Translate this page into:
Citrus limetta Risso peel mediated green synthesis of gold nanoparticles and its antioxidant and catalytic activity
⁎Corresponding authors. gannadurai@msuniv.ac.in (G. Annadurai), mahamed@ksu.edu.sa (Maqusood Ahamed)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Green synthesis of noble metal nanoparticles is now receiving great attention because of their numerous applications such as optics, catalysis, and biomedicine. In this work, gold nanoparticles were synthesized from peel extract of sweet lime (Citrus limetta Risso), which is usually discarded as a bio-waste. Bioactive components present in the fruit peel extract act as reducing and capping agents for the synthesis of gold nanoparticles from chloroauric acid (HAuCl4). The visual color change from light yellow to ruby red suggested the formation of gold nanoparticles, which was further confirmed by the maximum absorption peak at 530 nm of colloidal suspension measured by UV–vis spectroscopy. XRD spectra indicated the crystalline nature of prepared gold nanoparticles. The average crystallite size of gold nanoparticles measured from prominent peaks of XRD was found to be around 64 nm (size range: 50–80 nm), which was further supported by FESEM analysis. The components responsible for the reduction of chloroauric acid into gold nanoparticles were analyzed by FTIR, which suggested the presence of several phytochemicals e.g. phenol, carboxylic acid, amines, and alkynes in the fruit peel extract. Green prepared gold nanoparticle was further evaluated for its antioxidant and catalytic activity. Green gold nanoparticles exhibited excellent antioxidant activity evident by scavenging/inhibition of free oxygen/nitrogen radical generation. Furthermore, green gold nanoparticles demonstrated excellent catalytic activity indicated by the reduction of 4-nitrophenol into 4-aminophenol. This study highlights the significance of bio-waste for the production of nanostructure of noble metals for numerous applications.
Keywords
Sweet lime
Fruit peel extract
Biosynthesis
Gold nanoparticles
Free radicals
4-nitrophenol reduction
Phytochemicals
1 Introduction
Reactive oxygen/nitrogen species commonly known as pro-oxidants can cause oxidative damage to cell macromolecules (e.g. lipid, protein, and nucleic acid) that lead to various pathophysiological conditions and diseases (Ahamed et al., 2019 Zuo et al., 2022). Antioxidants are known to minimize oxidative damage induced by highly reactive free radicals. Pro-oxidants mediated several human diseases such as inflammation, cancer, and heart disease can be mitigated by the supplementation of antioxidants (Ahamed et al., 2017; Siddiqui et al., 2013). Since several decades herbo-mineral and herbo-metallic drugs are being synthesized by long heating that considerably reduces the particle size and it is the major constituent of Ayurveda (Deng et al., 2020). Colloidal gold (Swarnabhasma) and mercury are the two most popular medications in Ayurveda (Ansari et al., 2012). The Swarnabhasmas (gold ash) is spherical gold particles of nanoscale range that are applied in treatment of several diseases such as neural disease, asthma, diabetes, and arthritis (Priya Velammal et al., 2016).
In comparison to other metal nanoparticles, noble metal (e.g. Au, Ag, and Pt) nanostructures are considered one of the most ingenious forms of nanomaterials due to their unique physicochemical properties (Botteon et al., 2021). Among these, gold nanoparticles are one of the most stable nanostructures that possesses tunable optical property and can be prepared in a variety of shapes (Hu et al., 2020). Hence, gold nanoparticles have attracted great scientific and technological consideration in recent years. There are numerous physical, chemical, and biological methods are known for the synthesis of gold nanoparticles (Donga et al., 2020). Biological methods have the advantage over chemical and physical methods because of low cast and use of non-hazardous phytochemicals (Ahamed et al., 2021a). Microorganisms, plants, and viruses are usually used for the synthesis of nanoparticles in biological methods and are referred as a green route of synthesis (Pechyen et al., 2021). Various plant parts such as the leaf, fruit peel, root, seed, and flower are used to synthesize metallic nanoparticles (Yang et al., 2019). In this study, a simple, inexpensive, and environmentally-safe method was proposed to synthesize gold nanoparticles using Citrus limetta (Risso) fruit peel as a reducing and stabilizing agent. Citrus fruit peel (a biodegradable waste) contains several phytochemicals that can be exploited in the preparation of nanostructured materials (Ahamed et al., 2022). Synthesis of gold nanoparticles was confirmed by UV–vis spectroscopy, Fourier transform infrared spectroscopy (FTIR), X-ray diffraction (XRD), field emission scanning electron microscopy (FESEM), and atomic force microscopy (AFM). A series of assays were performed to examine the antioxidant activity and reducing power of green synthesized gold nanoparticles. Catalytic activity of gold nanoparticles was also explored.
