Translate this page into:
Comparative assessment of phenolic content, cellular antioxidant, antityrosinase and protective activities on skin cells of extracts from three sweet potato (Ipomoea batatas (L.) Lam.) cultivars
⁎Corresponding author at: Department of Plant Production Technology and Commodity Science, University of Life Sciences in Lublin, Poland, 20-950 Lublin, Poland. anna.kieltyka-dadasiewicz@up.lublin.pl (Anna Kiełtyka-Dadasiewicz)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objective
New raw materials are still being sought that could nurture the skin and protect it against various harmful factors, including free radicals responsible for ageing processes, cell mutagenesis and formation of cancerous lesions. Although the sweet potato (Ipomoea batatas (L.) Lam.) is not widely known as a medicinal plant, the available study findings suggest its multidirectional, health relevant properties, including anti-inflammatory, immunomodulatory, antimutagenic, and antimicrobial activities, as well as anticancer potential. However, the data on its antitirosinase and protective effects on skin cells are rather limited.
Methods
Our study evaluated the phenolic content, antioxidant, and antityrosinase activities, as well as cytotoxic effects of the extracts obtained from three sweet potato cultivars ('Beauregard', 'Purple' and 'Carmen Rubin') on two human cell lines - keratinocytes (HaCaT) and fibroblasts (BJ).
Results
The results evidenced that the cultivar characterized by the strongest antioxidant properties as well as the positive effect on the vitality of skin cells is the ‘Beauregard’ cultivar. Our findings showed that all three types of sweet potato extracts were tyrosinase inhibitors, yet their inhibitory capacities differed significantly.
Conclusions
Sweet potatoes can be considered a reservoir of biologically active substances with beneficial health properties.
Keywords
Ipomoea batatas
Plant extract
Medicinal plant
Polyphenols
Antioxidant
Tyrosinase inhibitors
1 Introduction
Human skin is continuously exposed to numerous harmful external factors, which lead to skin degenerative changes and accelerate skin ageing (Zhang and Duan, 2018). In addition to wrinkles, loss of elasticity and dryness, the clinical manifestations of skin ageing include hyperpigmentation disorders, in which an abnormal accumulation of melanin in the epidermis is an indicator of sun damage (the Glogau classification). Melanin, the natural skin pigment formed during complex biochemical changes in the melanocytes, mediated by tyrosinase, not only affects skin pigmentation but also plays an important role in photoprotection (Solano, 2014; d’Ischia et al., 2015; Yamaguchi et al., 2007; Hennessy et al., 2005). Melanin, located above the nuclei of keratinocytes, absorbs and disperses the UV radiation, but is also capable of interacting with reactive oxygen species (ROS), i.e. singlet oxygen, hydroxyl radical and superoxide anion radical, which can be generated in photochemical reactions. Therefore, melanin reduces their formation and protects the cells from damage to DNA, proteins, and lipids. The above-mentioned properties of melanin are essential for preventing the formation of mutations and the development of cancerous lesions, in particular basal cell carcinoma, squamous cell carcinoma or melanoma. The skin is equipped with natural anti-ROS defence mechanisms; however, in cases of excessive ROS production, the skin is exposed to the effects of oxidative stress (Masaki, 2010; Nichols and Katiyar, 2010). Free radical (FR) reactions lead to skin lesions characterized by impairment of defensive, regulatory, and regenerative mechanisms of the skin (Wölfle et al., 2014; Jadoon et al., 2015). FRs cause skin damage by destroying the lipid components of sebum, intercellular cement ceramides of the stratum corneum, oxidation of polyunsaturated fatty acids, cell membrane phospholipids, increased activity of metalloproteinases (MMPs) responsible for collagen and elastin degradation, as well as depolymerization of hyaluronic acid. Oxidative stress induced by ROS generated in the skin under the influence of solar radiation, is considered the main pathological mechanism causing damage to proteins of the extracellular matrix (ECM) and leading to photomutagenesis of skin cells (Wölfle et al., 2014; Pai et al., 2014).
Antioxidants play an important role in preventing and repairing skin damage caused by FRs. In recent years, numerous studies have focused on finding the antioxidants, including plant extracts as well as secondary metabolites of plants, that would scavenge FRs and support the defensive and regenerative mechanisms of the skin (Nichols and Katiyar, 2010; Wölfle et al., 2014; Jadoon et al., 2015; Pai et al., 2014). An example of such raw plant materials is the sweet potato (Ipomoea batatas (L.) Lam., Convolvulaceae family), whose tubers contain many beneficial phytochemicals, including carotenoids, minerals (zinc, potassium, sodium, manganese, calcium, magnesium, and iron), vitamins (vitamin A, B6, C, K), phenolic acids, flavonoids, anthocyanin, terpenoids, tannins, saponins, glycosides, alkaloids, and steroids (Sun et al., 2019; Krochmal-Marczak et al., 2014; Ayeleso et al., 2016). Although the sweet potato is not widely known as a medicinal plant, some studies have demonstrated its beneficial effects on the prevention or treatment of chronic diseases due to antioxidant, anti-inflammatory, antilipogenic, immunomodulatory, anticancer, antiulcer, and antimicrobial activities it exhibits (Tang et al., 2015; Ji et al., 2015; Li et al., 2013; Sugata et al., 2015; Mbaeyi-Nwaoha and Emejulu, 2013; Panda and Sonkamble, 2012). However, the studies on the potato protective effect on the skin cells are scarce. The evaluation of the chemical composition and bioactivity of extracts from different potato varieties is essential to thoroughly investigate and use the medicinal potential of this raw material. Such knowledge could promote sweet potatoes in the pharmaceutical and cosmetic industry, allowing to design new products containing sweet potato tubers.
