Translate this page into:
Comparative assessment of heavy metal bioaccumulation in skeletal muscles of softshell and hard-shell freshwater turtles
⁎Corresponding author. mushahid@ksu.edu.sa (Shahid Mahboob)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The heavy metal ions originating from the various point and nonpoint sources seriously threatening the lotic ecosystem by posing severe health crises to the aquatic organisms of higher trophic levels. The freshwater turtles with delicate body structure and devoid of any hardcovers are prone to higher heavy metal bioaccumulation risks. The present study was conducted to determine heavy metals bioaccumulation in three soft-shelled and hard-shelled freshwater turtle species in Pakistan. We collected the water samples and cadaver turtle bodies at both sites and transported them to the laboratory for heavy metal content evaluation. The skeletal muscles (1 g) and water samples (100 ml) were processed by acid digestion and prepared metals analyses by AAS. The results indicated that the mean concentrations of Copper (Cu), Zinc (Zn) and Cadmium (Cd) were significantly lower, while Nickel (Ni), Cobalt (Co), Lead (Pb) and Chromium (Cr) were significantly higher at both sites compared with world health organization (WHO, 2004) standards. Muscles of softshell turtle (Lissemys punctata) showed the maximum bioaccumulation of targeted metals than other two species of hard-shell turtles i.e. Cu (7.61 ± 1.88), Zn (49.17 ± 4.11), Ni (2.77 ± 0.41), Cd (0.21 ± 0.02), Co (3.31 ± 0.43), Pb (4.70 ± 0.62) and Cr (6.09 ± 0.39). The mean concentrations of Cu, Ni, Pb, and Cr were maximum in water and turtle species muscles collected from Trimmu Barrage. In conclusion, the heavy metal loads in water and bioaccumulation patterns in the skeletal muscles of freshwater turtle species illustrated species and location-based variations with overall similar pattern corroborations among the sequence of heavy presence levels.
Keywords
Freshwater turtle
Hard-shell
Softshell
Heavy metals
Zinc
Pakistan
1 Introduction
Freshwater turtles represent a unique group of Chelonian fauna. Eight species of freshwater turtles are present in Punjab, Pakistan, belonging to the two families (Geoemydidae and Trionychidae) of class Reptilia and order Testudines. Based on their outer body shell structure and composition, they are either soft-shelled or hard-shelled. The soft-shelled turtle species usually have lesser bones and more pores in their shells that render them prone to ambient pollutants and heavy metals (Haynes and Johnson, 2000).
Several vertebrate species have been reported to act as biomarkers of environmental contamination, especially metal pollution studies. Heavy metals have been detected in various organs (e.g. liver and kidney tissues) in marine mammals (Haynes and Johnson, 2000; Abdulaziz, 2020), freshwater turtles (De Solla and Fernie, 2004) and sea turtles (Fujihara et al., 2003). Heavy metals released from different geological processes and anthropogenic activities usually accumulate in river water, sediments and biota (Förstner and Wittmann, 1981; Kese-Relavins and Potapovics, 2009). The high concentration of heavy metals in the biosphere may cause oxidative damages to living organisms by changing their normal physiological functions, genetic information and mitochondrial activities at the cellular level (Burbure et al., 2006). At higher concentrations, heavy metals such as Zinc (Zn), copper (Cu), Lead (Pb), Cadmium (Cd), Nickel (Ni) and Chromium (Cr) become highly toxic to the aquatic organisms (Yılmaz et al., 2007).
Based on the bioaccumulation and bioconcentration factors, organisms at intermediate and higher trophic levels are frequently exposed to higher concentrations of chemical pollutants (Van Straalen and Ernst, 1991; Burger et al., 1992; Burger, 1993). Organisms at higher trophic levels are carnivores, and carnivorous species have shown higher levels of heavy metal accumulations than herbivores (Storelli et al., 1998; Storelli and Marcotrigiano, 2003). Therefore, the trace metals are most important because many of these metals are biologically essential nutrients when in lower concentrations; however, they become toxic if their concentrations exceed certain thresholds (O’Dell and Sunde, 1997; O’Shea and Geraci, 1999; Goldhaber, 2003; Mason, 2002; WHO, 2004; Sidra et al., 2019).
