicinal plant Cynanchum bungei Decne. Ultrason. Sonochem.. 2013;20:180-186.
- [Google Scholar]
- Isolation of Essential Oil from the Leaves of Cymbopogon martinii using Hydrodistillation: Effect on Yield of Essential Oil, Yield of Geraniol and Antimicrobial Activity. J. Essential Oil Bearing Plants. 2016;19:1943-1956.
- [Google Scholar]
- Biological lemon and sweet orange essential oil composition. Flavour Fragrance J.. 2004;19:544-548.
- [Google Scholar]
- Ionic liquids-based microwave-assisted extraction of active components from pigeon pea leaves for quantitative analysis. Sep. Purif. Technol.. 2013;102:75-81.
- [Google Scholar]
- Composition of the essential oils and volatile fractions of Artemisia absinthium by three different extraction methods: Hydrodistillation, solvent-free microwave extraction and headspace solid-phase microextraction combined with a novel QSRR evaluation. J. Essential Oil Bearing Plants. 2016;19:1561-1581.
- [Google Scholar]
- Development of an Ionic Liquid-Based Ultrasonic/Microwave-Assisted Simultaneous Distillation and Extraction Method for Separation of Camptothecin, 10-Hydroxycamptothecin, Vincoside-Lactam, and Essential Oils from the Fruits of Camptotheca acuminata Decne. Appl. Sci.. 2016;6:293.
- [Google Scholar]







