Translate this page into:
Co-immobilisation of superoxide dismutase and catalase using an in vitro encapsulation protocol
⁎Corresponding author at: Biochemistry Department, Faculty of Science, University of Jeddah, P.O box 80200, Jeddah 21589, Saudi Arabia. mialkhalaf@kau.edu.sa (Maha I. Alkhalaf)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The immobilisation of antioxidant enzymes using alginate gel beads has previously been reported. Upon immobilisation, the retention of enzyme activity, encapsulation efficiency and enzyme leakage from the capsules must be considered. The objective of this paper to improve the catalase (CAT) and superoxide dismutase (SOD) activity following encapsulation by co-immobilising the enzymes as a pair. CAT and SOD were partially purified from Bacillus licheniformis using (NH4)2SO4 and immobilised on beads of alginate. Further, the activities of the partially purified enzymes (both free and immobilised) were measured and characterised. The optimum pH and temperature for each of the enzymes when free and immobilised were determined. The optimum pH values for enzyme activities were 7 and 7.5 for free and immobilised CAT, respectively. The optimum temperature for activity was greater for immobilised than free enzymes. Immobilised SOD and CAT showed higher storage and thermal stabilities than free enzymes and remaining activities were about 40 and 70%, respectively, after eight cycles of use. The free CAT lost all activity within 60 days, while immobilised CAT lost 45% of its activity over a similar incubation period at 4 °C. In conclusion, we report the effects of synergistic immobilisation of the antioxidant enzymes SOD and CAT.
Keywords
Antioxidant enzymes
Superoxide dismutase
Catalases
Immobilised
Alginate
1 Introduction
Antioxidant enzymes have been isolated from many sources including plants, animals and microbes and have a wide range of applications in a variety of fields such as food processing, industrial processes and medicine. Free radicals can cause a lot of damage to cells due to the presence or variable numbers of electrons in the outermost shells of the atoms that can become highly reactive (Ferreira et al., 2002). Antioxidants therefore play a key role in cellular operations by protecting metabolic processes from being damaged by free radicals (Blokhina et al., 2003). Recent studies have focused on the antioxidant response of newly isolated strains of bacteria from various marine and soil-based locations, which are vital to bioremediation (Stentz et al., 2004).
Catalase (CAT) (EC: 1.11.1.6) and superoxide dismutase (SOD) (EC: 1.15.1.1) are two antioxidant enzymes that regularly coexist; they constitute the most effective method of regulating the number of free radicals in the body. Over previous decades, numerous methods of reducing the effect of oxidative stress have been developed. The use of naturally produced enzymes such as SOD and CAT through genetic overexpression has been shown to have good potential during in vitro and in vivo experiments (Howard et al., 2014; Shuvaev et al., 2016). Furthermore, SOD therapy can successfully be used to decrease the concentration of reactive oxygen species in the body following radiotherapy (Holley et al., 2014).
The metalloenzyme SOD acts by enabling the dismutation of superoxide anion radicals to release hydrogen peroxide, which can be degraded by CAT or reduced to a hydroxyl radical (Pastor et al., 2010; Perry et al., 2010). Catalase catalyses the decomposition of hydrogen peroxide to one molecule each of oxygen and water. CAT has been suggested as a potential therapeutic agent to be administered intraperitoneally (Arabaci and Usluoglu, 2013).
Antioxidant enzymes have been encapsulated in nano-carriers to increase their efficacy and half-lives (Perriotte-Olson et al., 2016). Immobilisation or encapsulation of single molecules of SOD or CAT have been used for purposes such as the manufacture of biosensors and in drug delivery (Giovagnoli et al., 2004). However, only a few studies focus on the multi-enzyme immobilisation of SOD and CAT (Villalong et al., 2005).
Immobilised enzymes contained in an insoluble matrix possess several advantages over free enzymes. For example, an increase in enzyme stability facilitates separation from a reaction medium by filtration or centrifugation. The resistance of these chemically dynamic protein structures to high temperatures, extraordinary pH values and other denaturing factors are important when using these enzymes in commercial and manufacturing applications (Dumitriu and Chornet, 1998).
The aim of this work was to isolate and characterise SOD and CAT from Bacillus licheniformis 15 and immobilise the molecules using alginate beads. The total production of these enzymes in this microbe was quantified. The unrivalled antioxidant capabilities of SOD and CAT coupled on alginate beads were also investigated for potential use in further applicable areas.