Varying levels of metal can be analyzed among various turtle species. In threatened and endangered wild species like turtles, the heavy metals toxicity level can be estimated in different tissues of deceased specimens (Burger et al., 2010). Wildlife species living close to or in the polluted sites are readily exposed to complex mixtures of pollutants by various point and nonpoint sources, which could hardly be evaluated in lab studies (Bernanke and Kohler, 2009). Turtles have numerous advantages when used as biomarkers of environmental contamination as compared to many other species. They are widely distributed, reside in various habitats, and are available in most terrestrial and aquatic ecosystems. Lissemys punctata (L. punctata) and Kachuga smithii (K. smithi) inhabit shallow, often stagnant water pools in rivers, streams, marshes, ponds, lakes, and irrigation canals and are omnivorous in their food habits.
In contrast, Kachuga tecta (K. tecta) is primarily herbivorous, with an occasional tendency of being carnivorous. Specifically, carnivorous species may accumulate higher concentrations of hazardous chemicals through trophic transfer as compared to herbivores. Both aquatic and terrestrial turtle species have been reported as useful biomonitors of heavy metals contamination in the environment. For example, box turtles (Terrapene carolina; Beresford et al., 1981) and common snapping turtles (Chelydra serpentina; Overmann and Krajicek, 1995) collected from sites contaminated with Lead showed significantly higher lead concentrations in tissues compared with turtles from reference sites. Although turtles could serve as biomarkers of environmental contamination, toxicological effects of contaminants are not sufficiently explored in turtles or other reptilian species than fish, birds, or mammals (Sparling et al., 2010; Sidra et al., 2019).
We recognize turtles for their multiple uses as they could act as scavengers in aquatic ecosystems. Their longer life span allows monitoring long-term environmental pollutants trends and has sufficient muscle tissue mass for multiple endpoint measurements (Meyers-Schöne et al., 1993). Turtle muscles are used in meals and eaten directly, while internal organs such as kidneys and liver are utilized for soup (Mack and Duplaix, 1982). Their body fat is used to extract oil, which is considered a cure for respiratory problems, especially in children, and the blood is drunk raw as a remedy for anemia and asthma (Caldwell, 1963; Felger and Moser, 1987).
The present assessment of heavy metals in the freshwater turtle muscles is relatively novel in Pakistan because little is known regarding heavy metal bioaccumulation in freshwater turtles. We planned this study to estimate the accumulation of selected heavy metals (Cu, Zn, Ni, Cd, Co, Pb, and Cr) regardless of species and locations. Furthermore, we analyzed species-wise bioaccumulation of heavy metals in skeletal muscles of deceased hard-shelled and soft-shelled freshwater turtle species and compared site based heavy metals bioaccumulation in skeletal muscles of freshwater turtle species from Balloki Headworks (BH) and Trimmu Barrage (TB).
