Combination of vitamin E and Lactobacillius plantarum reverses mercuric chloride-induced neurotoxicity: Implication of BDNF, CREB and MAPK proteins expressions
⁎Corresponding author. lfadda@ksu.edu.sa (Laila M. Fadda)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
It down-regulated BDNF protein expression, GSH level, and SOD activity.
Vitamin E and Lactobacillius plantarum ameliorated the previous signs.
Abstract
Mercury is the third most hazardous heavy metal and its toxicity causes a severe health risk through unfavorable detrimental pathological and biochemical effects. Mercury is widely found in many ecological and certain occupational settings.
Objectives
The aim of this study is to elucidate the neuroprotective role of vitamin E (VE) and Lactobacillus plantarum (LTB) either alone or in combination against a toxic sublethal dose of Mercuric chloride (MC).
Methods
First group served as a normal control group; rats from the second group were intoxicated with (5 mg/kg MC once daily); the third group was treated with VE; the fourth group was treated with LTB; and the fifth group was treated with VE and LTB. All treatments were given daily along with MC for fourteen days.
Results
The results of the current study confirmed that MC prompted an elevation in serum TNF-α, IL-6 and brain lipid peroxides, protein expression of mitogen-activated protein kinase (MAPK) and mRNA expression of Bax and caspase-3 level as well as DNA degradation. However, Brain-derived neurotrophic factor (BDNF) and cAMP response element-binding (CREB) protein expressions, GSH level and SOD activity were down-regulated. The intake of LTB and/or VE along with MC intoxication significantly mitigated the alteration in all the previous parameters. Moreover, histopathological analysis of brain sections confirmed that MC-induced brain injury and LTB or VE alone or together were capable of ameliorating brain artitechture.
Conclusions
The combination of LTB and VE was an effective therapy in the management of MC-induced neuroioxicity and this combination can be considered a useful therapeutic candidate against brain injury induced by MC. BDNF, MAPK and CREB protein expressions are implicated in MC -induced brain injury and its treatment.
Keywords
Mercuric chloride
Lactobacillus plantarum
Vitamin E
MAPK
BDNF
CREB
Bax and DNA fragmentation
1 Introduction
Mercury is an extensive metal in environments and there are unlimited populations that are currently exposed to this metal at low levels as a result of ever-present ecological factors (Eagles-Smith et al., 2018). It has been documented that low levels of mercury exposure caused immune system alterations (Nyland et al., 2011). Mercury toxicity is attributed to its capability to diminish free sulfhydryl groups of GSH and other antioxidants enzymes (Eagles-Smith et al., 2018).
Accumulating evidence illustrated that oxidative stress evokes numerous intracellular events, such as gene expression, cell-cycle arrest, and apoptosis (Wu et al., 2018). In addition, mercuric chloride (MC) has been found to damage function of many organelles (such as lysosomes that keep proton gradient through the membrane) and decline renal glutathione peroxidase activity (Buelna-Chontal et al., 2017). Some studies have found increased serious health effects among dental workers (Sahani et al., 2016). Mercury induces disruption of the cytochrome c oxidase system/ATP energy function (do Nascimento et al., 2008).
Numerous studies indicated that oxidative stress represents a dangerous event correlated to the neurotoxic effects of MC (Lohren et al., 2015). The levels of Reactive oxygen species (ROS) are dramatically increased upon MC exposure (Gökçe et al., 2018).
The central nervous system is one of the most susceptible organs affected by mercury toxicity. Both acute and chronic exposure to mercury is also known to cause a variety of neurological or psychiatric disorders (Moneim, 2015).
There is some evidence indicating that Mercury may be an etiological factor in Alzheimer’s disease (Siblerud et al., 2019). Thus, agents with the ability to suppress oxidative stress may be beneficial in reducing the risk of tissue injury and organ dysfunction during MC toxicity.
Brain-derived neurotrophic factor (BDNF) has emerged as a key neurotrophin regulating synaptic plasticity, neuronal differentiation and survival of new neurons and synapses. (Finkbeiner et al 1997). cAMP response element-binding (CREB) is a transcription activator that is implicated in the neuro-protection and survival-enhancing properties (Hatalski and Baram 1997; Conti et al 2002).
Mitogen-activated protein kinase (MAPKs) are a highly conserved family of structurally related serine/threonine protein kinases which is responsible for coordinating a variety of extracellular signaling pathways, regulating fundamental cellular processes involved in cell growth and survival (Johnson and Lapadat, 2002).
Alpha-tocopherol’s (vitamin E; VE) antioxidant activity plays a vital roles in the neuroprotection of mercury on the cerebellum and its movement-coordinating function (Owoeye et al., 2018).
The protective effect of VE on MC-induced hepatic and renal functions impairment and oxidative stress in male mice is well documented (Ali, 2018; Per, 2019; Owoeye et al., 2019).
Several studies have revealed that specific Lactobacillus strains alleviated heavy metal toxicity (Tian et al., 2017; Trinder et al., 2016; Yu et al., 2016; Alcantara et al., 2017). Recently, Majlesi et al., have proved the L. plantarum protected significantly against mercury toxicity in liver and kidney in rats by decreasing, creatinine, urea, bilirubin, ALT, and AST level, and preventing alterations in the levels of GPx and Superoxide dismutase (SOD) (Majlesi et al., 2017). It was previously reported that lactobacillus attenuates inflammation in mice by inhibiting NF-κB signaling pathway (Lim et al., 2017).