Therefore, the aim of the present study was to compare the protective effects of extracts from three sweet potato cultivars on cultured skin cell lines in vitro. The extracts were obtained from 'Beauregard' (B), 'Carmen Rubin' (CR), and 'Purple' (P), cultivated in Poland under identical conditions. The effect of extracts on exogenous FRs and the amount of FRs formed inside the skin cells treated with these extracts were assessed. Moreover, the inhibiting potential of tyrosinase was evaluated. The experiments were performed on two human cell lines: keratinocytes (HaCaT) and fibroblasts (BJ).
2 Materials and methods
2.1 Plant material
The plant material used in the study included tubers of three cultivars of sweet potato: 'Beauregard' (B), 'Purple' (P) and 'Carmen Rubin' (CR). To eliminate the influence of the environmental conditions of plant growth on the activity of extracts, tubers of all varieties were obtained from our cultivation conducted under identical conditions, in slightly acidic brown earth, in Żyznów (49°49′ N and 21°50′ E, Poland) in 2017. The fertilization was maintained on a fixed level and the cultivation was carried out in accordance with good agricultural practice.
2.2 Chemicals
2,2-diphenyl-1-picrylhydrazyl (Sigma-Aldrich, Poznan, Poland), 2′,7′-dichlorodihydrofluorescein diacetate (Thermo Fisher Scientific, Waltham, MA USA), acetic acid (≥99%, Sigma-Aldrich, Poznan, Poland), aluminum nitrate (Chempur, Piekary Slaskie, Poland), antibiotics (Penicillin-Streptomycin, Life Technologies, Bleiswijk, Netherlands), ascorbic acid (Sigma-Aldrich, Poznan, Poland), dimethyl sulfoxide (≥99.9% , Sigma-Aldrich, Poznan, Poland), DMEM (Dulbecco's Modification of Eagle's Medium, Biological Industries Israel Beit Haemek Ltd., Israel),ethyl alcohol (ethanol, 96%, Sigma-Aldrich, Poznan, Poland), FBS (Fetal Bovine Serum, Biological Industries, Genos, Lodz, Poland), Folin-Ciocalteu reagent (Sigma-Aldrich, Poznan, Poland), gallic acid (Sigma-Aldrich, Poznan, Poland), hydrogen peroxide solution (30%, Sigma-Aldrich, Poznan, Poland), kojic acid (Sigma-Aldrich, Poznan, Poland), L-DOPA (Sigma-Aldrich, Poznan, Poland), Neutral Red Solution (0.33%, Sigma-Aldrich, Poznan, Poland), phosphate buffered saline (PBS, pH 7.00 ± 0.05, ChemPur, Piekary Slaskie, Poland), potassium acetate (Sigma-Aldrich, Poznan, Poland), resazurin sodium salt (Sigma-Aldrich, Poznan, Poland), rutin (≥95.0%, Sigma-Aldrich, Poznan, Poland), quercetin (Sigma-Aldrich, Poznan, Poland), sodium carbonate (Sigma-Aldrich, Poznan, Poland), trypsin-EDTA solution (Sigma-Aldrich, Poznan, Poland) and tyrosinase from mushroom (Sigma-Aldrich, Poznan, Poland) were used as received.
2.3 Ultrasound-assisted extraction
The aqueous extracts from three sweet potato cultivars were obtained using the ultrasound-assisted extraction (UAE) method. UAE was performed according to Yang et al. (2009). Twenty grams of tubers were packed to the glass beakers and extracted with 200 mL of deionized water. Initially, the extractions were carried out at room temperature (about 22–24 °C) for 12 h at 500 rpm using a magnetic stirrer (Chemland, MS-H-S, Stargard, Poland). Subsequently, the mixture was homogenized for 50 min in an ultrasonic cleaner (Sonic-3, Polsonic, Warsaw, Poland). The obtained extracts were collected, filtered three times through Whatman filter paper No. 10 using a vacuum pump (Aga Labor 154 PL2/2, Warsaw, Poland) and stored in the dark at 4 °C for further analysis.
2.4 Total phenolic content determination
The total phenolic content (TPC) in extracts was determined by the Folin-Ciocalteu method according to the procedure described by Singleton et al. (1999) with modifications; 300 μL of the extract (25–5000 μg/mL) and 1500 μL of 1:10 Folin-Ciocalteu reagent were mixed. After 6 min of incubation, 1200 μL of sodium carbonate (7.5%) was added. After 2 h, the absorbance was measured spectrophotometrically at λ = 740 nm using DR600 UV–Vis spectrophotometer (Hach Lange, Wroclaw, Poland).
2.5 Total flavonoid content determination
The total flavonoid content (TFC) of sweet potato extracts was evaluated according to the procedure described by Woisky and Salatino with modifications (Matejić et al., 2013); 600 μL of extract solutions (25–5000 μg/mL) and 2400 μL of the mixture (80% C2H5OH, 10% Al(NO3)3 × 9 H2O and 1 M C2H3KO2) were mixed. After 40 min of incubation at room temperature, the absorbance was measured spectrophotometrically at 415 nm using DR600 UV–Vis spectrophotometer (Hach Lange, Wroclaw, Poland).
