Translate this page into:
Comb age significantly influences the productivity of the honeybee (Apis mellifera) colony
⁎Corresponding author. elkazafi.taha@agr.kfs.edu.eg (El-Kazafy A. Taha)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objectives
The honeybee mainly uses the wax comb for brood rearing and food storage. Repeated brood rearing in the comb changes the wax color and cell dimensions. Therefore, we aimed to study the decline of body size of the individual bees and its impact on colony productivity in relation to comb age.
Methods
Twenty colonies of hybrid Carniolan honeybees, each of 12,000 bees, were used. Combs aged 1–3 years were used as new combs, and combs aged 4–6 years as old combs. The weight of the worker, queen, drone, and royal jelly (RJ)/queen cell, storing pollen and honey, and rearing worker and drone brood were determined.
Results and conclusions
The body weights of the newly emerged worker, drones, queens, and nurse and forager workers of colonies with the new combs were significantly heavier than those of the colonies with the old combs. Colonies with the new combs were significantly more active in storing pollen and honey, RJ production, and rearing workers and drones. We concluded that the body sizes of the individual bees were declined, and the productivity was decreased in the colonies with the old combs. Replace combs after three years with new others is recommended to encourage colony growth and increase productivity.
Keywords
Brood
Comb
Honey
Honeybee
Pollen
Queen
Worker
1 Introduction
Several factors influence the activity of the honeybee colony, but the availability of food resources has been reported as the most effective (Taha and Bayoumi, 2009; Awad et al., 2017; El-Seedi et al., 2020; Taha and Al-Kahtani, 2020). Feeding on proteinaceous diets, e.g., brewer's yeast, defatted soybean flour, pollen candy, and skimmed powder milk (Taha, 2015b; Puškadija et al., 2017) has considered. Also, the population size of the colony, i.e., the strong colonies produced more brood and honey compared with the weak colonies (Taha and Al-Kahtani, 2013; Kasangaki et al., 2018). Besides, the activity of the honeybee colony has reportedly affected by the bee species/subspecies, e.g., the Yemeni bee in Saudi Arabia stored more pollen and reared more worker brood than the Carniolan one, while the Carniolan bees produced more honey (Taha et al., 2016; Taha and Al-Kahtani, 2019). In addition, seasonal variations of colony activities have been reported (Taha and Al-Kahtani, 2020).
A large scale of beekeepers worldwide continues using combs in the colony for about 4–6 years (Taha, 2013). This practice resulted in an accumulation of cocoons, pollen, and propolis in the cells (Taha et al., 2010), and the cell dimensions declined (Shawer et al., 2020). The age of the comb has reportedly affected the brood survivorship and the colony growth (Taha and Al-Kahtani, 2020), and the morphometric characteristics of the workers (Al-Kahtani, 2018; Shawer et al., 2020). Also, the honey yield (Taha and Al-Kahtani, 2020) and the physicochemical composition of the honey (Taha and El-Sanat, 2007; Taha et al., 2010) have been affected.
Because of the accumulation of larval cocoons and other detritus, the cell walls become thicker, and overtime, the internal cell diameter declines and becomes smaller (Karihaloo et al., 2013). Compared with the inner cell diameter of the new combs, more than a 15% reduction in the inner cell diameter of the old combs has occurred (Shawer et al., 2020). Taha and Al-Kahtani (2020) have examined each comb age in a separate colony as a treatment to investigate the effect on colony performance; they have found a significant effect on the activity and productivity of the colony. Applicably, it is difficult to use one comb age separately in a colony, and actually, the hive consists of combs of different ages, so in this study, we used combs aged 1–3 years as new combs and 4–6 years as old combs. A reduction of cell size overtime have expected; this reduction influences the size of worker bees (Shawer et al., 2020), and consequently, a decline of nectar and pollen collection, brood rearing, honey yield, and other hive products can occur. Here we aimed to study the decline of the body size of the individual bees and its effect on the colony productivity in relation to the age of the comb.