Although mercury is broadly sulfhydryl reactive, yet signaling cascade implicated in mediating MC-induced neurotoxicity is not fully investigated. This initiates the interest of the present study to investigate the new mechanistic role of MC neurotoxicity at the molecular level and to implement a trail for its amelioration using a combination of vitamin E (VE) and the probiotics Lactobacillus plantarum (LTB).
The current research highlights the potential impact of VE and/or LTB against brain injury induced by MC. The assessment of the efficacy of the antioxidants in question against brain injury was performed biochemically through measuring oxidative status biomarkers Malondialdehyde (MDA), Glutathione (GSH) levels and Superoxide dismutase (SOD) activity, inflammatory markers such as tumor necrosis factor-alpha (TNF-α) and interleukin 6 (IL-6), the apoptotic marker, cysteine-aspartic acid protease (caspase-3) and the expression of apoptosis regulator (Bax). At the molecular level protein expression of the MAPK and the neurotrophin BDNF and CREB were performed, DNA fragmentation was conducted as well. The previous parameters were supported by histopathological examination.
2 Materials and methods
2.1 Chemicals
LCB, VE, and MC were obtained from Sigma Chemical Co. (Sigma, St. Louis, MO, USA). MAPK, CREB and BDNF kits were obtained from Santa Cruz (Santa Cruz Biotechnology, CA, USA).
2.2 Experimental animals
Thirty Wistar adult male albino rats weighing 170–210 g were kept at a temperature of 20–22 °C, they were fed with standard rat pellet chow with free access to tap water ad libitum.
2.2.1 Experimental design
Rats were divided into five groups, six rats each. Rats were obtained from the Animal House, Faculty of Pharmacy, King Saud University. The Experimental protocol was approved by the Research Ethics Committee, King Saud University (KSU, SE, 19-38). They were treated as follows: the first group served as a normal control group and administered distilled water; the second group was intoxicated (s.c.) with 5 mg/kg MC (Peixoto et al., 2003) once daily; the third group was treated with VE at a dose of 100 mg/kg/day; orally (Yousef et al., 2012); the fourth group was orally treated with 6 × 1010 CFU of LTB in 1 mL of sterile normal saline (Li et al., 2017); and the fifth group was treated with VE and LTB at the previously mentioned doses. All treatments were given daily along with MC for fourteen days.
After the experimental period, the rats were fasted overnight, then they were subjected to CO2 gas and sacrificed with decapitation. Blood samples were collected then sera were separated by centrifugation at 3000 rpm for 20 min and the brain tissues were excised. Some samples of the brain were homogenized in phosphate buffer to yield 20% homogenates. The homogenates were centrifuged for 20 min at 3000 rpm at 4 °C and the supernatants were kept at − 80 °C. Other samples of the brain were rapidly frozen under liquid nitrogen and stored at −80 °C for Western blotting. Three samples from each group were kept in 10% formalin for histopathological examination.
2.3 Biochemical analysis
2.3.1 Determination of lipid peroxidation, glutathione levels, and Superoxide dismutase (SOD) activity
Malondialdehyde (MDA) levels were estimated in the brain tissues following the method of Uchiyama and Mihara, (1978). Glutathione (GSH) was determined using the method of Ellman (1959). Superoxide dismutase (SOD) activity was evaluated following the procedure of Marklund and Marklund (1974).
2.3.2 Determination of serum tumor necrosis factor-alpha (TNF-α), interleukin 6 (IL-6) and cysteine-aspartic acid protease (caspase-3)
Serum tumor necrosis factor-alpha (TNF-α) and interleukin 6 (IL-6) levels were measured using a sensitive rat ELISA kit (Immuno-Biological Laboratories Co., Ltd. Takasaki-Shi, Gunma, 370-0831, JAPAN). Caspase-3 was evaluated using a colorimetric assay kit obtained from Randox; UK.
2.4 Histological analysis
Brain tissues samples were stored in 10% formaldehyde embedded in paraffin wax. Thin sections were used for histopathological examination using Haemotoxylin and Eosin (H&E) stain.
2.5 DNA fragmentation
DNA fragmentation was quantitated by measuring oligonucleosome bound DNA using an ELISA kit (Boehringer, Mannheim, Germany) as described by Sedlackova et al. (2013).
2.6 Western blot analysis
Western blots of the extracts were performed to determine the protein expressions of MAPK, CREB, and BDNF. Proteins bands were visualized using the ECL-Plus detection system (Amersham Life Sciences, Little Chalfont, Buckinghamshire, UK) according to the manufacturer’s instructions. Positive immunoreactive bands were quantified densitometrically and compared with the control (Jackson et al., 2000).