2 Materials and methods
2.1 Study area and sampling
We collected dead specimens of turtle species (Kachuga smithii, Kachuga tecta and Lissemys punctata) to procure the body muscle samples from two headworks located on two major rivers of Pakistan i.e., River Ravi and River Chenab). The Balloki Headworks is constructed on River Ravi, while Trimmu Barrage is erected on River Chenab. (Fig. 1). For species identification, Minton’s (1966)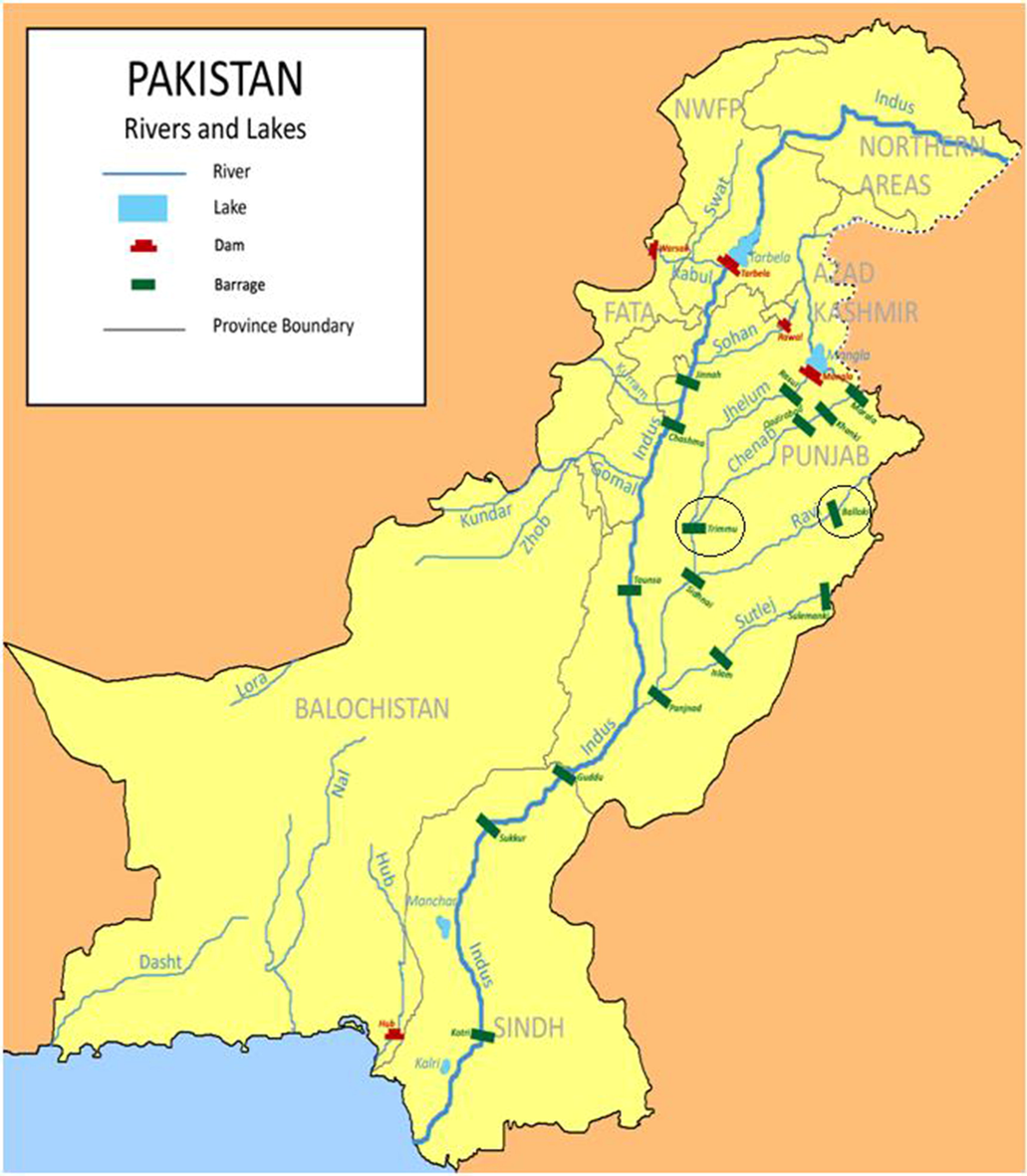
Water Storage Reservoirs and Barrages of Pakistan.
Both study sites encircled on map manual was followed. The straight carapace length and width, and plastron length were measured using a digital calibrated tape, while body size was calculated using a measuring tape. Dead turtle body weight could not be recorded due to the loss of most body mass and damages due to decomposition or consumption by predators. Water samples were also collected from both sites to corroborate the corresponding levels of targeted metals analyses.
2.2 Samples preparation and metal content analysis
Skeletal muscles were collected after the dissection of dead turtles. Muscle tissues were washed with distilled water, wrapped in aluminum foil, and packed in polyethylene bags before storage at −20 °C before analysis. Samples were allowed to thaw at room temperature prior to examination. Acid-washed laboratory materials were used to prevent contamination of samples (Páez-Osuna et al., 2010). Muscles and water samples were prepared using a wet digestion protocol for metals analyses (Hseu, 2004; S.M.E.W.W., 1989). We targeted the evaluation of seven heavy metals viz. Cu, Zn, Cd, Co, Ni, Pb and Cr, and detection was carried out by AAS (atomic absorption spectrometry; Aurora AAS, Al-1200, Canada) by following the method described in AOAC (1990).