2 Materials and methods
2.1 Experimental site
The study was conducted at the apiary of the Faculty of Agriculture, Kafrelsheikh University, Kafrelsheikh, Egypt, from the begging of February 2019 to the end of January 2020. Kafrelsheikh lies at longitude 30° 56′ 45″ E, latitude 31° 6′ 42″ N, and an altitude of 17 m above sea level.
2.2 Experimental colonies
Twenty colonies of hybrid Carniolan (Apis mellifera carnica Pollmann) honeybees were selected, equalized to be in the same strength (12,000 bees on six combs for each), and requeened by newly mated sister queens. The colonies were divided into two groups of 10 colonies. The combs were replaced by empty combs aged 1–3 years (Photo 1) in group 1 (new combs) and 4–6 years (Photo 2) in group 2 (old combs). Each colony of group 1 consists of two combs of age 1, 2, and 3 years, and group 2 consists of two combs of age 4, 5, and 6 years.

Photo (1): New combs.

Photo (2): Old combs.
2.3 Body weight of individual bees and royal jelly/queen cell
The body weights of the newly emerged, nurse, and forager workers were determined at the begging and end of the experiment. Fifty newly emerged workers of each group were collected within three hours of emergence. Fifty nurse workers (4–12 days) of each group were collected from the brood nest area. Fifty foragers workers (>21 days) of each group were collected from the flaying board. We selected March and July to examine the amount of royal jelly (RJ)/queen cell and the weight of the newly emerged queen and drone. The queens were removed from the colonies at the begging of March and July to stimulate building the emergency queen cells. From each group, we harvested RJ from 50 queen cells after three days of the larval age. The mean yield of RJ (mg)/queen cell was calculated as described by Al-Kahtani and Taha (2020). Fifty queen cells of each group were left to ripe and caging one day before emergence. The newly emerged queens were weighed within three hours of emergence. Fifty drones of each group were weighed within three hours of emergence. The individual bees were chilled for 4 min to facilitate the determining of the fresh body weight (mg) using an electronic balance.
2.4 Stored pollen, honey, and brood production
The areas of worker and drone sealed brood and stored pollen were measured using a standard frame divided into square inches at 12 days intervals, and the monthly area was calculated. The monthly area of sealed honey in each colony was measured using a standard frame divided into square inches.
2.5 Statistical analysis
The differences between the new and old combs were tested using a two-way analysis of variance (ANOVA), which indicated significant differences between the new and old combs. The normality in data was tested by the Shapiro-Wilk normality test, which indicated the normal distribution of the data. Therefore, the analysis was performed on the original data. The ANOVA was used to assess differences between the new and old combs via the PROC GLM function in SAS version 9.1 (SAS Institute, 2003). Duncan’s Multiple Range Test (Duncan, 1955) was used to compare the mean weights of workers, drones, queens, and RJ/queen cells from the new and old combs.
3 Results
The body weights of the newly emerged (114.89 and 109.45 vs. 88.05 and 78.21 mg), nurse (95.70 and 91.65 vs. 84.95 and 61.83 mg), and forager (85.75 and 81.75 vs. 78.00 and 57.05 mg) workers reared in the new combs were significantly (P < 0.01) heavier than those of the old combs at the begging and end of the experiment, respectively (Table 1). Values are the mean ± S.D. The means of each worker caste followed by a different letter are significantly different. ** indicate P < 0.01.
Worker caste
Experiment
Combs
Significance
New
Old
Newly emerged
Begging
114.89 ± 2.85a
88.05 ± 2.06c
**
End
109.45 ± 3.01b
78.21 ± 2.89d
Nurse
Begging
95.70 ± 0.67a
84.95 ± 3.21c
**
End
91.65 ± 0.52b
61.83 ± 1.10d
Forager
Begging
85.35 ± 0.49a
78.00 ± 3.14c
**
End
81.75 ± 0.50b
57.05 ± 0.69d
The weights of the RJ/queen cell (209.01 and 180.67 vs. 132.18 and 108.07 mg), newly emerged queen (162.18 and 146.89 vs. 137.41 and 114.78 mg), and drone (235.12 and 228.64 vs. 191.50 and 186.16 mg) in the new combs vs. old combs on March and July, respectively (Table 2). Values are the mean ± S.D. The means of each parameter followed by a different letter are significantly different. ** indicate P < 0.01.