2 Materials and methods
2.1 Preparation of fruit peel extract
Sweet lime (Citrus limetta Risso) was purchased from the local market of Tenkasi district of Tamil Nadu, India. Peel from fruits was taken off, washed thoroughly in distilled water, and dried in shade. Dried fruit peels were boiled in distilled water for 30 min. After cooling macerated in distilled water and filtered through a clean muslin cloth. Then, chilled acetone was added and the resulting supernatant was collected by centrifugation at 10,000 rpm for 10 min. The collected supernatant (extract) was stored at 4 °C for further experiments.
2.2 Green preparation of gold nanoparticles
Gold nanoparticles were synthesized by boiling 1 mM chloroauric acid (HAuCl4) (Sigma-Aldrich, St. Louis, USA) in double distilled water and fruit peel extract for 10 min. The reaction mixture was monitored until the light-yellow solution turned into ruby red (UV–vis spectroscopy). At the end reaction time, the sample was dried at 100 °C for 2 h, then ground into fine powder for characterization and study of different activities.
2.3 Green gold nanoparticles characterization
The colloidal suspension of green synthesized gold nanoparticles was characterized by UV–vis spectroscopy (PerkinElmer). Crystallinity of gold nanoparticles was analyzed by XRD (Diffractometer system XPERT-PRO). Functional groups present on the surface of green synthesized gold nanoparticles were examined by FTIR (Bruker TENSOR 27). The morphology of the prepared sample was characterized by FESEM (Tescan VEGA) and AFM (Nanosurf Easyscan 2).
2.4 Various assays to examine the antioxidant activity of green gold nanoparticles
DPPH radical scavenging activity of gold nanoparticles was assayed using the protocol of Shimada et al. (1992). Protocol of Halliwell and Gutteridge (1981) was applied to examine the hydroxyl radical scavenging potential of green gold nanoparticles. Nitric oxide radical inhibition assay was done using the method of Ebrahimzadeh et al. (2010) with slight modifications. Hydrogen peroxide (H2O2) scavenging activity of gold nanoparticles was determined by following the method of Nabavi et al. (2008). Superoxide anion scavenging activity of gold nanoparticles was assayed as described by Nishikimi et al. (1992). Lipid peroxidation inhibition ability of gold nanoparticles was evaluated by the ammonium thiocyanate method (Mitsuda et al 1966). ABTS radical scavenging assay was performed according to the method of Siddhuraju and Becker (2003).
2.5 Assay of reducing power
Reducing power of gold nanoparticles was determined as reported earlier (Yen and Chen, 1995). Different concentration of gold nanoparticles (20–100 μg/ ml) was added into 2.5 ml phosphate buffer (0.2 M, pH 6.6) and 2.5 ml potassium ferricyanide (1 %w/v). The reaction mixture was incubated at 50 °C for 20 min. Then, 2.5 ml trichloroacetic acid (10 % w/v) was added to terminate the reaction. Reaction mixture was then centrifuged at 3000 rpm for 10 min and collected the supernatant. After that 2.5 ml of supernatant was mixed with 2.5 ml distilled water, and 0.5 ml ferrous chloride (0.1 %, w/v). The absorbance of the reaction mixture was measured at 700 nm. Higher absorbance indicates higher reducing power of the gold nanoparticles. Ascorbic acid was used as a positive control.
2.6 Catalytic reduction of 4-nitrophenol
Briefly, 0.2 ml of 4-nitrophenol (1 mM) was mixed with 3.6 ml of freshly prepared sodium borohydride (16.5 mM) and 0.2 ml of gold nanoparticles suspension in a 10-mm quartz cuvette. The UV–vis Spectroscopy was used to record the progress of reaction at different time intervals (0–80 min) and absorbance was recorded between 200 and 600 nm to calculate the reduction of 4-nitrophenol to 4-aminophenol.
3 Results and discussion
3.1 UV–vis spectroscopy
Formation of gold nanoparticles by the fruit peel extract was observed with change of color of solution from light yellow to ruby red. The excitation of surface plasmon vibrations in gold nanoparticles is the reason for this characteristic color change. The size and shape of the metal nanoparticles are responsible for the optical properties of the metal nanoparticles. According to the Mie theory, the small gold nanoparticles exhibit only one surface plasmon resonance (SPR) absorption band, whereas anisotropic particles show two or three SPR bands (Pechyen et al., 2021). Fig. 1 shows UV–vis spectra of the gold nanoparticle solution and the SPR band appears at 530 nm. Colloidal suspension gold nanoparticles were stable without agglomeration for several weeks (See Fig. 2).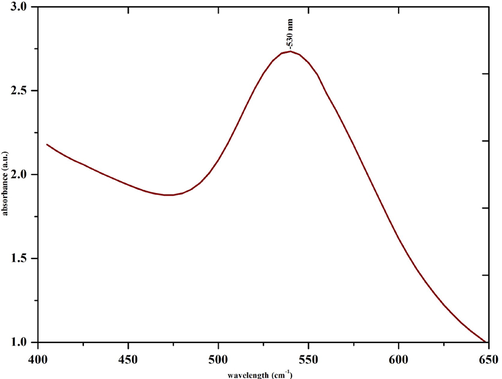
UV–vis spectrum of green synthesized gold nanoparticles.