2.3 Statistical analysis
Before data analyses, the obtained dataset was subjected to a data normality check. Further, the data were statistically analyzed by one-way analysis of variance (ANOVA) method (Steel et al., 1996) followed by the Duncan’s Multiple Range (DMR) test to compare means in the species-wise and site-based levels of heavy metals concentrations in freshwater turtle species (P < 0.05). The student’s t-test was used for comparing metals concentration with standard values.
3 Results and discussion
3.1 Body size comparisons
During the study period, we collected 35 dead freshwater turtles, out of which fifteen procured from Balloki Headworks (K. smithi = 4, K. tecta = 4 and L. punctate = 7) and twenty from Trimmu Barrage (K. smithi = 7, K. tecta = 10 and L. punctata = 3). Comparison of body size of deceased turtles from both study sites illustrated non-significant differences (Table 1). We collected the muscle samples from all dead turtle pieces from BH, whereas out of twenty dead turtles collected from TB, 18 muscle samples were collected. This discrepancy occurred due to the whole consumption of the turtle muscles of K. tecta by predators who also left their internal body organs almost entirely damaged and eaten away. Similar observations were reported by Akbar et al., (2006), that feral animals such as wandering dogs patrol extensively during the canal closure period and hunt for alive and dear turtles and trapped fish as their food items. Being slow-moving creatures, the freshwater turtles are highly prone to predation of feral terrestrial animals. NS = Non-significant (P > 0.05); * = Significant (P < 0.05); ** = Highly significant (P < 0.01). N = Number of observations.
Site
N
Mean ± SE
t-value
Probability
Balloki Headworks (BH)
15
303.18 ± 34.49
0.59
0.557
Trimmu Barrage (TB)
20
276.14 ± 29.87
3.2 Heavy metals assessment
The concentrations of targeted heavy metals showed varying levels in water samples at the study sites. The presence levels of Zn, Cu and Cd were significantly lower, while Ni, Co, Pb and Cr displayed significantly higher loads at both localities in comparison with the given standards of WHO (2004) in riverine waters of Pakistan (Table 2). This alluded to a varying degree of anthropogenic activities in study areas as we estimated the concentrations of these heavy metals in water samples collected from both sites and compared using the student t-test. * = Significant (P < 0.05); ** = highly significant (P < 0.01).
Sites/Metals
Cu
Zn
Ni
Cd
Co
Pb
Cr
Balloki Headworks (BH)
0.073 ± 0.060
0.169 ± 0.0248
0.045 ± 0.0044
0.0042 ± 0.0005
0.018 ± 0.0034
0.06 ± 0.004
0.121 ± 0.0066
Trimmu Barrage (TB)
0.084 ± 0.0074
0.145 ± 0.0240
0.05 ± 0.0049
0.0041 ± 0.0005
0.026 ± 0.0031
0.06 ± 0.0043
0.127 ± 0.0064
Permissible limits (WHO, 2004)
2
5
≤0.02
0.01
0.001
≤0.05
≤0.05
t- value in comparison of BH
−321.17
−194.80
5.68
−11.60
5
2.5
10.76
Probability
**
**
**
**
**
**
**
t- value in comparison of TB
−258.91
−202.29
6.12
−11.8
8.06
1.39
12.03
Probability
**
**
**
**
**
*
**
Table 3 shows species-wise and locality-based concentrations of Cu, Zn and Ni in the freshwater turtle muscles sampled from BH and TB. The mean absorption of Cu was high in skeletal muscles of L. punctata (7.61 ± 1.88) mg Kg−1 while lower in K. smithi (5.07 ± 1.12) and K. tecta (5.05 ± 0.52 mg Kg−1). Species and the locality-based mean concentration of Cu showed non-significance (P > 0.05) statistically. The mean concentration of Cu in the muscles BH was lower than at TB i.e 5.03 ± 0.74 and 6.64 ± 1.19 mg Kg−1, respectively. Species-wise mean levels of Zn varied highly significant (P < 0.01), whereas location-based mean concentration was non-significant (P > 0.05). The mean concentration of Zn was maximum in L. punctata (49.17 ± 4.11 mg Kg−1) and minimum in K. smithi (29.98 ± 1.27 mg Kg−1). The location-wise mean concentration of Zn in the freshwater turtle muscles at BH was 41.64 ± 4.28 mg Kg−1, and was recorded as 30.75 ± 1.70 mg Kg−1 at TB. * = values significantly different (P < 0.05); ** = values highly significantly different (P < 0.01). Superscript letters show comparison of mean values using conventional standard a, b for P < 0.05 and A, B for P < 0.01. Mean values sharing similar letter in a column are statistically non-significant (P > 0.05).