Parameters
New combs
Old combs
Significance
RJ/queen cell
March
209.01 ± 5.53a
132.18 ± 3.62c
**
July
180.67 ± 6.05b
108.07 ± 4.99d
Newly emerged queen
March
162.18 ± 2.45a
137.41 ± 4.69c
**
July
146.89 ± 2.84b
114.78 ± 1.34d
Newly emerged drone
March
235.12 ± 2.67a
191.50 ± 2.45c
**
July
228.64 ± 1.49b
186.16 ± 1.11d
Colonies with the new combs stored significantly (P < 0.01) more pollen and honey than colonies with the old combs (1031.55 and 1719.70 in.2/colony/year vs. 616.43 and 910.73 in.2/colony/year, respectively). The largest monthly area of stored pollen and sealed honey (238.47 and 563.20 in.2/colony, respectively) were found in colonies with the new combs during May. The lowest monthly area of stored pollen and sealed honey (1.07 and 3.73 in.2/colony, respectively) were recorded in colonies with the old combs during January (Figs. 1 and 2).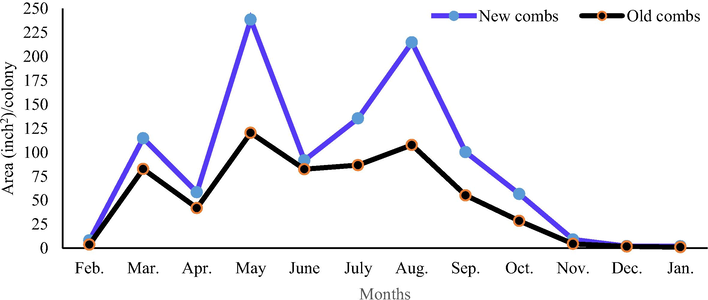
Effect of comb age on the monthly stored pollen area.
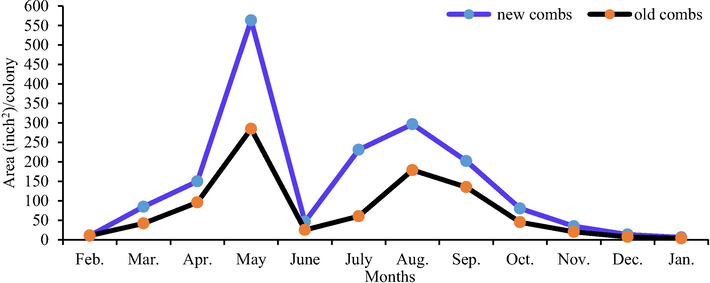
Effect of comb age on the monthly sealed honey area.
Colonies with the new combs reared significantly (P < 0.01) more workers and drones in comparison with colonies of the old combs (3930.49 and 66.09 in.2/ colony/year vs. 1996.46 and 49.09 in.2/colony/year, respectively). The largest monthly area of worker sealed brood (484.10 in.2/colony) was found in colonies with the new combs during July. The lowest monthly area of worker sealed brood (50.50 in.2/colony) was recorded in the old combs colonies during December (Fig. 3). The largest monthly area of drone sealed brood (18.93 in.2/colony) was found in colonies with the new combs during March, while all colonies did not rear drone brood from October to January (Fig. 4).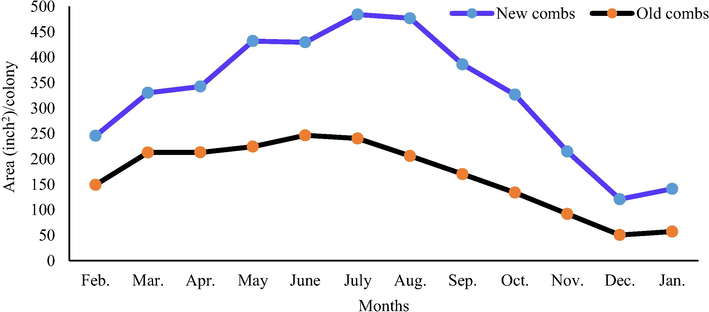
Effect of comb age on the monthly worker sealed brood area.