2.6 DPPH radical scavenging activity
The ability of study extracts to scavenge FRs was evaluated using the DPPH (2,2-diphenyl-1-picrylhydrazyl) assay according to Brand-Williams et al. (1995); 167 µL of 4 mM ethanol solution of DPPH was mixed with 33 µL of samples of different concentrations (25–5000 µg/mL). Ascorbic acid, rutin and quercetin at a concentration of 100 µg/mL were used as positive controls. The absorbance was measured at λ = 516 nm every 5 min for 30 min using a microplate reader (FilterMax F5 Molecular Devices, San Jose,CA, USA). The DPPH solution mixed with an equal volume of distilled water was used as a control. The percentage of DPPH radical scavenging was calculated using the following equation:
2.7 Cell culture
The BJ cells (fibroblasts, ATCC®CRL-2522™) used in the study were obtained from the American Type Culture Collection (Manassas, VA 20108, USA). The HaCaT cells (normal human keratinocytes) were purchased from CLS Cell Lines Service (Germany). The cells were maintained in a Dulbecco’s Modified Essential Medium (DMEM), Gibco) with L-glutamine, supplemented with 10% (v/v) FBS (foetal bovine serum, Gibco), and 1% (v/v) antibiotic (100 U/ml Penicillin and 1000 µg/mL Streptomycin, Gibco). The fibroblasts were maintained in modified minimal essential medium (MEM) supplemented in the same way as DMEM. The cultured cells were kept at 37 °C in a humidified atmosphere of 95% air and 5% carbon dioxide (CO2).
2.8 Cell viability assay
The Neutral Red Uptake (NRU) assay (Sigma Aldrich) was used to assess the viability of HaCaT and BJ cells. After 24 h of pre-culture in 96-well plates, the medium was aspirated and different concentrations (25–500 μg/mL) of extracts were added into each well and cultured for another 24 h. The control group consisted of unexposed cells. Following the exposure to sweet potato tuber extracts, the cells were incubated for 2 h with a neutral red dye (40 μg/mL) dissolved in the serum-free medium and washed with phosphate-buffered saline (PBS); subsequently, 150 μL of the Destain solution (EtOH/AcCOOH/H2O2, 50%/1%/49%) was added per well and gently shaken for 10 min, until the NR was extracted from the cells and formed a homogenous solution. The NR uptake was determined by measuring the optical density (OD) of the eluted dye at 540 nm using a microplate reader (FilterMax F5 Molecular Devices, San Jose,CA, USA). The experiments were performed in triplicates and presented as the percentage of control values.
2.9 Detection of intracellular levels of ROS
The cells were seeded in 96–well plates at a density of 1 × 104 cells per well and cultured for 24 h prior to the experiment. Then, the culture medium was replaced with 10 µM 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA) (Sigma Aldrich) in the serum-free medium. The cells were incubated with H2DCFDA for 45 min and exposed to different concentrations of aqueous sweet potato 'Beauregard' extract (25–500 μg/mL). The cells treated with 1 mM hydrogen peroxide (H2O2) were used as a positive control. The 2′,7′-dichlorofluorescein (DCF) fluorescence was measured every 30 min for a total of 90 min at maximum excitation of 485 nm and emission spectra of 530 nm using a microplate reader (FilterMax F5 Molecular Devices, San Jose,CA, USA).
2.10 Inhibitory effects against tyrosinase
Phosphate buffer (pH 6.8) was added to each well containing the extract solution diluted in DMSO (25–500 μg/mL), followed by mushroom tyrosinase solution (450 U/mL). After each well was mixed and pre-incubated at 25 °C for 10 min, L-DOPA was added to the mixture and incubated for 20 min.
The ability of extracts obtained from sweet potato tubers to inhibit tyrosinase enzyme activity was measured using the procedure with L–DOPA as a substrate (Wang et al., 2011). Phosphate buffer (pH 6.8) was added to each well containing the extract solution diluted in DMSO (25–500 μg/mL; then the mushroom tyrosinase solution (450 U/mL) was also added. After each well was mixed and pre-incubated at 25 °C for 10 min, L-DOPA was added to the mixture and incubated for 20 min. The absorbance of each well was measured at 492 nm using a microplate reader (FilterMax F5 Molecular Devices, San Jose,CA, USA). Kojic acid was used as s positive control. The experiments were performed in triplicates for each concentration of sweet potato extracts. The inhibitory effects of the test samples were expressed as the percentage of tyrosinase inhibition as follows:
where:
A – optical density of the mixture without a test sample,
B – optical density of the mixture without a test sample and an enzyme,
C – optical density of the mixture with a test sample and an enzyme,
D – optical density of the mixture without an enzyme.
2.11 Statistical analysis
The values of parameters were expressed as the mean ± standard deviation (SD). The two-way analysis of variance (ANOVA) and Bonferroni post-test were performed at p < 0.05 to evaluate the significance of differences. Statistical analyses were conducted using GraphPad Prism 5.0 (GraphPad Software, Inc., San Diego CA).