Species
Cu
Zn
Ni
TB
BH
Mean
TB
BH
Mean
TB
BH
Mean
K. tecta
4.59 ± 0.52
5.79 ± 1.05
5.05 ± 0.52
26.66 ± 1.00
38.48 ± 8.84
**31.21 ± 3.62B
1.19 ± 0.61
1.55 ± 0.34
*1.41 ± 0.30b
K. smithi
5.22 ± 1.21
5.00 ± 1.64
5.07 ± 1.12
30.60 ± 0.90
28.91 ± 3.35
**29.98 ± 1.27C
2.17 ± 0.21
1.35 ± 0.45
*1.65 ± 0.31ab
L. punctata
9.59 ± 2.71
4.14 ± 0.77
7.61 ± 1.88
41.97 ± 6.83
52.76 ± 4.84
**49.17 ± 4.11A
3.07 ± 0.41
2.17 ± 0.93
*2.77 ± 0.41a
Overall mean
6.64 ± 1.19
5.03 ± 0.74
5.91 ± 0.73
30.75 ± 1.70
41.64 ± 4.28
35.70 ± 2.32
2.20 ± 0.33
1.58 ± 0.26
1.86 ± 0.21
Species-wise mean concentrations of Ni differed significantly (P < 0.05), whereas location-wise- mean concentration did not show significant (P > 0.05) loadings. The mean concentration of nickel was maximum in the muscle of L. punctata (2.77 ± 0.41 mg Kg−1), while the minimum records noted in K. tecta (1.41 ± 0.30 mg Kg−1). The mean concentration of nickel in muscle of K. smithi was 1.65 ± 0.31 mg Kg−1. The mean concentration of nickel in the muscle tissues at TB was greater than those from BH. The present study's findings are in line with Nisa et al. (2015) who analyzed metals in the blood samples of three different freshwater turtle species and reported the highest bioaccumulation of Cu, Zn, Ni, Cd and Cr in L. punctata than other species.
Table 4 shows species and location-wise concentration of Cd, Co, Pb and Cr in freshwater turtle’s muscle collected from BH and TB. Species-wise and the locality-wise mean concentrations of Cd did not differ significantly (P > 0.05). The mean concentration of cadmium was higher in L. punctata (0.21 ± 0.02 mg Kg−1) and lower in K. smithi 0.15 ± 0.02 mg Kg−1. The mean concentration in the muscle of K. tecta was (0.17 ± 0.03 mg Kg−1). The mean concentration of cadmium in the muscle of freshwater turtles at BH was greater than TB i.e. 0.19 ± 0.02 mg Kg−1 and 0.16 ± 0.02 mg Kg−1, respectively. Species-wise and locality-wise mean concentrations of Co were non-significant (P > 0.05). The mean Co concentrations were lower in K. smithi (1.68 ± 0.70 mg Kg−1) and higher in K. tecta (3.33 ± 0.47 mg Kg−1). However, the mean cobalt concentration recorded in the muscle tissues of L. punctata was intermediate between two hard shell species i.e. 3.31 ± 0.43 mg Kg−1. The mean concentration of cobalt in the muscle of freshwater turtles at BH was 2.49 ± 0.48 mg Kg−1 and TB was 3.37 ± 0.69 mg Kg−1. The present study results are parallel to the findings of Nisa et al. (2015), Nisa et al. (2019), who concluded maximum bioaccumulation of Ni, Cd, Co and Cr in the liver of L. Punctata than other species of freshwater turtles (Mehana et al., 2020). Letters are used for the overall mean.