2.7 Gene expression of apoptosis regulator (Bax)
Bax gene expression, in brain tissues, was estimated using the Livak and Schmittgen method (2001). It involves using quantitative real-time polymerase chain reaction (qRT-PCR) with specific primers of the Bax gene (Al-Rasheed et al., 2016) and cyber green dye (Table 1).
| Gene name | Primer sequence | Primer size (bp) |
|---|---|---|
| Refer-actin | Forward 5′ GAGACCTTCAACACCCCAGC 3′ Reverse 5′ ATGTCACGCACGATTTCCC 3′ |
263 |
| Bax | Forward 5′ GTTGCCCTCTTCTACTTTG 3′ Reverse 5′ AGCCACCCTGGTCTTG 3′ |
194 |
2.8 Statistical analysis
Data were expressed as means ± SEM for quantitative measures. The statistical comparison was performed using a one-way analysis of variance (ANOVA) followed by Tukey-Kramer multiple comparisons test. The level of significance was set at p ≤ 0.05, p ≤ 0.01 and p ≤ 0.001. Statistical tests were conducted using GraphPad Prism 5.00 (GraphPad Prism, San Diego, California, USA) and SPSS 21 (IBM, USA).
3 Results
The outcomes of the current study confirmed MC-induced elevation in serum TNF-α, IL 6 levels and Caspase 3 activity compared to the control group (P ≤ 0.001). The intake of VE and/or LTB for fourteen consecutive days along with MC intoxication significantly reduced the increase in serum TNF-α, IL-6 levels and Caspase 3 activity comparad to the MC injected group (P ≤ 0.001) (Table 2). Similarly, MC injection produced a significant increase in MDA level and a decline in GSH level and SOD activity in the brain tissues (P ≤ 0.001) matched with the control group. Oral treatment of MC injected rats with VE and/or LTB significantly modulated the MDA and SOD activity (P ≤ 0.001),while the GSH level was significantly up regulated only in the combination group (P ≤ 0.001) and moderately increased in VE treated rats (P ≤ 0.05), as depicted in Fig. 1. MC exhibited a significant increase in MAPK (Fig. 2), while protein expression for BDNF, CREB (Fig. 3), were significantly down regulated (P ≤ 0.001). BAX expressions were unregulated (P ≤ 0.001) (Fig. 4) compared to the normal group. On the other hand, oral treatment with VE and/or LTB significantly down regulated MAPK and BAX expression (P ≤ 0.001), contrary protein expressions of BDNF as well as CREB were significantly over expressed (P ≤ 0.001) matched with MC – intoxicated rats. Fig. 5 reveals that DNA fragmentation significantly increased in MC injected rats compared to the control group (P ≤ 0.001), in contrast, there was a significant decrease in DNA fragmentation post VE and/or LTB treatments (P ≤ 0.001).
| Groups | TNF-α (pg/ml) |
IL-6 (pg/ml) |
Caspase-3 (ng/ml) |
|---|---|---|---|
| Con | 27.5 ± 0.6 | 15.87 ± 0.8 | 2.5 ± 0.2 |
| MC | 127 ± 0.9+++ | 106.5 ± 3.9+++ | 11.5 ± 0.4+++ |
| VE | 55.3 ± 1.06*** | 66.44 ± 1.12*** | 8.5 ± 0.2***π |
| LTB | 59.7 ± 0.8*** | 72.8 ± 0.3*** | 6.89 ± 0.4*** |
| VE + LTB | 42.7 ± 1.7*** | 33.8 ± 1.2*** | 4.8 ± 0.2*** |
Data are Mean ± SEM (n = 6). +++P < 0.001 Vs control, ***P < 0.001 Vs MC group, πP < 0.05 Vs the combination group.
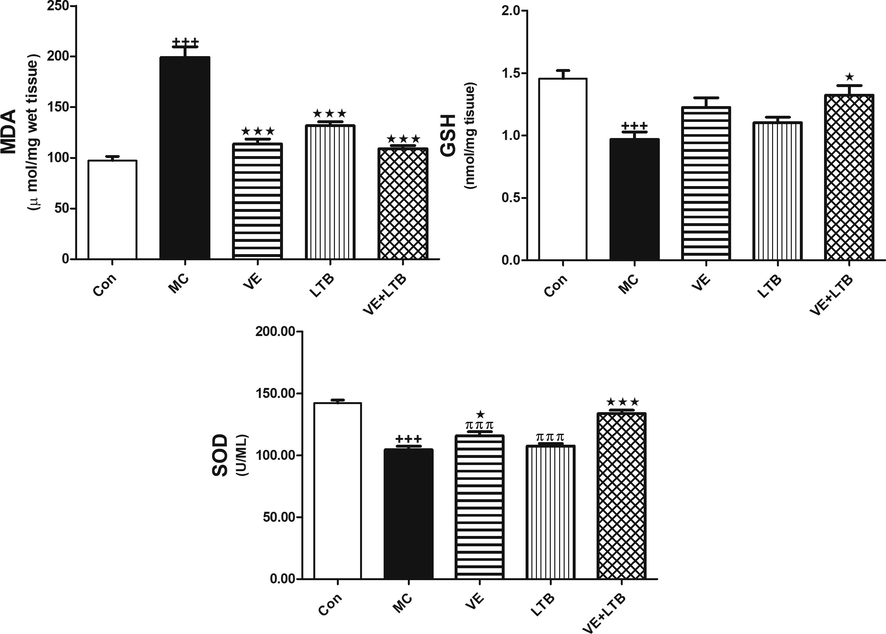
- GSH, SOD and MDA in brain tissue in control, MC and in all treated groups. The animals were treated with either distilled water (control). Data are Mean ± SEM (n = 6). +++P ≤ 0.001 Vs control, *P ≤ 0.05, ***P ≤ 0.001 Vs MC group, πππP < 0.001 Vs the combination group.