3 Results and discussion
3.1 Total phenolic and total flavonoid content
Plant polyphenols are bioactive compounds that have protective effects against oxidative stress-induced damage in the human body. These secondary plant metabolites present a broad-spectrum of health-promoting properties and play an important role in the treatment and prevention of many diseases. The antioxidant properties of plants are attributed to the presence of phenolic compounds (Hannan et al., 2016). Many studies have shown that a high dietary content of fruits and vegetables is extremely beneficial for human health and can delay the ageing processes. Moreover, such a high content can reduce inflammation and oxidative stress, which are closely related to the pathogenesis of many health conditions, e.g., cancer, diabetes mellitus, cardiovascular diseases, and neurological disorders (Aryaeian et al., 2017). The flavonoid compounds are the most abundant and diverse group of phenolic compounds present in plants. They act through the mechanism of scavenging or chelating (Cook and Samman, 1996).
Sweet potato tubers contain different bioactive substances, including phenolic compounds, such as phenolic acids, flavonoids, anthocyanins (Table 1) (Sun et al., 2019). antioxidant capacity modulatory effects on the immune system anti-inflammation, anti-mutation, anti-tumor, antimicrobial, antifungal activity wound healing and antiulcer properties reduced risks of cardiovascular disorders, diabetes, aged-related macular degeneration and others - can serve as natural, safe and effective colorants
Phenolic compounds
Example
Function
flavonoids
isoquercetin, isorhamnetin, hyperoside, catechin, epicatechin, rutin hydrate, luteolin
phenolic acids
chlorogenic acid, isochlorogenic acid, cinammic, and hydroxycinammic acids, caffeic acid, quinic acid, syringic acid, cumaric acid, ferulic and trans-ferulic acid
anthocyanins
cyanidin-3-sophoroside-5-glucoside, peonidin-3-sophoroside-5-glucoside, peonidin-3-feruloyl sophoroside-5-glucoside, peonidin-3-caffeoyl sophoroside-5-glucoside, peonidin-3-caffeoyl-p-hydroxy benzoyl sophoroside-5-glucoside, peonidin-3-caffeoyl-feruloyl sophoroside-5-glucoside
In the present study, the TPC and the TFC were determined based on the calibration curves of gallic acid and quercetin (y = 0.0046x + 0.0452, R2 = 0.9989; y = 0.0153x-0.0053, R2 = 0.9996, respectively). The results showed that the extracts tested were characterized by a high phenolic and flavonoid content. The highest content of flavonoids and phenolic compounds was found in the B cultivar, i.e. 4.58 ± 0.33 mg/g and 38.43 ± 0.96 mg/g, respectively. A slightly lower content was demonstrated in the P cultivar while the smallest content was observed in the CR cultivar (Table 2). The inter-cultivar differences in the content of biologically active compounds were also reported by other authors. Comparative analyses of cultivars with tubers of different colours demonstrated the differences in the content of chemical compounds and biological properties. According to Ju et al. (2017), the purple sweet potato had the highest antioxidant content, as compared to white or orange-fleshed potatoes, which was also confirmed in our study. Moreover, the experiments carried out on other plant extracts have evidenced that a high content of pigments in the plant material affects its potent antioxidant properties (Bao et al., 2005). Padda and Picha (2007) and Ju et al. (2017) have also demonstrated that the size of sweet potato tubers has a huge impact on their properties. Smaller size tubers are found to be a richer source of biologically active compounds. Furthermore, the content of biologically active compounds and antioxidant properties of sweet potato are significantly affected by genotype, growing conditions and geographical region (Ju et al., 2017). Abbreviations: UAE, ultrasound-assisted extraction; GA, gallic acid; SD, standard deviation; Qu, quercetin.
Sweet potato cultivars
Total phenolic content (mg/g GA ± SD)
Total flavonoid content (mg/g Qu ± SD)
'Beauregard'
38.43 ± 0.96
4.58 ± 0.33
'Purple'
26.14 ± 0.32
2.91 ± 0.21
'Carmen Rubin'
14.37 ± 0.12
2.24 ± 0.29
3.2 Free radical scavenging activity
Our study showed that the content of active compounds in the analysed extracts was closely related to their antioxidant properties. The significant correlations observed by us support the hypothesis of other authors that the phenolic concentration highly contributes to the total antioxidant capacity of plant extracts (Subramanya et al., 2015). In the present study, eight different concentrations (25–5000 µg/mL) of the tested extracts were evaluated by the DPPH scavenging assay. The findings demonstrated that the B cultivar was characterized by the strongest DPPH radical scavenging activity (up to 70%), followed by the P cultivar (over 60%). Otherwise, the CR cultivar, in which the content of these compounds was significantly lower, showed much weaker antioxidant properties (DPPH radical scavenging activity up to 30%) (Fig. 1). Furthermore, the results disclosed that the extract potency to reduce DPPH was directly dependent on the concentration used. The antioxidant properties increased with increasing concentrations of extracts. In order to compare the obtained results with the antioxidant activity of commonly known antioxidants, the evaluation of DPPH radical scavenging was also carried out for ascorbic acid, rutin and quercetin at a concentration of 100 µg/mL. The percentage of DPPH radical inhibition by these compounds was 78.2, 66.1 and 62.3%, respectively, which indicates that the extracts tested in this study, especially the B and P cultivar, can be perceived as strong antioxidants.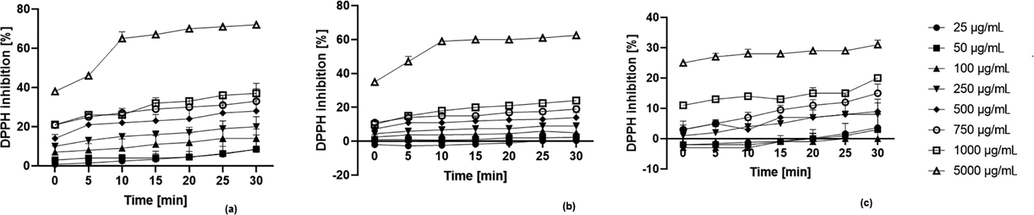
Kinetics of the absorbance changes in DPPH solutions in the presence of sweet potato tuber extract of (a) 'Beauregard', (b) ‘Purple’, (c) ‘Carmen Rubin’ cultivar (n = 3).