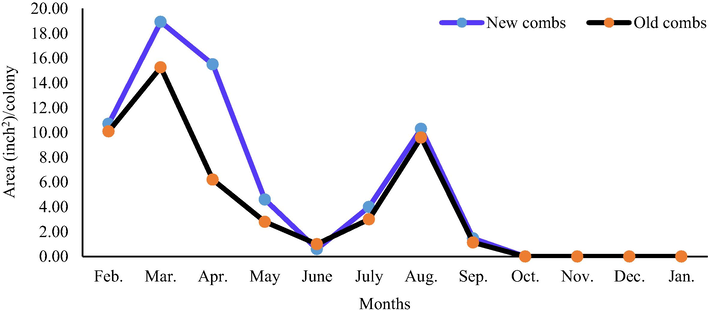
Effect of comb age on the monthly drone sealed brood area.
4 Discussion
The worker body weight has positively correlated with morphometric characteristics (Al-Kahtani and Taha, 2014, 2021), and colony productivity (Kolmes and Sam, 1991; Taha, 2013). Body characteristics have been used to indicate colony productivity because workers with larger legs and wings gather greater amounts of nectar and pollen than smaller workers (Mostajeran et al., 2006). The body weight of workers reared in the new combs exceeded the body weight of workers reared in the old comb by 30.48 and 39.95%, 12.65 and 48.23%, and 9.42 and 43.30% for the newly emerged, nurse, and forager workers at the begging and end of the experiment, respectively. Therefore, we expected better performance for workers produced from the new combs in nursing brood and gathering nectar, pollen, propolis, and water; and consequently high yields of the hive products. The differences between workers reared in the new and old combs may be due to the differences in cell dimensions of the combs, which were larger in the new combs. Taha and Al-Kahtani (2017) and Shawer et al. (2020) reported the superiority of body weight and other morphometric characteristics related to the colony productivity of workers reared in the new combs compared to workers reared in the old combs.
The reduction percentages in the body weight of workers reared in the old combs in comparison with those from the new combs at the begging vs. the end of the experiment were 4.97 vs. 12.58%, 4.42 vs. 37.39, and 4.40 vs. 36.72% for the newly emerged, nurse, and forager workers, respectively. These percentages proved that the decline of worker body size from the old combs was many folds of the decline occurred in the new combs.
The colonies with the new combs surpassed colonies with the old combs in RJ/queen cell (58.13 and 67.18%), body weight of the newly emerged queen (18.03 and 27.98%), and drone (22.78 and 22.82%) in March and July, respectively. These reductions were due to the decrease in the dimensions of comb cells in the old combs. The reduced weight of the queens and drones possibly resulted from poor feeding of the drone and queen larvae by the nurse bees because of the reduced cell volume. The findings of Taha and Al-Qarni (2013), and Szentgyörgyi et al. (2017) can explain the reduction in drone weight as they reported that the reduction of feeding the drone larva has resulted in a small drone.