2 Results and discussion
2.1 Isolation of SOD- and CA-producing bacteria
Representative results from the partial purification of SOD and CAT from Bacillus licheniformis 15 are summarised in Table 1. The activities of SOD and CAT isolated from the bacterial culture were 72 and 16 U/ml, respectively. The best precipitation yield from the partial purification of the crude extract was acquired by adding 80% (NH4)2SO4 to the supernatant. The activities of precipitated SOD and CAT at this stage were found to be 88 and 23 U/ml respectively. These data were in agreement with those of Svetlana et al. (2004), who suggested that further SOD precipitation using 50–80% (NH4)2SO4 saturation enabled the highest amount of enzyme to be retrieved. Additionally, Arabaci and Usluoglu (2013) reported that CAT enzyme extraction was most efficient when fractionation was undertaken by adding (NH4)2SO4 to the supernatant to give 80% saturation.
Stages
Enzyme activity U/ml
Purification level
Yield (%)
SOD
CAT
SOD
CAT
SOD
CAT
Crude enzyme
72
16
1
1
100
100
(NH4)2SO4 precipitate
88
23
1.2
1.4
83
71
Immobilised enzyme
57
17.6
0.8
1.1
79
91
2.2 Effect of experimental variables
In this study, the CAT and SOD enzyme mixture was immobilised on to the alginate beads. The free and immobilised enzymes were compare in terms of their kinetic parameters. However, enzymes entrapped in gels tend to leach out over time, making long-term conservation of enzyme activity very difficult. There are, however, many reports describing the covalent immobilisation of different enzymes on various supports (Canh Le et al., 2004).
2.3 Influence of pH on enzymes activities
As seen in Fig. 1, the maximum activity was observed for free SOD and CAT at pH 6.5 and 7, respectively. However, the optimum pH for immobilised SOD and CAT ranged from pH 6–7 and 6.5–7.5, respectively. The optimum pH values for both immobilised and free enzymes showed some similarities; however, the immobilised enzymes were stable under a wider range of pH values than the free enzymes. The lower sensitivity to pH of the immobilised enzyme may be due to the immobilisation method or the basic characteristics of the substance carrier (Lee et al., 2000). The choice of matrix is important and bespoke to the domain in which the enzyme is being used, i.e. medicine, pharmaceuticals or food processing. In each case, the matrix should generally be non-toxic and biocompatible. Several studies have suggested alginate as an interesting matrix due to its low toxicity and biocompatibility. Also, it forms beads easily by ionotropic gelation (Shapiro and Cohen, 1997).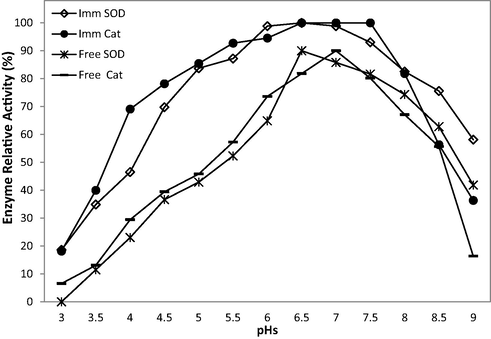
Relative activities of free and immobilised (SOD and CAT) enzymes at various levels of pH. A relative activity of 100% was considered enzyme activity under the same conditions.
The immobilised CAT did not show any change in stability at different pH values, suggesting that the immobilisation method preserved the enzyme activity (Yakup Arca, 2000).