3.3 Effect of sweet potato extracts on skin cell viability
The biological activity of aqueous sweet potato extracts was assessed using in vitro cell models of immortalized human keratinocytes (HaCaT) and fibroblasts (BJ). The cells were exposed to various concentrations (25–500 µg/mL) of extracts. The cell viability was evaluated using the neutral red uptake assay, based on the ability of viable cells to incorporate and bind the neutral red in the lysosomes (Repetto, Peso, & Zurita, 2008). Thanks to the quantification of the dye retained within the lysosomes, the viability of keratinocytes treated with sweet potato extracts was estimated. The B cultivar extracts were found to show the highest ability to stimulate keratinocyte and fibroblast proliferation (Fig. 2).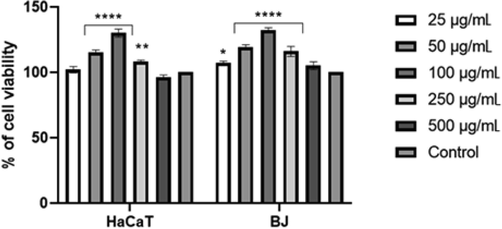
The effect of 'Beauregard' cultivar extract on neutral red (NR) uptake. Data are expressed as the mean ± SD of 3 independent experiments, each consisting of 3 replicates per treatment group ****p < 0.0001, **= 0.0017 *p < 0.0145 versus the control (100%).
The effect of the study extracts on the cell lines tested was strictly dependent on their concentration. The most beneficial effect was found in the extracts at a concentration of 100 µg/mL. In the case of the B cultivar, this concentration resulted in a statistically significant increase in the viability of cells by over 30%, as compared to the control (untreated cells). The same concentration of the P extract caused a slightly smaller proliferative effect; the viability of cells increased by over 20% (Fig. 3).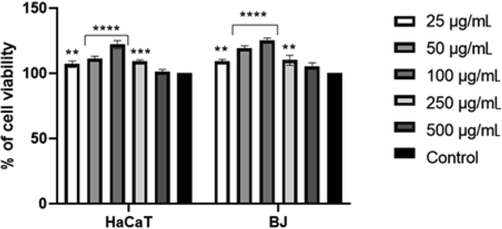
The effect of 'Purple' cultivar extract on neutral red (NR) uptake. Data are expressed as the mean ± SD of 3 independent experiments, each consisting of 3 replicates per treatment group. ****p < 0.0001, ***= 0.0002, **p < 0.0024 versus the control (100%).
Otherwise, the CR cultivar extract had no significant effect on HaCaT cells. The concentration of 100 µg/mL caused only a slight increase in cell viability while the remaining concentrations did not statistically significantly affect HaCaT cells. In fibroblasts, the CR extract resulted in a statistically significant increase in cell viability in the concentration range of 25–250 µg/mL (Fig. 4).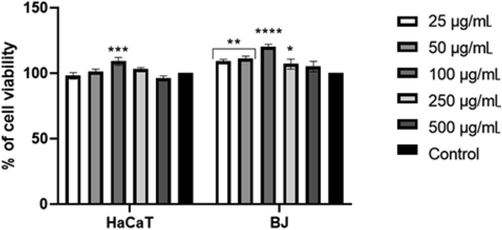
The effect of 'Carmen Rubin' cultivar extract on neutral red (NR) uptake. Data are expressed as the mean ± SD of 3 independent experiments, each consisting of 3 replicates per treatment group. ****p < 0.0001,***= 0.0007, **p < 0.0049, *p = 0.0256 versus the control (100%).
All the extracts analyzed showed no statistically significant toxic effects on human HaCaT and BJ cells within the range of concentrations applied (25–500 µg/mL). Considering a decrease in cell viability observed at concentrations higher than 100 µg/mL, it can be assumed that such concentrations may have toxic effects on HaCaT cells.
3.4 Intracellular ROS levels in skin cells
The ability of Ipomoea batatas extracts to generate FRs was assessed using H2DCFDA assay, which is widely used to determine intracellular ROS levels in skin cells (Dikalov and Harrison, 2014). Prior to measurements in cell lines, potential effects of the sweet potato extract without cells on the H2DCFDA fluorescence were tested. Additionally, in another experiment we showed no interactions between the sweet potato extract and H2DCFDA substrate in the medium.