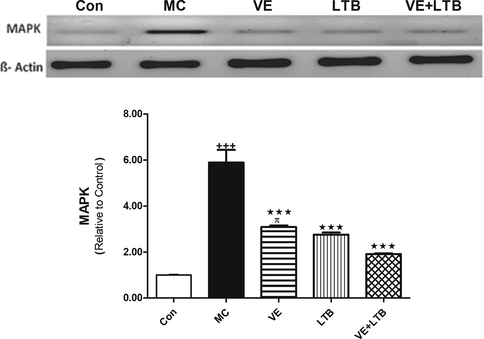
- Protein expression of MAPK in control, MC and in all treated groups. Data are Mean ± SEM (n = 6). +++P ≤ 0.001 Vs control, ***P ≤ 0.001 Vs MC group, πP ≤ 0.05 Vs the combination group.
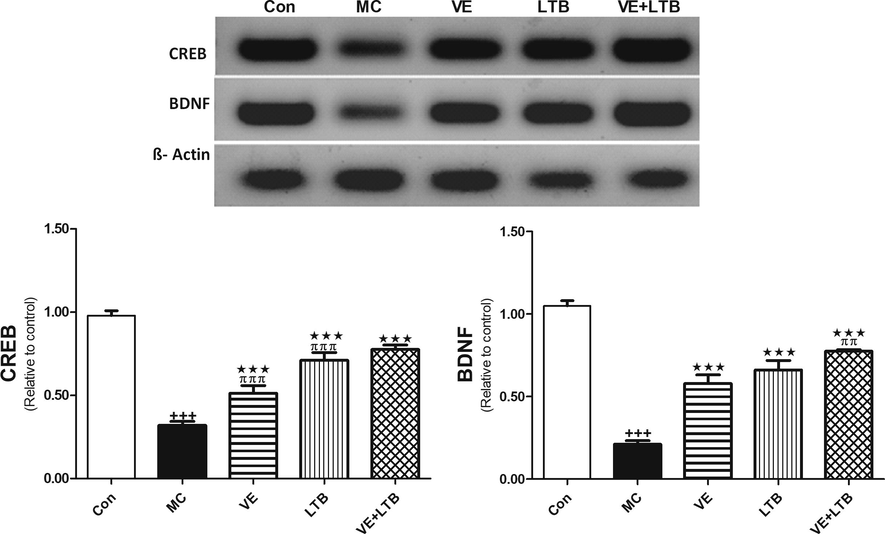
- Protein expression of BDNF and CREB in control, MC and in all treated groups. Data are Mean ± SEM (n = 6). +++P ≤ 0.001 Vs control, ***P ≤ 0.001 Vs MC group, ππP < 0.01, πππP < 0.001 Vs the combination group.
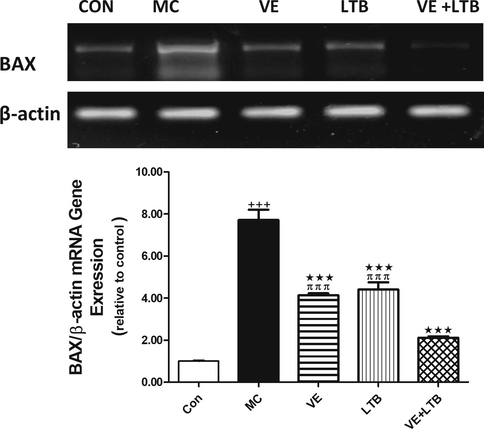
- mRNA gene expression of BAX in control, MC and all treated groups. The animals were treated with either distilled water (control). Data are Mean ± SEM (n = 6). +++P ≤ 0.001 Vs control, ***P ≤ 0.001 Vs MC group, πππP ≤ 0.001 Vs the combination group.
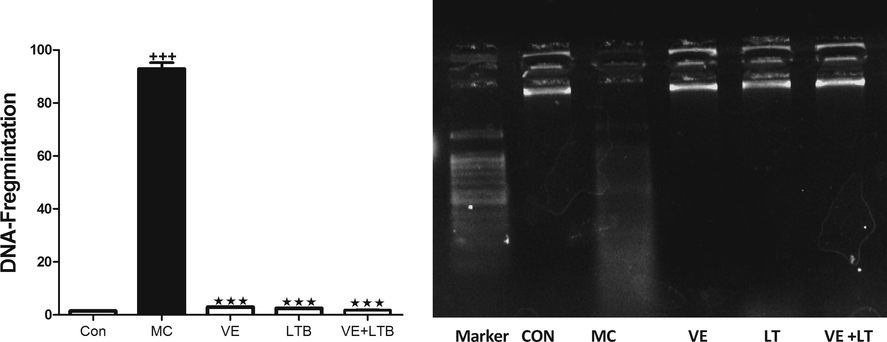
- DNA Fragmentation in control, MC and all treated groups. Data are Mean ± SEM (n = 6). +++P ≤ 0.001 Vs to control, ***P ≤ 0.001 Vs to MC group.
Histopathological examination of the H&E-stained brain sections illustrated that the cerebellum was characterized by a reduction in the cellular size of the molecular layer, distortion of granular cell layer and scattered, sparse cell distribution of Purkinje layers in MC injected rats compared to the normal brain tissues (Fig. 6B). On the other hand, examining sections of the brains treated with VE and LTB either alone or together; the cerebellum revealed almost normal histological features, illustrating well-defined molecular, granular and Purkinje layers (Fig. 6C–E). The combination of VE and LTB was the most effective regimen in the improvement of brain architecture in MC-induced brain injury.