The amount of stored pollen in the colonies depends upon the amount of gathered pollen and the rate of consumption by brood and adult bees (Taha, 2015c). Colonies with the new combs stored 67.34% more pollen than colonies with the old combs. We observed three peaks of storing pollen in the combs; the first was during March, the second and the biggest was during May, and the third was during August. These peaks coincided with the flowering of faba bean (Vicia faba L.) and flax (Linum usitatissimum L.) during March, Egyptian clover (Trifolium alexandrinum L.) during May, maize (Zea mays L.), and sunflower (Helianthus annuus L.) during August (Taha et al., 2019). Relatively similar results were reported by Shawer et al. (2003), Taha et al. (2019) in Egypt, Taha (2015a), Taha (2015b) and Taha and Al-Kahtani (2019) in Saudi Arabia. We observed a very low stored pollen area from November to February due to the decrease of gathering pollen because of the scarcity of pollen resources, colony weakness, and bad weather. According to Shawer et al. (2003), Taha and Al-Kahtani (2013), Taha (2014), and Taha and Al-Kahtani (2020), the amount of pollen in the colony increased in parallel to the amount of the brood.
Colonies with the new combs stored 88.83% more honey than the old combs colonies. The findings of Taha and El-Sanat (2007), Dizaji et al. (2008), and Taha and Al-Kahtani (2020) confirmed our results since they harvested the largest honey yield from the new combs colonies. The superiority of the new combs colonies in honey production may be due to the workers with large size, wings, legs, and proboscis, which gather more nectar and pollen (Dizaji et al., 2008; Taha and Al-Kahtani, 2020). The honey yield was significantly correlated with the legs and wings characteristics (Mostajeran et al., 2006), and the corbicula area (Kolmes and Sam, 1991). Also, the large colony population can gather more nectar and produced a high honey yield (Taha and Al-Kahtani, 2020). Furthermore, the small amounts of the brood in colonies with the old combs resulted in weak colonies that consume a large part of the collected nectar during the early part of the flow season in building up its population (Taha and Al-Kahtani, 2013).
We observed the largest area of sealed honey in May coincided with the flow season of Egyptian clover during May, followed by August coincided with the cotton (Gossypium spp.) flow season (Taha et al., 2019). Our results were confirmed by Shawer et al. (2003) and Taha et al. (2019).
The strength of the colony was estimated by the area of the sealed brood and population of adult bees (Taha, 2007; Taha et al., 2006). In addition to the food supply, brood rearing was influenced by the colony population size and the egg-laying ability of the queens (Taha, 2014). At the beginning of the experiment, all colonies were headed by sister queens, and the colony strength was relatively similar in all colonies, so any differences should be due to the age of combs. Colonies with the new combs reared 96.87% more worker brood than colonies with the old combs. Our results were confirmed by Dizaji et al. (2008) and Taha and Al-Kahtani (2020).
The largest monthly worker sealed brood area was obtained during July and August, which formed the major peak, followed by May and June, which formed the second peak. The peaks of the brood rearing were in parallel to the peaks of stored pollen. In a previous study in the same area, Taha (2000) and Shawer et al. (2003) recorded the largest area of the worker sealed brood during May, followed by March and April. The variation of the brood rearing was dependent on the amount of the collected nectar and pollen (Taha, 2005), which depends upon the floral resource supply and the availability of the young and foraging workers. In Saudi Arabia, the worker sealed brood area showed three peaks: the major was during August and September, the second during May, and the third occurred during March (Taha, 2014; Taha and Al-Kahtani, 2019, 2020). The lowest area of the worker sealed brood was recorded in November and December due to the sharp shortage of nectar and pollen supply. On the contrary, Musa et al. (1989) recorded that brood rearing was increased in November, the main season of the nectar flow in Sudan.
Drone production in the colony is seasonal and depends on the environmental conditions (Taha et al., 2007; Taha and Al-Kahtani, 2019). In the present study, drone production in the colonies continued throughout the year from February to September. It formed two major peaks during March and August, coincided with the peaks of the stored pollen area. These results agree with the findings of Taha (2005) since he found a highly positive correlation between collected pollen and drone brood rearing rates. The high rate of drone production during March compared with the other months may be due to the period of swarming season accompanied by the need for more drones to fertilize queens. Helal et al. (2003), Taha et al. (2007) in Egypt, Taha and Al-Kahtani (2019) in Saudi Arabia reported relatively similar results.