Moreover, during incubation the study extracts were demonstrated to generate intracellular reactive oxygen species in a time- and dose-dependent manner. The activity of extracts was also specific to the cell model. HaCaT treated with the B extract disclosed a correlation between the dose and the level of intracellular free reactive oxygen species. When HaCaT were treated with the extract at a concentration of 25–100 µg/mL, the intracellular ROS level decreased below the level observed in controls (cells unexposed to the extract). In a dose > 100 μg/mL, the production of ROS was increasingly high. At the highest concentration used, the amount of ROS was about 1.8-fold higher, as compared to the untreated cells (Fig. 5).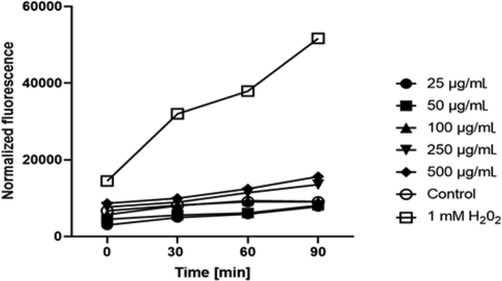
The effect of 'Beauregard' cultivar extract on the DCF fluorescence in HaCaT cells. The data are expressed as the mean ± SD of 3 independent experiments, each consisting of 3 replicates per treatment group.
Furthermore, the results of determinations of intracellular ROS levels in fibroblasts treated with the study extracts were comparable. The two highest concentrations of sweet potato extracts increased the production of ROS. At the concentration of 500 μg/mL, the level of intracellular reactive oxygen species was about 1.5 times higher, as compared to the unexposed cells (Fig. 6).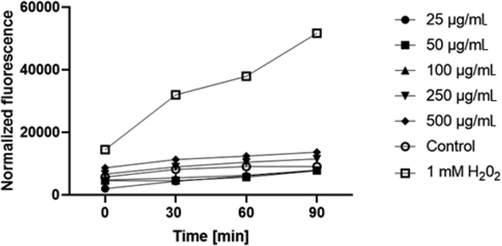
The effect of 'Beauregard' cultivar extract on the DCF fluorescence in fibroblasts. The data are expressed as the mean ± SD of 3 independent experiments, each consisting of 3 replicates per treatment group.
3.5 Tyrosinase inhibition
Nowadays, pigmentation disorders are extremely common and affect many people. They are difficult to treat; hence the ongoing search for natural, safe, and effective skin lightening agents (Halder and Richards, 2004). The use of tyrosinase inhibitors to regulate melanogenesis is an important strategy to treat skin disorders associated with abnormal skin pigmentation. The inhibition of this enzyme is relevant because it catalyses the two initial sequential oxidations of L-tyrosine in melanin biosynthesis (Bang et al., 2018). The melanin-related disorders include lentigines, melasma and post-inflammatory hyperpigmentation. To obtain new, efficient tyrosinase inhibitors, different types of compounds from natural sources have been intensively studied. An important issue is to find the depigmenting agents that could be an essential constituent of pharmaceutical and cosmetic products used to treat the discoloration process (Kim and Uyama, 2005). In the available studies, kojic acid was used as a positive control due to its well-documented tyrosinase inhibitory capacity. It is the most thoroughly studied naturally occurring tyrosinase inhibitor currently used as a skin-whitening agent (Hashemi and Emami, 2015). In the present study, the anti-tyrosinase activities of extracts were examined. To our knowledge this is the first report assessing the tyrosinase inhibitory activity of sweet potato. Our findings showed that all three types of sweet potato extracts were tyrosinase inhibitors, yet their inhibitory capacities differed significantly. The percentage of inhibition ranged from 10.8 to 35.5%, depending on the type of extract (Fig. 7).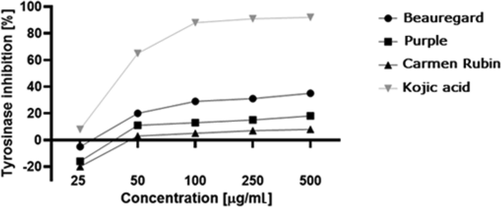
Inhibition of tyrosinase by extracts from three sweet potato cultivars. Values are the mean of 3 replicate determinations (n = 3) ± SD.
3.6 Comparative assessment of sweet potato cultivars
Consistently with other studies assessing antioxidant properties and effects of extracts on skin cells, our findings demonstrated that the B cultivar extract was the one most effectively inhibiting tyrosinase, which suggests that extracts from Ipomoea batatas can be used as skin-whitening agents. Moreover, the B cultivar was characterized by the highest antioxidant properties and the most positive effects on cell vitality. The scavenging activity and reducing power of DPPH were positively correlated with the flavonoid and phenolic content, which was also observed by other authors (Padda and Picha, 2007; Ji et al., 2015; Karna et al., 2011; Sun et al., 2019). In our study, the extracts obtained from cultivars with a higher content of biologically active compounds were characterized by a greater ability to scavenge FRs and stimulate the proliferation of healthy in vitro skin cells. Bao et al. (2005) have demonstrated that highly pigmented plant cultivars are richer in antioxidant compounds, which was also observed in our determinations. Our findings suggest that besides some obvious benefits associated with the consumption of various sweet potato cultivars, which are a valuable source of many biologically active compounds, these plants can also have a positive effect on skin cells. Their consumption can also be part of chemo-preventive activities that protect the body against the development of various types of cancer. Other authors have demonstrated the inhibitory effect of sweet potato extracts on the viability of prostate cancer cells, which might suggest its potential use as a plant with anticancer activity (Karna et al., 2011). The antitumor effect of sweet potato protein has also been shown by Li et al. (2013) in their study on human colon cancer cells. The abovementioned research and its results clearly indicate that sweet potatoes should be further investigated, as they seem to be very promising raw materials of natural origin. Although numerous studies have been focused on the positive effect of their consumption, further research is needed regarding their possible wider applications outside the food industry. Availability and relatively low price of the raw materials in question, as compared to some raw materials commonly used in the pharmaceutical and cosmetic industry, are likely to increase interest of skin care manufacturers.