2.4 Influence of temperature on enzymes activities
The temperature profiles for free and immobilised SOD and CAT are shown in Fig. 2. Free CAT exhibited maximum activity at 30 °C, while immobilised CAT maintained activity up to 50 °C. The results indicated that the activity of the immobilised CAT was more stable than that of free catalase at 30–50 °C. However, the optimum temperature for SOD activity was 30 °C for both the free and immobilised enzymes. Alginate is a natural polysaccharide and co-polymer of alternating sequences of β-D-mannuronic and α-L-guluronic acid residues linked by 1 → 4 glycosidic bonds. Alginate beads may offer protection to enzymes at temperatures that would normally lead to deactivation (Lee et al., 2000). Hence, SOD and CAT enzymes immobilised in alginate beads may be more robust (Engasser and Horvath, 1976).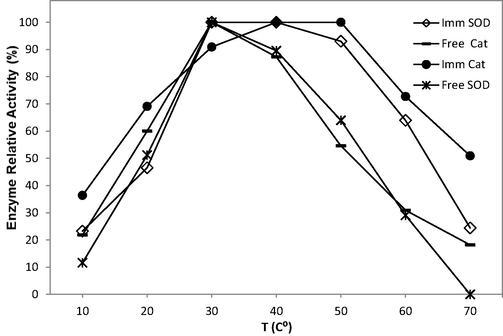
Optimum temperature profiles of free and immobilised SOD and CAT indicating the thermal stability of the enzymes.
2.5 Thermal stability of enzymes
The thermal stability of the free and immobilised enzymes is presented in Figs. 3 and 4, respectively. Inactivation occurred at a lower temperature in the immobilised enzymes than the free enzymes at first. However, storing the immobilised SOD and CAT in phosphate buffer (50 mM, pH 7.4) greatly improved stability compared with that of the free SOD and CAT at 40 °C for 240 min. After 180 min at 50 °C, the activity of free SOD enzymes was reduced by about half, while the immobilised enzymes were better protected and lost only 15% of their activity over the same time period. At 60 °C, the activity of free CAT was reduced by about 50% after 210 min. Under similar conditions, immobilised CAT retained 70% of its activity. These findings suggest that the activity of immobilised enzymes is greater at higher temperatures (Yasar Mahlicli et al., 2015). It is notable that enzyme immobilisation offers an improvement that extend the scope of conditions under which an enzyme can act. Immobilisation improves enzyme stability by covalent linkage to an insoluble matrix or by entrapment in a gel support under mild conditions (i.e. by ionotropic gelation or by polymerisation) (Canh Le et al., 2004). The increased heat stability of immobilised enzymes also broadens their possible applications (Jürgen-Lohmann and Legge, 2006; Alptekin et al., 2010). The differences in the enzyme activities and stabilities in the gel globules may be explained by the adaptation of the enzymes in attaching to the beads; basically, the conformation changes that the enzymes undergo because of the connections between enzyme molecules or the ionic effect of the phosphate buffer during stockpiling (Yasar Mahlicli et al., 2015).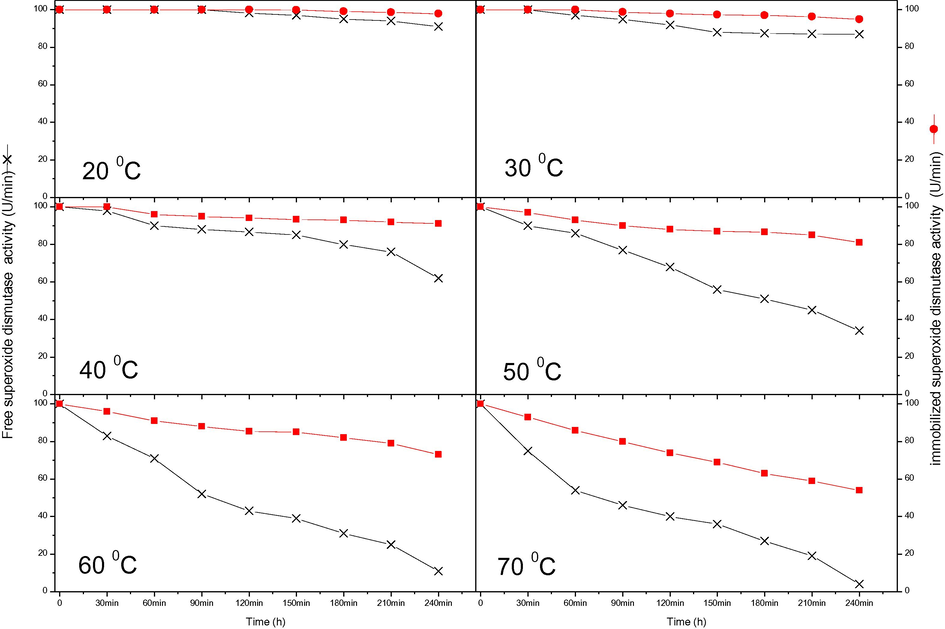
Stabilities of free and immobilised SOD at various temperatures for 240 min.