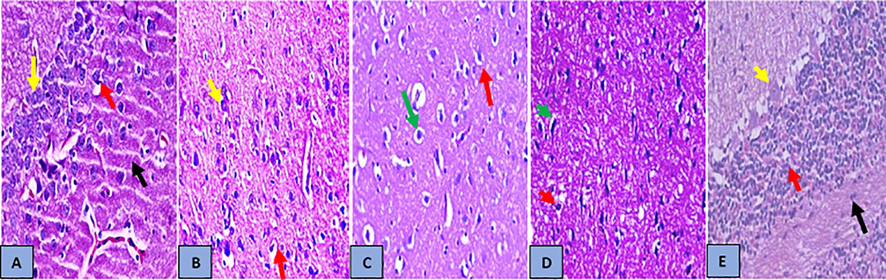
- Histopathology analysis of brain sections (A) Brain section of normal control group, showed normal histological features of the cerebellum (black arrow) with the presence of numerous closely packed small cells in the granular layer (red arrow) and Purkinje layers (large Purkinje cells) (yellow arrow) with normal structure of neuronal cells. (B) Brain section of MC group, showed the cerebellum with thin, reduction in cellular size of the molecular layer (black arrow), distortion of granular cell layer (red arrow) and scattered, sparse cell distribution of Purkinje layers (yellow arrow), (C) In brain section treated with VE, the cerebellum showed almost normal histological features, illustrating a well-defined molecular (green arrow), granular (presence of numerous closely packed small cells in the granular) (red arrow) (D) Brain section of treated with LTB, the cerebellum showed almost normal histological features, illustrating a well-defined molecular (yellow arrow), granular (presence of numerous closely packed small cells in the granular) (red arrow) and Purkinje layers (large Purkinje cells) (yellow arrow) with vacuolization and edema of neuronal cells of the frontal cortex (green arrow). (E) Brain section of treated with VE + LTB cerebellum showed almost normal histological features, illustrating a well-defined molecular (black arrow), granular (presence of numerous closely packed small cells in the granular) (red arrow) and Purkinje layers (large Purkinje cells) (yellow arrow) with normal structure of neuronal cells, (H&E stain, ×400).
4 Discussion
The central nervous system is one of the most vulnerable organs affected by mercury toxicity, which causes a variety of neurological or psychiatric disorders (Moneim, 2015; Sakamoto et al., 2015, 2017). MC caused significant behavioral alteration and induced Purkinje neuron lesion in the cerebellum. VE has antioxidant roles in the reducing the toxic effects of mercury on the cerebellum and its movement-coordinating function. It is well known that LTB has antioxidative potential and able to decrease oxidative stress-mediated atherogenicity in humans, (Kullisaar et al., 2003). However, the mechanisms underlying the neuroprotective efficacy of VE or LTB are not fully understood.
In this study, the effect of VE and/or LTB on oxidative stress, inflammation and apoptosis in brain of MC-intoxicated rats was investigated. Rats received MC exhibited brain injury evidenced by the significant increase in brain LPO and diminished GSH level and SOD activity indicating oxidative stress. Moreover, MC showed cerebellum with reduction in cellular size of the molecular layer, distortion of granular cell layer and scattered, sparse cell distribution of Purkinje layers. In accordance, previous studies have demonstrated diminished the expression of SOD, reduced glutathione, and catalase and increased LPO in testes of MC -intoxicated rats (Muthu and Krishnamoorthy, 2012).
It was reported that the prime mechanism related to mercuric toxicity is the formation of ROS and nitrogen species that exhibit an imbalance between – oxidants/anti-oxidants in the body (Moneim, 2015).
ROS provoke injury through oxidizing lipids and proteins, inactivating antioxidant enzymes and triggering DNA damage (Martindale and Holbrook, 2002). In the current study, VE and/or LTB reduced LPO and boosted cellular antioxidants in brain of MC-intoxicated rats, demonstrating a potent antioxidant efficacy. Upon treatment with VE, the cerebellum showed almost normal histological features. Treatement with LTB revealed that the cerebellum showed almost normal histological features, large Purkinje cells with vacuolization and edema of neuronal cells of the frontal cortex, while brain sections of rats treated with VE & LTB cerebellum showed large Purkinje cells with normal structure of neuronal cells.
In accordance, Celikoglu et al. (2015) found that supplementation of VE alleviated MC-induced oxidative stress and increased the activities of SOD, catalase, glutathione peroxidase that were depleted as a result of the toxicity induced by mercury. Beside its antioxidant activity, VE plays a vital role in decreasing the absorption of mercury from the gastrointestinal tract (Su et al., 2008). Matched with the obtained results, it was documented that Lactobacillus brevis 23,017 relieves mercury toxicity in the colon by modulation of oxidative stress and inflammation through the interplay of MAPK and NF-κB Signaling Cascades (Jiang et al., 2018).
Besides oxidative stress, inflammation has also been implicated in the adverse toxic effects of MC (Rao and Purohit, 2011). In the current study, MC induced an inflammatory response illustrated by the production of inflammatory mediators, including TNF-α, IL-6 and increased DNA fragmentation and enhanced MAPK signaling pathways.