Metals/Species
Cd
Co
Pb
Cr
Species
TB
BH
Overall
TB
BH
Overall
TB
BH
Overall
TB
BH
Overall
K. tecta
0.17 ± 0.04
0.17 ± 0.03
0.17 ± 0.03
3.37 ± 0.69
3.28 ± 0.60
3.33 ± 0.47
2.59 ± 1.13
2.58 ± 0.89
2.58 ± 0.66
6.21 ± 0.44
5.45 ± 0.08
5.92 ± 0.29
K. smithi
0.15 ± 0.01
0.14 ± 0.04
0.15 ± 0.02
2.62 ± 1.69
1.22 ± 0.67
1.68 ± 0.70
3.57 ± 1.01
4.58 ± 0.62
3.94 ± 0.67
5.81 ± 0.41
5.00 ± 0.11
5.51 ± 0.28
L. punctata
0.16 ± 0.04
0.23 ± 0.01
0.21 ± 0.02
3.25 ± 0.47
3.40 ± 0.96
3.31 ± 0.43
5.00 ± 0.66
4.22 ± 1.27
4.70 ± 0.62
7.15 ± 0.83
5.56 ± 0.26
6.09 ± 0.39
Overall Means
0.16 ± 0.02
0.19 ± 0.02
0.17 ± 0.01
3.37 ± 0.69
2.49 ± 0.48
2.88 ± 0.32
4.04 ± 0.54
3.66 ± 0.59
3.87 ± 0.39
6.21 ± 0.29A
5.38 ± 0.12B
5.83 ± 0.18
Species-wise and locality-wise Pb concentrations in the muscle tissues of freshwater turtle showed non-significant (P > 0.05) variations among the study sites. The Pb level was higher in L. punctata (4.70 ± 0.62 mg Kg−1) and lower in K. tecta (2.58 ± 0.66 mg Kg−1). The mean concentration of Pb in the muscle tissue samples of K. smithi was (3.94 ± 0.67 mg Kg−1). However, the mean concentration of Pb in the muscle of freshwater turtles at BH was 3.66 ± 0.59 mg Kg−1 and at TB, it was recorded as 4.04 ± 0.54 mg Kg−1. Nisa et al. (2015), Nisa et al. (2019) reported the estimated Pb level in blood and liver of freshwater turtle at TB compared to BH.
The locality-wise concentration of Cr in the skeletal muscle tissues of the freshwater turtle was highly significant (P < 0.01), whereas according to the turtle species, it was statistically non-significant (P > 0.05). Cr's mean concentration was higher in L. punctata (6.09 ± 0.39) and lower in K. smithi (5.51 ± 0.281 mg Kg−1). The mean concentration of Cr in the muscle of K. tecta was (5.92 ± 0.29). Overall, the maximum mean concentration of Cr detected in the freshwater turtle's skeletal muscle tissues was higher (6.21 ± 0.29 mg Kg−1) at TB than recorded at BH (5.38 ± 0.12 mg Kg−1).
4 Conclusion
Overall heavy metals loading array led this (Zn > Cr > Cu > Pb > Ni > Co > Cd) trend in water samples, which also nearly corroborated (Zn > Cu > Cr > Pb > Co > Ni > Cd) with their bioaccumulation tendencies in the skeletal muscles of freshwater turtles. All heavy metals showed higher bioaccumulation in skeletal muscles of softshell turtle species (L. punctata) than hard shell species. Location-based mean concentrations of Cu, Ni, Pb and Cr were maximum in water and turtle skeletal muscles collected from Trimmu Barrage compared to Balloki Headworks. However, the locality-wise mean Co concentration was somewhat similar in skeletal muscle tissue samples at both sites, but in greater magnitude in the soft-shelled turtle species. The Zn and Cd mean concentrations were maximum in water and consequently bioaccumulated higher in the turtle skeletal muscles collected from Balloki Headworks, with higher bioaccumulation in soft-shelled turtles. In conclusion, the hard-shell provided a protective covering against predators and rescued its keeper from unseen hazards, i.e., comparatively less intake of metals through cutaneous routes. It also established that the freshwater hard-shell and softshell turtle species could be used as suitable bioindicator species to assess the various degrees of pollution and pollutants.