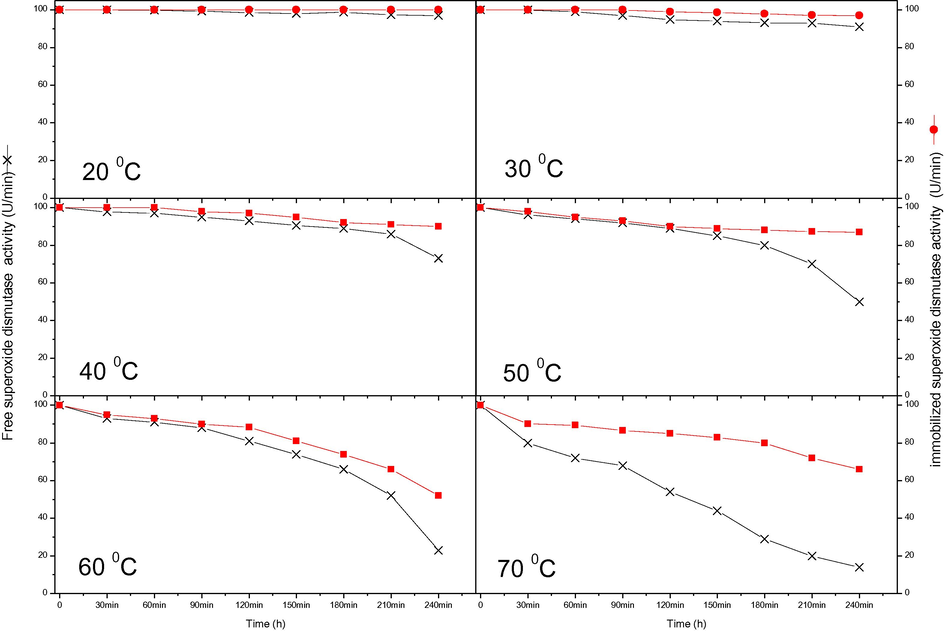
Stabilities of free and immobilised CAT at various temperatures for 240 min.
2.6 Reusability of immobilised enzymes
The results presented in Fig. 5 show that SOD lost 15% of its activity within the first five uses, while CAT loss 10% of its activity. The activities of immobilised SOD and CAT enzymes were maintained at 50 and 75%, respectively, after being reused seven times. After 10 cycles of repeated use, the immobilised alginate beads containing SOD and CAT enzymes retained about 25 and 50% of their initial activities, respectively. A small amount of enzyme leakage was confirmed but the stability of the immobilised enzymes was good, and the immobilised enzymes could be reused. These outcomes are equivalent to those found in an earlier study by Gholami-Borujeni et al. (2011). However, another study found that loss of activity because of enzyme leakage occurred and enzymes were deactivated after repeated uses (Bayramoglu and Arica, 2010).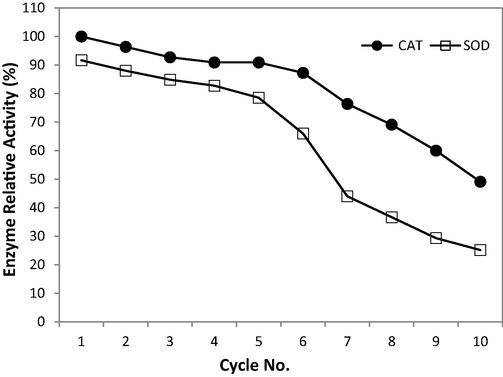
Repeated use of immobilised capsules of SOD and CAT enzymes.