TNF-α is released by activated macrophages and participates in both local and systemic inflammation (Turner et al., 2014). Betti et al. (1993) showed that administration of MC impairs the cells, cell membrane, and DNA which leads to cell necrosis. The increased level of ROS production, inflammatory cytokines, and NO represent a main culprit behind MC toxicity in different tissues (Ahmad and Mahmood, 2019). In accordance, MC induces an autoimmune syndrome and necrotizing leucocytoclastic vasculitis in the Brown Norway rat (Qasim et al., 1994). Mercury stimulated the expression of TNFα, and increased LPS-induced TNFα and interleukin-6 mRNA expression, activated p38 mitogen-activated protein kinase (Kim et al., 2002). In addition, excessive release of TNF-α and IL −1β was recorded in response to MC intoxication (Almeer et al., 2019).
Long exposure of vascular smooth muscle cells (VSMC) to low doses of mercury activates MAPK signaling pathways resulting in activation of inflammatory proteins such as NADPH oxidase and COX-2 that in turn induces proliferation of VSMC and changes in cell size (Aguado et al., 2013). Other researches have revealed that oral mercury exposure affects the gut ecology, increases inflammation and susceptibility to colitis in mice (Stejskal, 2013) (Toomey et al., 2014; Eaton et al., 2017). In the present study, treatment with VE and/or LTB reduced TNF-α, interleukin (IL)-6 levels, decreased DNA fragmentation and down regulated MAPK signaling pathways. It was shown that VE ameliorated oxidative damage in vivo in systemic vasculitides. Numerous researches indicated that lactobacillus attenuates inflammation in mice by inhibiting NF-κB and p38 MAPK signaling pathways (Chon et al., 2010; Saito et al., 2012) that leads to suppress oxidative stress and inflammation (Lim et al., 2017). CREB and BDNF are the major proteins that play a vital role in brain function and regulation. BDNF has emerged as a key neurotrophin regulating synaptic plasticity, neuronal differentiation and survival of new neurons and synapses. CREB, is a transcription activator that is implicated in both stress- (Hatalski and Baram 1997) and antidepressant-induced transcriptional regulation (Conti et al 2002). The cAMP-CREB signalling cascade is critical to the generation of new neurons in the rodent hippocampus (Nakagawa et al 2002), and also facilitates their subsequent morphological maturation (Fujioka et al 2004). Thus, given its neuro-protective and survival-enhancing properties. Shared interactions between BDNF and CREB were well documented: BDNF promotes the phosphorylation of CREB, which, in turn, promotes the transcription of BDNF gene. BDNF activates CREB, in part, by increasing intracellular Ca2+ leading to the activation of CaMKIV, which ‘phosphorylates CREB (Finkbeiner et al 1997). In the present study, MC intoxication induces down regulation of protein expression of both CREB and BDNF in the brain tissues. Previous studies demonstrated that MC induced Purkinje neuron lesion in the cerebellum, thus caused significant behavioural alteration (Owoeye and Arinola, 2017; Owoeye et al., 2018).
VE supported its antioxidant roles in neuroprotection, thus reducing the toxic effects of mercury on the cerebellum and its movement-coordinating function.
The current study introduced new information that modulation of CREB and BDNF signaling plays a role in the protective mechanism of VE against MC neurototoxicity.
Treatment with the antioxidants in question alone or together mitigated the alteration in CREB and BDNF signaling pathways.
In the present study, MC intoxication induces up regulation of caspase-3 activity and mRNA expression of Bax in brain tissues. Previous studies demonstrated that pro-apoptotic proteins was favored following MC intoxication. Caspase-3 activity was higher with MC apoptosis, as indicated by the elevation of Bax/Bcl-2 ratio in brain tissues (Moneim, 2015).
It was reported that VE has an antioxidant defense with anti-inflammatory and anti-apoptotic activities against MC-induced neurotoxicity (Moneim, 2015).
In the present study, the intake of VE and/or LTB alleviated the increase in the studied apoptotic markers. Interestingly, the combination regimen exhibited the most admirable results in all the studied parameters
5 Conclusions
This study demonstrates that VE prevents MC neurotoxicity by attenuating oxidative stress, inflammation, DNA damage and cell death. The neuroprotective efficacy of VE was reinforced by the intake of LTB. The obtained results introduced new information that BDNF and CREB/MAPK signaling is implicated in MC -induced brain injury and dysfunction.
Acknowledgment
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding this work through (RG-1440-017).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Mercury chloride toxicity in human erythrocytes: enhanced generation of ROS and RNS, hemoglobin oxidation, impaired antioxidant power, and inhibition of plasma membrane redox system. Environmental Science and Pollution Research. 2019;26(6):5645-5657.
- [Google Scholar]
- Characterization of the binding capacity of mercurial species in Lactobacillus strains. Journal of the Science of Food and Agriculture. 2017;97:5107-5113.
- [CrossRef] [Google Scholar]
- Down-regulation of NFkB, Bax,TGF-β, Smad-2mRNA expression in the livers of carbon tetrachloride treated rats using different natural antioxidants. Brazilian Archives of Biology and Technology. 2016;59:e16150553. Epub March 22
- [CrossRef] [Google Scholar]
- Mercury induces proliferation and reduces cell size in vascular smooth muscle cells through MAPK, oxidative stress and cyclooxygenase-2 pathways. Toxicology and Applied Pharmacology. 2013;268(2):188-200.