Acknowledgements
‘‘The authors (SM, KAG) express their sincere appreciation to the Deanship of Scientific Research at the King Saud University for its funding of this research through the Research Group Project No. 1440-0138.”
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Official Methods of Analysis (15th Edition). Washington DC: Association of Official Analytical Chemist; 1990.
- Akbar, M., Hassan, M., & Nisa, Z. 2006. Distribution of freshwater turtles in Punjab, Pakistan. Caspian Journal of Environmental Sciences, 4 (2), 142–146. (9) (PDF) Diversity and conservation of amphibians and reptiles in North Punjab, Pakistan. Available from: https://www.researchgate.net/publication/263662699_Diversity_and_conservation_of_amphibians_and_reptiles_in_North_Punjab_Pakistan [accessed May 11 2021].
- Abdulaziz, M. S., 2020. Histopathology and heavy metal bioaccumulation in some tissues of Luciobarbus xanthopterus collected from Tigris River of Baghdad, Iraq.“ Egypt. J. Aquat. Res. 46.2, 123-129.
- The sea turtle fishery of Baja California, Mexico. California Fish and Game. 1963;49:140-151.
- [Google Scholar]
- Characterization of contaminants in snapping turtles (Chelydra serpentina) from Canadian Lake Erie Areas of Concern: St. Clair River, Detroit River and Wheatley Harbour. Environ. Pollut.. 2004;132(1):101-112.
- [Google Scholar]
- Trace element risk assessment: Essentiality vs. toxicity. Regul. Toxicol. Pharmacol.. 2003;38(2):232-242.
- [Google Scholar]
- Organochlorine, heavy metal and polyaromatic hydrocarbon pollutant concentrations in the Great Barrier Reef (Australia) environment: a review. Mar. Pollu.t Bull.. 2000;41(7-12):267-278.
- [Google Scholar]
- Evaluating heavy metal contents in nine composts using four digestion methods. Bioresource Technol.. 2004;95(1):53-59.
- [Google Scholar]
- Arsenic accumulation in livers of pinnipeds, seabirds and sea turtles: Subcellular distribution and interaction between arsenobetaine and glycine betaine. Comp. Biochem. Physiol.. 2003;136(4):287-296.
- [Google Scholar]
- Förstner U., Wittmann G.T.W., eds. Metal Pollution in the Aquatic Environment. Berlin, Heidelberg: Springer Berlin Heidelberg; 1981.
- Introduction. In: O’Dell B.L., Sunde R.A., eds. Handbook of nutritionally essential mineral elements. New York: Marcel Dekker; 1997. p. :1-12.
- [Google Scholar]
- Toxicology in marine mammals. In: Fowler M.E., Miller R.E., eds. Zoo & Wild Animal Medicine: Current Therapy. Saunder Company, Philadelphia: W. B; 1999. p. :412-478.
- [Google Scholar]
- The impact of environmental chemicals on wildlife vertebrates. Rev. Environ. Contam. Toxicol.. 2009;198:1-47.
- [Google Scholar]
- Lead in the bone and soft tissues of box turtles caught near smelters. Bull. Environ. Contam. Toxicol.. 1981;27-27(1):349-352.
- [Google Scholar]
- Renal and neurologic effects of cadmium, lead, mercury and arsenic in children: evidence of early effects and multiple interactions at environmental exposure levels. Environ. Health Perspect.. 2006;114(4):584-590.
- [Google Scholar]
- Heavy metal and selenium levels in young Cattle Egrets from nesting colonies in the Northeastern United States, Puerto Rico and Egypt. Arch. Environ. Contam. Toxicol.. 1992;23:435-439.