2.7 Storage stability
Free and immobilised SOD and CAT enzymes were stored in phosphate buffer (50 mM, pH 7.4) at 4 °C and estimations of activity were carried out for 60 days. As shown in Fig. 6, after 7 days, 70% of the free SOD activity was maintained, while the immobilised enzyme retained 99% of its activity. Immobilisation gave a preferred conformation to the enzymes and they were more stable, particularly over longer periods (Arabaci and Usluoglu, 2013). The free SOD and CAT enzymes lost all of their activity within 35 and 42 days, respectively. Conversely, immobilised SOD and CAT lost 35 and 20% of their activity over the same period. Fig. 6 shows that there were significant decreases in the activities of the enzymes over time. The half-life of free SOD and CAT was 30 days at 4 °C, however, the half-life of immobilised CAT was about 60 days. Similar findings have previously been reported using CAT immobilised on other support materials (Bayramoglu and Arica, 2010; Alptekin et al., 2010). The instability of free enzymes in long-term storage has been referred to in terms of the harmful action of microbial contamination (Engasser and Horvath, 1976). The successful use of immobilised enzymes in different applications requires stability in storage as well as in operation (Tukel and Alptekin, 2004). The results summarised in Table 2 show that the co-immobilised enzymes exhibited a higher level of stability compared to that of their free counterparts.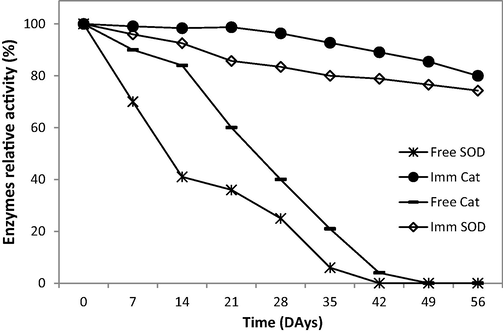
Stability of free and immobilised (SOD and CAT) enzymes.
Kinetics property
Free Enzyme
Immobilised Enzyme
SOD
CAT
SOD
CAT
Enzyme Activity
88 U/ml
23 U/ml
57 U/ml
17.6 U/ml
Optimum pH
6.5
7
6–7
6.5–7.5
Optimum T°
30 °C
30 °C
30–40 °C
40–50 °C
Thermal stability 70 °C
<30 min
<30 min
>240 min
>240 min
Storage at 4 °C/(day)
<35
<42
>60
>60
Reusability (Cycle)
1
1
10 with 25%
10 with 50%
3 Materials and methods
3.1 Enzymes
3.1.1 Extraction and partial purification of enzymes
100 ml of fermentation medium included with Bacillus licheniformis 15 for 3 days was pelleted by centrifugation at 14.000g for 30 min then the supernatant was collected for (SOD and CAT) antioxidant enzymes activities Zaushitsyna et al. (2014). The supernatant aqueous solution with activities 72 and 16 U/mL for SOD and CAT respectively was used as the crude enzymes. Partial Purification was fractionated by adding (NH4)2SO4 salt to the supernatant to obtain 80% saturation solution. The mixture was kept in a fridge at 4 °C over night and centrifuged at 14,000g for 30 min. The precipitate was dissolved in a small amount of phosphate buffer 50 mM at pH 7.4 and then dialyzed at 4 °C in the same buffer for 24 h with three changes of the buffer during dialysis (Arabaci, 2011).
3.2 Immobilisation of enzymes
The immobilized enzymes were prepared by mixed 1 ml of ready partial purified enzyme (prepared in previous step) to 25 ml of 3% sodium alginate solution , then the mixture were disturbed for 5 min to homogenize Wu et al. (2013). The resultant mixtures were transported one by one to a beaker with CaCl2 and the material in the beaker was moved by means of a magnetic stirrer. A syringe with a 1.2-mm needle, positioned in an infusion pump was used for the drop wise transporting of the viscous sodium alginate solutions. The replacement of sodium ions with calcium ions caused in immediate gelation of the alginate drops. The resulting biocatalyst beads were detached from the CaCl2 solution by means of a mesh and were kept in a fridge at 4 °C Zaushitsyna et al. (2014).
3.3 Superoxide dismutase activity
The experiments were conducted in 3 ml disposable cuvettes at 30 °C. Each 3 ml mixture contained 2 ml Sodium phosphate buffer (PBS) of 50 mM at pH 7.4, 200 μl 75 μ MNBT, 13 mM Methionine, 100 nM EDTA and 0.1 ml of suspension respective crud SOD enzyme solution. Lastly, 200 μl of 2 mM riboflavin solution was added, the cuvettes were shaken, and the reaction was started under illumination of a15W fluorescent lamp. Each sample was kept under the light for10 min before the lamp was switched off in order to stop the reaction. Two absorbance data points were collected for each sample: before adding the riboflavin solution into the cuvette and after the10 min time period was completed. The absorbance of each sample was measured by a UV-pectro photometer (PerkinElmer, model no: Lambda45) at 560 nm against a 3 ml solution of PBS. Each measurement was repeated five times for each test. To determine immobilized SOD activity, instead fusing free enzyme, 10 beads of alginate were immersed into 3 ml reaction mixture. Definition of one enzyme unit was the amount of enzyme which inhibited 50% the reduction of nitro blue tetrazolium to blue formazan at 30 °C and pH 7.4. SOD activity is expressed as the percentage of inhibition in reduction NBT (inhibition of the formazan production) per ml of SOD (or beads of alginate) (Siyu et al., 2017).