5 Conclusion
Data from this study showed that the body sizes of the individual bees (queens, drones, and workers) were declined, and storing pollen and honey, brood rearing, and RJ production were decreased in the colonies with the old combs compared to the colonies with the new combs. Replace combs after three years with new others is recommended to encourage colony growth and increase colony productivity.
Acknowledgements
The authors extend their appreciation to Taif University for funding the current work by Taif University Researchers Supporting Project number (TURSP – 2020/59), Taif University, Taif, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Morphometrical characteristics of Carniolan honeybee workers in relation to age of comb. Sci. J. King Faisal Univ. (Basic Appl. Sci.). 2018;19:47-54.
- [Google Scholar]
- Morphometric studies on dwarf honeybee Apis florea F. workers in Saudi Arabia. J. Apic. Sci.. 2014;58:127-134.
- [Google Scholar]
- Post grafting time significantly influences royal jelly yield and content of macro and trace elements. PLoS One. 2020;15(9):e0238751
- [Google Scholar]
- Morphometric study of the Yemeni (Apis mellifera jemenitica) and Carniolan (A. m. carnica) honeybee workers in Saudi Arabia. PLoS One. 2021;16:e0247262
- [Google Scholar]
- Performance of two honeybee subspecies during harsh weather and Acacia gerrardii nectar–rich flow. Scientia Agricola. 2017;74:474-480.
- [Google Scholar]
- Effects of comb wax age on the brood and honey product performance in honeybee. Asian J. Anim. Vet. Adv.. 2008;3:51-53.
- [Google Scholar]
- Honeybee products: an updated review of neurological actions. Trends Food Sci. Technol.. 2020;101
- [Google Scholar]
- Effect of moving the apiaries on activity of honeybee colonies. 2-Flight activity, gathering of nectar and sugar concentration contents and honey. J. Agric. Res. Tanta Univ.. 2003;29:268-282.
- [Google Scholar]
- Honeybee combs, how the circular cells transform into rounded hexagons. J. R. Soc. Interface. 2013;10:20130299.
- [Google Scholar]
- Assessment of honeybee colony performance in the agro-ecological zones of Uganda. Curr. Invest. Agric. Curr. Res.. 2018;1:122-127.
- [Google Scholar]
- Relationships between sizes of morphological features in worker honeybees (Apis mellifera) J. New York Entomol. Soc.. 1991;99:684-690.
- [Google Scholar]
- Analysis of colony and morphological characteristics in honeybees (Apis mellifera meda) Pakistan J. Biol. Sci.. 2006;9:2685-2688.
- [Google Scholar]
- Studies on feeding colonies of honeybees in Sudan. In: Proc. 4th Inter. Conf. Apis Tropical Climate, Cairo, Egypt, 6–10 Nov. 1988. London Uk: Inter. Bee Res. Assoc.; 1989. p. :27-28.
- [Google Scholar]
- Late winter feeding stimulates rapid spring development of Carniolan honeybee colonies (Apis mellifera carnica) Poljoprivreda. 2017;23:73-77.
- [Google Scholar]
- SAS/STAT User’s Guide Release 9.1. Cary, NC 27513: SAS Institute Inc.; 2003.
- Effect of moving the apiaries on activity of honeybee colonies. 1 – Gathering and storing pollen, brood rearing and wax secretion. J. Agric. Res. Tanta Univ.. 2003;29:250-267.
- [Google Scholar]
- Impact of different comb age on morphological and biological characteristics of honeybee workers (Apis mellifera L.) J. Fac. Agric. Kyushu Univ.. 2020;65:277-282.
- [Google Scholar]
- The effects of starvation of honeybee larvae on reproductive quality and wing asymmetry of honeybee drones. J. Apic. Sci.. 2017;61:233-243.