- [Google Scholar]
- Ziziphus spina-christi leaf extract attenuates mercury chloride-induced testicular dysfunction in rats. Environmental Science and Pollution Research 2019:1-12.
- [Google Scholar]
- Mitigative role of garlic and vitamin E against cytotoxic, genotoxic, and apoptotic effects of lead acetate and mercury chloride on WI-38 cells. Pharmacological Reports. 2018;70(4):804-811.
- [Google Scholar]
- Comparative studies on cytotoxic and genotoxic effects of two organic mercury compounds in lymphocytes and gastric mucosa cells of sprague-dawley rats. Environmental and Molecular Mutagenesis. 1993;22(3):172-180.
- [Google Scholar]
- CDP-choline circumvents mercury-induced mitochondrial damage and renal dysfunction. Cell Biology International. 2017;41(12):1356-1366.
- [Google Scholar]
- Vitamin E and sodium selenite against mercuric chloride-induced lung toxicity in the rats. Brazilian Archives of Biology and Technology. 2015;58(4):587-594.
- [Google Scholar]
- Suppression of proinflammatory cytokine production by specific metabolites of Lactobacillus plantarum 10hk2 via inhibiting NF-kappaB and p38 MAPK expressions. Comparative Immunology, Microbiology and Infectious Diseases. 2010;33:e41-e49.
- [CrossRef] [Google Scholar]
- cAMP response element-binding protein is essential for the upregulation of brain-derived neurotrophic factor transcription, but not the behavioral or endocrine responses to antidepressant drugs. Journal of Neuroscience. 2002;22(8):3262-3268.
- [Google Scholar]
- Methylmercury neurotoxicity & antioxidant defenses. Indian Journal of Medical Research. 2008;128:373-382.
- [Google Scholar]
- Modulators of mercury risk to wildlife and humans in the context of rapid global change. Ambio. 2018;47(2):170-197.
- [Google Scholar]
- Primary human polarized small intestinal epithelial barriers respond differently to a hazardous and an innocuous protein. Food and Chemical Toxicology. 2017;106:70-77.
- [CrossRef] [Google Scholar]
- CREB: a major mediator of neuronal neurotrophin responses. Neuron. 1997;19(5):1031-1047.
- [Google Scholar]
- Activation of cAMP signaling facilitates the morphological maturation of newborn neurons in adult hippocampus. Journal of Neuroscience. 2004;24(2):319-328.
- [Google Scholar]
- Ergothioneine prevents endothelial dysfunction induced by mercury chloride. Experimental and Therapeutic Medicine. 2018;15(6):4697-4702.
- [Google Scholar]
- Stress-induced transcriptional regulation in the developing rat brain involves increased cyclic adenosine 3′, 5′-monophosphate-regulatory element binding activity. Molecular Endocrinology. 1997;11(13):2016-2024.
- [Google Scholar]
- Cyclooxygenase (COX) 1 and 2 in normal, inflamed, and ulcerated human gastric mucosa. Gut. 2000;47(6):762-770.
- [Google Scholar]
- Lactobacillus brevis 23017 relieves mercury toxicity in the colon by modulation of oxidative stress and inflammation through the interplay of MAPK and NF-κB signaling cascades. Frontiers in Microbiology. 2018;9:2425.
- [Google Scholar]
- Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298(5600):1911-1912.
- [Google Scholar]
- Mercury inhibits nitric oxide production but activates proinflammatory cytokine expression in murine macrophage: differential modulation of NF-κB and p38 MAPK signaling pathways. Nitric Oxide. 2002;7(1):67-74.
- [Google Scholar]
- Antioxidative probiotic fermented goats’ milk decreases oxidative stress-mediated atherogenicity in human subjects. British Journal of Nutrition. 2003;90:449-456.
- [Google Scholar]
- Safety assessment of Lactobacillus helveticus KLDS1. 8701 based on whole genome sequencing and oral toxicity studies. Toxins. 2017;9(10):301.
- [Google Scholar]
- Lactobacillus fermentum IM12 attenuates inflammation in mice by inhibiting NF-kappaB-STAT3 signalling pathway. Beneficial Microbes. 2017;8:407-419.
- [CrossRef] [Google Scholar]
- Analysis of relative gene expression data using real-time quantitative PCR and the 2 (−Delta Delta C (T)) Method. Methods. 2001;25:402-408.
- [Google Scholar]
- Toxicity of organic and inorganic mercury species in differentiated human neurons and human astrocytes. Journal of Trace Elements in Medicine and Biology. 2015;32:200-208.
- [Google Scholar]
- Effect of probiotic Bacillus Coagulans and Lactobacillus Plantarum on alleviation of mercury toxicity in rat. Probiotics and Antimicrobial Proteins. 2017;9:300-309.
- [CrossRef] [Google Scholar]
- Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. European Journal of Biochemistry. 1974;47(3):469-474.
- [Google Scholar]
- Cellular response to oxidative stress: signaling for suicide and survival. Journal of Cellular Physiology. 2002;192:1-15.
- [Google Scholar]
- The neuroprotective effect of berberine in mercury-induced neurotoxicity in rats. Metabolic Brain Disease. 2015;30(4):935-942.