3.4 Catalase activity
The antioxidant activities of free and immobilized CAT were determined at 30 °C according to Aebi (1984). The reaction mixture contained 40 mM H2O2 in a 50 mM phosphate buffer pH 7.4 and 0.1 ml enzyme in a total volume of 3 ml. CAT activity was estimated by decreased in absorbance of H2O2 at 240 nm. Activities were carried out at optimum conditions. Approximately 100 mg of catalase immobilize alginate beads were mixed 10 ml of 10 mM H2O2 solution in 0.05 M phosphate buffer (pH 7.4) 30 °C. After 5 min, the reaction was determinate by removal of the alginate beads from the reaction mixture. The absorbance of each cuvette was measured before and after the reaction by a UV-spectrophotometer (PerkinElmer, mode no: Lambda45) at 240 nm against 2.0 ml PBS. Unite the absorbance of the reaction mixture was determined, and the immobilized catalase activity was calculated.
3.5 Influence of pH
The enzyme activities were measure in a pH range of 3–9. The activities of free and immobilised (SOD and CAT) enzyme preparations with respect to pH were investigated using 50 mM–5.5 mM acetate and phosphate buffers at pH 3 and pH 6–9, respectively at 30 °C.
3.6 Influence of temperature
The effects of temperature on free and immobilised (SOD and CAT) enzyme activities were examined in the range 20–70 °C.
3.7 Estimation of thermal stability
Determine the stability of free and immobilised (SOD and CAT) enzymes was assayed by soaking the enzymes in 25 ml of 50 mM phosphate buffer solution (pH 7.4) at temperatures between 20 and 70 °C for 4 h. The enzymatic activities of free and immobilised enzymes (SOD and CAT) were measured using same procedure described above.
3.8 Reusability of immobilised enzymes
To analyse the reuse capabilities of enzymes beads, the enzymes adsorption and desorption cycle was carried out ten times. Then beads were washed many times using the same buffer and reused for enzyme immobilisation. Enzyme immobilisation was carried out as described above.
3.9 Storage stability
The free and immobilised SOD or CAT enzymes with activities of 88 and 23 U/ml and 57 and 17.6 U/ml, respectively were stored at 4 °C for 60 days in buffer.
4 Conclusions
The present study investigated microbial antioxidant enzyme conjugates of SOD and CAT on alginate beads. The experiments showed that SOD and CAT activities were retained when immobilised on alginate beads. The co-immobilised enzymes exhibited a higher level of stability compared to that of their free counterparts. As far as we know, SOD and CAT enzymes have not previously been utilised together. Here, we demonstrated the differences in the adaptation and action of these enzymes when co-immobilised on alginate beads. The advantages of using alginate to co-immobilise the two enzymes are: (i) it is a polypeptide; (ii) the immobilised enzymes are more stable and can be reused; and (iii) a high level of activity can be achieved. Finally, the immobilised enzymes may be useful for various biotechnological applications in, for example, the food and textile industries.
Acknowledgements
This project was funded by the Deanship of Scientific Research (DSR) at King Abdulaziz University, Jeddah, under grant no. G:282-363-1439. The authors, therefore, acknowledge with thanks the DSR for technical and financial support.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Immobilization of catalase onto Eupergit C and its characterization. J. Mol. Catal. B. 2010;64:177-183.
- [Google Scholar]
- Partial purification and some properties of catalase from dill (Anethum graveolens L.) J. Biol. Life Sci.. 2011;2:11-15.
- [Google Scholar]
- Catalytic properties and immobilization studies of catalase from Malva sylvestris L. J. Chem.. 2013;2013:1-6.