- [Google Scholar]
- Effect of Transferring the Apiaries on Activity of Honeybee Colonies. Egypt: Fac. Agric. Tanta Univ. Kafrelsheikh; 2000. p. :117. (M.Sc. Thesis)
- Studies on Honeybee (Apis mellifera L.). Unpublished. Kafrelsheikh, Egypt: Fac. Agric. Tanta Univ.; 2005. p. :151. (Ph.D. Thesis)
- Importance of banana Musa sp. (Musaceae) for honeybee Apis mellifera L. (Hymenoptera, Apidae) in Egypt. Bull. Entomol. Soc. Egypt. 2007;II:125-133.
- [Google Scholar]
- Honeybee and Modern beekeeping. Translation. Al-Ahsa, Saudi Arabia: Authoring and Publishing Center-King Faisal University; 2013. (In Arabic)
- Seasonal variation of foraging activity, pollen collection and growth of honeybee colonies in Al-Ahsa, Saudi Arabia. Bull. Entomol. Soc. Egypt. 2014;91:163-175.
- [Google Scholar]
- A study on nectar and pollen sources for honeybee Apis mellifera L. in Al-Ahsa, Saudi Arabia. J. Entomol. Zool. Stud.. 2015;3:272-277.
- [Google Scholar]
- Chemical composition and amounts of mineral elements in honeybee-collected pollen in relation to botanical origin. J. Apic. Sci.. 2015;59:75-81.
- [Google Scholar]
- The impact of feeding certain pollen substitutes on maintaining the strength and productivity of honeybee colonies (Apis mellifera L.) Bull. Entomol. Soc. Egypt Econ. Ser.. 2015;41:63-74.
- [Google Scholar]
- Effect of combs age on honey production and its physical and chemical properties. Bull. Entomol. Soc. Egypt. 2007;II:9-18.
- [Google Scholar]
- The value of honeybee (Apis mellifera L.) as pollinator of summer seed watermelon (Citrullus lanatus colothynthoides L, Cucurbitaceae) in Egypt. Acta Biol. Szeg.. 2009;53:33-37.
- [Google Scholar]
- Relationship between population size and productivity of honeybee colonies. J. Entomol.. 2013;10:163-169.
- [Google Scholar]
- Morphometrical characteristics of Carniolan honeybee workers in relation to age of comb. In: Proc. 32nd Meeting Saudi Biol. Soc. 6–8 Mar. 2017.
- [Google Scholar]
- Comparison of the activity and productivity of Carniolan (Apis mellifera carnica Pollmann) and Yemeni (Apis mellifera jemenitica Ruttner) subspecies under environmental conditions of the Al-Ahsa oasis of eastern Saudi Arabia. Saudi J. Biol. Sci.. 2019;26:681-687.
- [Google Scholar]
- The relationship between comb age and performance of honeybee (Apis mellifera) colonies. Saudi J. Biol. Sci.. 2020;27:30-34.
- [Google Scholar]
- Morphometric and reproductive organs characters of Apis mellifera jemenitica drones in comparison to Apis mellifera carnica. Int. J. Sci. Eng. Res.. 2013;4:411-415.
- [Google Scholar]
- Loofah (Luffa aegyptiaca Mill., Cucurbitaceae), a source of nectar and pollen for honeybee Apis mellifera L. Hymenoptera, Apidae) in Egypt. Bull. Entomol. Soc. Egypt. 2006;83:337-345.
- [Google Scholar]
- Effect of nectar and pollen flora on drones of honeybee. In: Nat. Conf. Env. Prot. Poll. Fac. Sci. Qassim Univ. Saudi Arabia, 18–20 Mar. 2007. p. :71.
- [Google Scholar]
- The relationship between comb age and the amounts of mineral elements in honey and wax. J. Apic. Res. Bee World. 2010;49:202-207.
- [Google Scholar]
- Insect pollinators and foraging behavior of honeybees on alfalfa (Medicago sativa L.) in Saudi Arabia. J. Kansas Entomol. Soc.. 2016;89:92-99.
- [Google Scholar]
- Nectar and pollen resources for honeybees in Kafrelsheikh, northern Egyp. Saudi J. Biol. Sci.. 2019;26:890-896.
- [Google Scholar]