4 Conclusions
Based on the above-mentioned literature reports and the results of our experiments, sweet potatoes can be considered a reservoir of biologically active substances with beneficial health properties. Due to increasingly growing interest in skin care, new raw materials, being valuable sources of active substances are being searched for to be used in preparations designed for care and treatment of various skin conditions. The present study highlights the differences in the biological activity of extracts from various varieties of Ipomoea batatas. The results suggest that extracts from sweet potato tubers may positively affect the in vitro skin cells and protect them against free radicals. The inhibitory effects of extracts on tyrosine expression observed in our study suggest that they may be useful as effective ingredients of low skin toxicity preventing hyperpigmentation changes. Moreover, new natural compounds and resources that can have beneficial effects on the skin can limit the use of some common synthetic compounds that are often associated with numerous side effects. Our research on three sweet potato cultivars seems to be useful as the sweet potato is now considered a rich source of valuable chemical compounds, particularly in the food industry.
Funding
This research was funded by the Scholarship Fund of Stanisława Pigon for students and employees of the State Higher Vocational School in Krosno with the number PDR.SP.0041.6.2020.
Declaration of Competing Interest
The authors declare that they have no known competing finan-cial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Aryaeian, N., Sedehi, S. K., & Arablou, T. (2017). Polyphenols and their effects on diabetes management: A review. Medical Journal of the Islamic Republic of Iran, 31, 134. https://doi: 10.14196/mjiri.31.134
- A review of therapeutic potentials of sweet potato: Pharmacological activities and influence of the cultivar. Trop. J. Pharm. Res.. 2016;15(12):2751-2761.
- [CrossRef] [Google Scholar]
- Evaluation of the novel synthetic tyrosinase inhibitor (Z)-3-(3-bromo-4-hydroxybenzylidene) thiochroman-4-one (MHY1498) in vitro and in silico. Molecules. 2018;23:3307.
- [CrossRef] [Google Scholar]
- Anthocyanins, flavonols, and free radical scavenging activity of Chinese bayberry (Myrica rubra) extracts and their color properties and stability. J. Agric. Food Chem.. 2005;53(6):2327-2332.
- [CrossRef] [Google Scholar]
- Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol.. 1995;28(1):25-30.
- [Google Scholar]
- Flavonoids-Chemistry, metabolism, cardioprotective effects, and dietary sources. J. Nutr. Biochem.. 1996;7(2):66-76.
- [CrossRef] [Google Scholar]
- Melanins and melanogenesis: from pigment cells to human health and technological applications. Pigment Cell & Melanoma Research. 2015;28(5):520-544.
- [CrossRef] [Google Scholar]
- Methods for detection of mitochondrial and cellular reactive oxygen species. Antioxid. Redox Signal.. 2014;20(2):372-382.
- [CrossRef] [Google Scholar]
- Topical agents used in the management of hyperpigmentation. Skin Therapy Letter. 2004;9(6):1-3.
- [Google Scholar]
- Hannan, P. A., Khan, J. A., Ullah, I., & Ullah, S. (2016). Synergistic combinatorial antihyperlipidemic study of selected natural antioxidants; modulatory effects on lipid profile and endogenous antioxidants. Lipids in Health and Disease, 15(1), 151. http://doi: 10.1186/s12944-016-0323-3
- Hashemi, S., & Emami, S. (2015). Kojic acid-derived tyrosinase inhibitors: synthesis and bioactivity. Pharmaceutical and Biomedical Research, 1(1), 1–17. http://doi: 10.18869/acadpub.pbr.1.1.1
- Hennessy, A., Oh, C., Diffey, B., Wakamatsu, K., Ito, S., & Rees, J. (2005). Eumelanin and pheomelanin concentration in human epidermis before and after UVB irradiation. Pigment Cell Research, 18(3), 220-223. http://doi: 10.1111/j.1600-0749.2005.00233.x
- Anti-aging potential of phytoextract loaded-pharmaceutical creams for human skin cell longetivity. Oxidative Medicine and Cellular Longevity. 2015;2015:1-17.
- [CrossRef] [Google Scholar]
- Analysis on the nutrition composition and antioxidant activity of different types of sweet potato cultivars. Food and Nutrition Sciences. 2015;6(1):161-167.
- [CrossRef] [Google Scholar]
- Ju, D., Mu, T., & Sun, H. (2017). Sweet potato and potato residual flours as potential nutritional and healthy food material. Journal of Integrative Agriculture, 16(11), 2632–2645. http://doi. org/10.1016/S2095-3119(16)61601-5
- Polyphenol-rich sweet potato greens extract inhibits proliferation and induces apoptosis in prostate cancer cells in vitro and in vivo. Carcinogenesis. 2011;32(12):1872-1880.