- [Google Scholar]
- Reversible immobilization of catalase on fibrous polymer grafted and metal chelated chitosan membrane. J. Mol. Catal. B. 2010;62:297-304.
- [Google Scholar]
- Antioxidants, oxidative damage and oxygen deprivation stress: a review. Ann. Bot.. 2003;91:179-194.
- [Google Scholar]
- Modified alginate matrice for the immobilization of bioactive agents. Biotechnol. Appl. Biochem.. 2004;38:1-10.
- [Google Scholar]
- Polysaccharides: Structural Diversity and Functional Versatility. New York: Marcel Dekker Inc.; 1998. p. :629-748.
- Applied Biochemistry and Bioengineering: Immobilized Principles. NY: Academic Press; 1976. p. :127-220.
- Changes in antioxidant enzyme activities in soybean under cadmium stress. J. Plant Nutr.. 2002;25:327-342.
- [Google Scholar]
- Application of immobilized horseradish peroxidase for removal and detoxification of azo dye from aqueous solution. Resjchemenviron. 2011;15:217-222.
- [Google Scholar]
- Biodegradable microspheresas carriers for native superoxide dismutase and catalase delivery. AAPSpharmSciTech. 2004;5:e51
- [Google Scholar]
- Redox-modulated phenomena and radiation therapy: the central role of superoxide dismutases. Antioxid. Redox Signal.. 2014;20:1567-1589.
- [Google Scholar]
- Endothelial targeting of liposomes encapsulating SOD/catalase mimetic EUK-134 alleviates acute pulmonary inflammation. J. Control. Release. 2014;177:34-41.
- [Google Scholar]
- Immobilization of bovine catalase in sol-gels. Enzyme Microb. Technol.. 2006;39:626-633.
- [Google Scholar]
- Degradation behavior of covalently cross-linked poly (aldehyde guluronate) hydrogels. Macromolecules. 2000;33:97-101.
- [Google Scholar]
- Multi-enzymatic system immobilization in sol–gel slides: fluorescent superoxide biosensors development. Biosensbioelectron. 2010;25:1526-1529.
- [Google Scholar]
- Nano formulated copper/zinc superoxide dismutase Reduces Adipose Inflammation in Obesity. Obesity. 2016;24:148-156.
- [Google Scholar]
- Novel alginate sponges for cell culture and transplantation. Biomaterials. 1997;18:583-590.
- [Google Scholar]
- Size and targeting to PECAM vs ICAM control endothelial delivery, internalization and protective effect of multimolecular SOD conjugates. J. Control. Release. 2016;234:115-123.
- [Google Scholar]
- Co-mobilization of superoxide dismutase with catalase on soft microparticles formed by self-assembly of amphiphilic poly (aspartic acid) Catalysts. 2017;7:2-11.
- [Google Scholar]
- Pro inflammatory cytokines, markers of cardiovascular risks, oxidative stress, and lipid peroxidation in patients with hyperglycemic crises. Diabetes. 2004;53:2079-2086.
- [Google Scholar]
- Purification and partial characterization of superoxide dismutase from the thermophilic bacteria Thermothrix sp. J. Serb. Chem. Società. 2004;69:9-16.
- [Google Scholar]
- Immobilization and kinetics of catalase onto magnesium silicate. Process Biochem.. 2004;39:2149-2155.
- [Google Scholar]
- Supra- molecular assembly of cyclodextr in-modified gold nano-particles and Cu, Zn- superoxidedismutaseoncatalase. J. Mol. Catal. B: Enzymologia. 2005;35:79-85.
- [Google Scholar]
- Enhanced stability of catalase covalently immobilized on functionalized titania submicrospheres. Mater. Sci. Eng., C. 2013;33:1438-1445.
- [Google Scholar]
- Immobilization of polyphenol oxidase on carboxymethyl cellulose hydrogel beads: preparation and characterization. Polym. Int.. 2000;49:775-781.
- [Google Scholar]
- Immobilization of superoxide dismutase/catalase onto polysulfone membranes to suppress hemodialysis-induced oxidative stress: A comparison of two immobilization methods. J. Membr. Sci.. 2015;479:175-189.
- [Google Scholar]
- Cryostructured and crosslinked viable cells forming monoliths suitable for bioreactor applications. Top Cat. 2014;57:339.
- [Google Scholar]