- [Google Scholar]
- Effect of vitamin C and vitamin E on mercuric chloride-induced reproductive toxicity in male rats. Biochemical Pharmacology. 2012;1(102):2167-0501.
- [Google Scholar]
- Regulation of neurogenesis in adult mouse hippocampus by cAMP and the cAMP response element-binding protein. Journal of Neuroscience. 2002;22(9):3673-3682.
- [Google Scholar]
- Low-dose inorganic mercury increases severity and frequency of chronic coxsackievirus-induced autoimmune myocarditis in mice. Toxicological Sciences. 2011;125(1):134-143.
- [Google Scholar]
- A Vegetable, Launaea taraxacifolia, mitigated mercuric chloride alteration of the microanatomy of rat brain. Journal of Dietary Supplements. 2017;14(6):613-625.
- [Google Scholar]
- Bromocriptine and vitamin E were protective against mercury-induced purkinje neuron injury in male wistar rats. African Journal of Biomedical Research. 2018;21(2):193-199.
- [Google Scholar]
- Comparative neuroprotective effect of Celosia argentea Linn. and vitamin E on mercury-induced oxidative and histological parameters of rat brain. Nigerian Journal of Physiological Sciences: Official Publication of the Physiological Society of Nigeria. 2019;34(2):167-175.
- [Google Scholar]
- Protective effect of vitamin E and sodium selenite in mercury chloride-induced toxicity in human leukocytes in vitro. Fresenius Environmental Bulletin. 2019;28(11):7971-7981.
- [Google Scholar]
- Effects of zinc and cadmium on HgCl2-δ-ALA-D inhibition and Hg levels in tissues of suckling rats. Toxicology letters. 2003;146(1):17-25.
- [Google Scholar]
- Use of methyl prednisolone and antioxidants in mercuric chloride-induced experimental vasculitis. Clinical & Experimental Immunology. 1994;98(1):66-70.
- [Google Scholar]
- Neuroprotection by melatonin on mercury induced toxicity in the rat brain. Pharmacology & Pharmacy. 2011;2(04):375.
- [Google Scholar]
- Mercury in dental amalgam: Are our health care workers at risk? Journal of the Air & Waste Management Association. 2016;66(11):1077-1083.
- [Google Scholar]
- Phosphodiesterase inhibitors suppress Lactobacillus casei cell-wall-induced NF-kappaB and MAPK activations and cell proliferation through protein kinase A–or exchange protein activated by cAMP-dependent signal pathway. Scientific World Journal. 2012;2012:748572.
- [CrossRef] [Google Scholar]
- Significance of fingernail and toenail mercury concentrations as biomarkers for prenatal methylmercury exposure in relation to segmental hair mercury concentrations. Environmental Research. 2015;136:289-294.
- [CrossRef] [Google Scholar]
- Stable and episodic/bolus patterns of methylmercury exposure on mercury accumulation and histopathologic alterations in the nervous system. Environmental Research. 2017;152:446-453.
- [CrossRef] [Google Scholar]
- Fragmentation of DNA affects the accuracy of the DNA quantitation by the commonly used methods. Biological Procedures Online. 2013;15:5.
- [Google Scholar]
- A hypothesis and evidence that mercury may be an etiological factor in Alzheimer’s disease. International Journal of Environmental Research and Public Health. 2019;16(24):5152.
- [Google Scholar]
- Mercury-induced inflammation: yet another example of ASIA syndrome. Israel Medical Association Journal. 2013;15:714-715.
- [Google Scholar]
- The interaction of selenium and mercury in the accumulations and oxidative stress of rat tissues. Ecotoxicology and Environmental Safety. 2008;70(3):483-489.
- [Google Scholar]
- The therapeutic protection of a living and dead Lactobacillus strain against aluminum-induced brain and liver injuries in C57BL/6 mice. PloS One. 2017;12(4):e0175398.
- [Google Scholar]
- Cathepsin B regulates the appearance and severity of mercury-induced inflammation and autoimmunity. Toxicological Sciences. 2014;142:339-349.
- [CrossRef] [Google Scholar]
- Probiotic Lactobacillus rhamnosus reduces organophosphate pesticide absorption and toxicity to drosophila melanogaster. Applied and Environmental Microbiology. 2016;82:6204-6213.
- [CrossRef] [Google Scholar]
- Cytokines and chemokines: at the crossroads of cell signalling and inflammatory disease. Biochimica et Biophysica Acta. 2014;1843:2563-2582.
- [Google Scholar]
- Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Analytical Biochemistry. 1978;86(1):271-278.
- [Google Scholar]
- Oxidative stress, apoptosis and abnormal expression of apoptotic protein and gene and cell cycle arrest in the cecal tonsil of broilers induces by dietary methionine deficiency. Research in Veterinary Science. 2018;121:65-75.
- [CrossRef] [Google Scholar]
- Ascorbic acid protects against the nephrotoxicity and apoptosis caused by colistin and affects its pharmacokinetics. J Antimicrob Chemother.. 2012;67:452-459.
- [Google Scholar]
- Potential of Lactobacillus plantarum CCFM639 in protecting against aluminum toxicity mediated by intestinal barrier function and oxidative stress. Nutrients. 2016;2:8.
- [CrossRef] [Google Scholar]