- [CrossRef] [Google Scholar]
- Tyrosinase inhibitors from natural and synthetic sources: structure, inhibition mechanism and perspective for the future. Cell. Mol. Life Sci.. 2005;62(15):1707-1723.
- [CrossRef] [Google Scholar]
- Nutrition value of the sweet potato (Ipomoea batatas (L.) Lam) cultivated in south–eastern Polish conditions. Int. J. Agronomy Agric. Res.. 2014;4(4):169-178.
- [Google Scholar]
- Research advances of purple sweet potato anthocyanins: extraction, identification, stability, bioactivity, application, and biotransformation. Molecules. 2019;24:3816.
- [CrossRef] [Google Scholar]
- Anticancer effects of sweet potato protein on human colorectal cancer cells. World J. Gastroenterol.. 2013;19(21):3300-3308.
- [CrossRef] [Google Scholar]
- Role of antioxidants in the skin: anti-aging effects. Journal of Dermatological Science. 2010;58(2):85-90.
- [CrossRef] [Google Scholar]
- Total phenolic and flavonoid content, antioxidant and antimicrobial activity of extracts from Tordylium maximum. Journal of Applied Pharmaceutical Science. 2013;3(1):55-59.
- [CrossRef] [Google Scholar]
- Evaluation of phytochemical composition and antimicrobial activity of sweet potato (Ipomoea batatas) leaf. Pakistan Journal of Nutrition. 2013;12(6):575-586.
- [CrossRef] [Google Scholar]
- Anthocyanins in purple sweet potato (Ipomoea batatas L.) varieties. Fruit, Vegetable and Cereal Science and Biotechnology. 2011;5:19-24.
- [Google Scholar]
- Skin photoprotection by natural polyphenols: anti-inflammatory, antioxidant and DNA repair mechanisms. Arch. Dermatol. Res.. 2010;302(2):71-83.
- [CrossRef] [Google Scholar]
- Antioxidant activity and phenolic composition in 'Beauregard' sweet potato are affected by root size and leaf age. Journal of the Amican Society for Horticultural Science. 2007;132(4):447-451.
- [CrossRef] [Google Scholar]
- Antioxidants in dermatology. Indian Dermatology Online Journal. 2014;5(2):210-214.
- [CrossRef] [Google Scholar]
- Anti-ulcer activity of Ipomoea batatas tubers (sweet potato) The Functional Foods in Health and Disease. 2012;2(3):48-61.
- [CrossRef] [Google Scholar]
- Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat. Protoc.. 2008;3(7):1125-1131.
- [CrossRef] [Google Scholar]
- Singleton, V.L., Orthofer, R., & Lamuela-Raventós, R. M. (1999). Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods in Enzymology, 299, 152–178. doi.org/10.1016/S0076-6879(99)99017-1
- Melanins: skin pigments and much more - types, structural models, biological functions, and formation routes. New Journal of Science. 2014;2014:1-28.
- [CrossRef] [Google Scholar]
- Total polyphenolic contents and in vitro antioxidant properties of eight Sida species from Western Ghats, India. Journal of Ayurveda and Integrative Medicine. 2015;6(1):24-28.
- [CrossRef] [Google Scholar]
- Sugata, M., Lin, C. Y., & Shih, Y. C. (2015). Anti-inflammatory and anticancer activities of Taiwanese purple-fleshed sweet potatoes (Ipomoea batatas L. Lam) extracts. BioMed Research International, doi.org/10.1155/2015/768093
- Antioxidant and prebiotic activity of five peonidin-based anthocyanins extracted from purple sweet potato (Ipomoea batatas (L.) Lam.) Sci. Rep.. 2018;8:5018.
- [CrossRef] [Google Scholar]
- Comparative assessment of phenolic profiles, cellular antioxidant and antiproliferative activities in ten varieties of sweet potato (Ipomoea Batatas) storage roots. Molecules. 2019;24:4476.
- [CrossRef] [Google Scholar]
- Profiles of phenolics, carotenoids and antioxidative capacities of thermal processed white, yellow, orange and purple sweet potatoes grown in Guilin, China. Food Science and Human Wellness. 2015;4(3):123-132.
- [CrossRef] [Google Scholar]
- Antioxidant and antityrosinase activity of aqueous extracts of green asparagus. Food Chem.. 2011;127(1):141-146.
- [CrossRef] [Google Scholar]
- Reactive molecule species and antioxidative mechanisms in normal skin and skin aging. Skin Pharmacology and Physiology. 2014;27(6):316-332.
- [CrossRef] [Google Scholar]
- The regulations of skin pigmentation. J. Biol. Chem.. 2007;282(38):27557-27561.
- [CrossRef] [Google Scholar]
- Extraction optimization of bioactive compounds crocin, geniposide and total phenolic compounds from Gardenia jasminoides Ellis fruits with response surface methodology. Innovative Food Sci. Emerg. Technol.. 2009;10(4):610-615.
- [CrossRef] [Google Scholar]
- Fighting against skin aging. The way from bench to bedside. Cell Transplant.. 2018;27(5):729-738.
- [CrossRef] [Google Scholar]