- [Google Scholar]
- Metals in avian feathers: bioindicators of environmental pollution. Rev. Environ. Toxicol.. 1993;5:203-311.
- [Google Scholar]
- Arsenic, cadmium, chromium, lead, mercury, and selenium levels in blood of four species of turtles from the Amazon in Brazil. J. Toxicol. Environ. Health A.. 2010;73:33-40.
- [Google Scholar]
- Mack, D., Duplaix, N. and Wells, S. 1982. Sea turtles, animals of divisible parts: international trade in sea turtle products. In: Bjorndal, K. (Ed.), Biology and Conservation of Sea Turtles, , Washington, DC: Smithsonian Institution Press, pp 545–565.
- Biology of freshwater pollution (4rd ed.). England: Essex Univ; 2002. p. :387.
- Biomonitoring of heavy metal pollution using acanthocephalans parasite in ecosystem: an updated overview. Animals Basel. 2020;10(5):811.
- [CrossRef] [Google Scholar]
- A contribution to the herpetology of West Pakistan. Bull. Am. Mus. of Nat. Hist.. 1966;134:49-70.
- [Google Scholar]
- Lead in blood and eggs of the sea turtle, Lepidochelys olivacea, from the Eastern Pacific: concentration, isotopic composition and maternal transfer. Mar. Pollut. Bull.. 2010;60(3):433-439.
- [Google Scholar]
- S. M. E. W. W. (Standard Methods for Examination of Water and Wastewater), 1989. 17th ed. A. P. H. A., Washington, DC.
- Principles and Procedures of Statistics: A Biomaterical Approach (2nd edition). Singapore: McGraw Hill Book Co.; 1996.
- Comparison of two freshwater turtle species as monitors of radionuclide and chemical contamination: DNA damage and residue analysis. Environ Toxicol Chem. 1993;12(8):1487-1496.
- [Google Scholar]
- Snapping turtles (Chelydra serpentina) as biomonitors of lead contamination of the Big River in Missouri’s old lead belt. Environ. Toxicol. Chem.. 1995;14(4):689-695.
- [Google Scholar]
- Fecal matter as a bio-indicator tool for heavy metal pollution. J. Wildlife and Ecol.. 2019;3:1-7.
- [Google Scholar]
- Recent advances in amphibian and reptile toxicology. In: Sparling D.W., Linder G., Bishop C.A., Krest S.K., eds. Ecotoxicology of amphibians and reptiles (2nd edn.). Boca Raton: CRC Press; 2010. p. :1-11.
- [Google Scholar]
- Distribution of heavy metal residues in some tissues of Caretta caretta (Linnaeus) specimen beached alongthe Adriatic Sea (Italy) Bull. Environ. Contam. Toxicol.. 1998;60(4):546-552.
- [Google Scholar]
- Heavy metal residues in tissues of marine turtles. Mar. Pollut. Bull.. 2003;46(4):397-400.
- [Google Scholar]
- Guidelines for Drinking Water Quality. third edition; 2004.
- Metal biomagnification may endanger species in critical pathways. Oikos. 1991;62(2):255.
- [CrossRef] [Google Scholar]
- Yilmaz, F., Ozdemir, N., Demirak, A. and Tuna, A. L., 2007. Heavy metal levels in two fish species Leuciscus cephalus and Lepomis gibbosus. Microchem. J., 95, 341-44.
- Zaib-Un-Nisa, Sultana, S., Sultana T., 2015. Accumulation of heavy metals (Cu, Zn, Ni, Cd, Co, Pb and Cr) in blood of Freshwater Turtles from Balloki Headworks and Trimmu Barrage, Punjab, Pakistan. Pure Appl. Biol., 4(3), 280-287.
- Zaib-Un-Nisa, Sultana, S. Sultana, T., Al-Ghanim, K. A., Al-Ghanem, M. K., Al-Misned, F. and Mahboob, S. 2019. Environmental exposure to metals and bioaccumulation in the liver of three freshwater species of turtles from two different rivers. Pol. J. Environ. Stud. 28(5), 1-8.