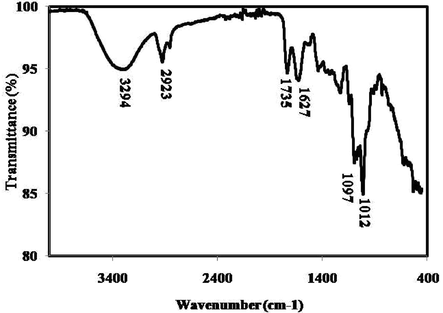
FTIR spectra of green synthesized gold nanoparticles.
3.2 X-ray diffraction analysis
Crystallite size and structure of green prepared gold nanoparticles were determined by XRD spectra. Sweet lime peel extract mediated synthesized gold nanoparticles showed four strong diffraction peaks at the 2θ values of 38.52°, 44.03°, 64.53°, and 78.65°, corresponding to plane (1 1 1), (2 0 0), (2 2 0), and (3 1 1), respectively. These planes indicate the gold nanoparticles have face cantered cubic (fcc) crystalline structure. The spectrum of synthesized gold nanoparticles was compared with standard spectrum published by Joint Committee on Powder Diffraction Standards (JCPDS file: 04–0783). The comparison of this spectrum with the standard confirmed that green prepared gold nanoparticles have crystalline nature. The Debye-Scherrer’s equation (Patterson, 1939) was used to calculate the crystallite size of green synthesized gold nanoparticles. where D is the average crystallite domain size perpendicular to the reflecting planes, λ is the X-ray wavelength (1.5418 Å), β is the width of the XRD peak at half height, θ is the diffraction angle, and K is the Scherrer coefficient (0.89). The average crystallite size of the as synthesized gold nanoparticles was found to be around 64 nm (size range: 50–80 nm). The present XRD report is well matched with the standard gold and previous report (Shukla et al., 2010).
3.3 Fourier transform infrared spectroscopy
FTIR spectra of green synthesized gold nanoparticles is presented in Fig. 3. Functional groups responsible for the synthesis of gold nanoparticles are denoted by the corresponding peaks arising due to the infrared radiations (Table 1). FTIR data of green gold nanoparticles are well matched with previous report (Donga et al., 2020; Yang et al., 2019).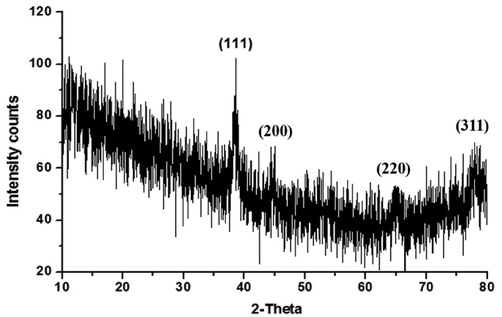
XRD pattern of green synthesized gold nanoparticles.
Peak position
Respective functional groups
3294
O—H stretch of alcohols, Phenols, N—H stretch of 1°, 2° amines, amides,
—C≡CvH: C—H stretch of alkynes
2923
C—H stretch of alkanes, O—H stretch of carboxylic acids
1735
C⚌O stretch of carbonyls, carboxylic acids, esters, aldehydes, saturated aliphatics
1627
N—H bend of 1° amines
1097
C—O stretch of carboxylic acids, alcohols, esters, ethers, C—N stretch of aliphatic amines
1012
C—O stretch of carboxylic acids, alcohols, esters, ethers
3.4 Field emission scanning electron microscopy and atomic force microscopy
Field emission scanning electron microscope (FESM) was used to identify the surface morphology of green synthesized gold nanoparticles. Fig. 4A–D show the FESEM micrographs of gold nanoparticles at different scale bar. These micrographs suggested that green prepared gold nanoparticles were uniformly distributed and most of the particles were irregular in shape with the size range of 50–80 nm (average size 64 nm). Atomic force microscopy (AFM) images of green gold nanoparticles are shown in Fig. 5. The particles are viewed at 2 and 4 µm distances and also in 3D pattern. AFM images further supported the FESEM results.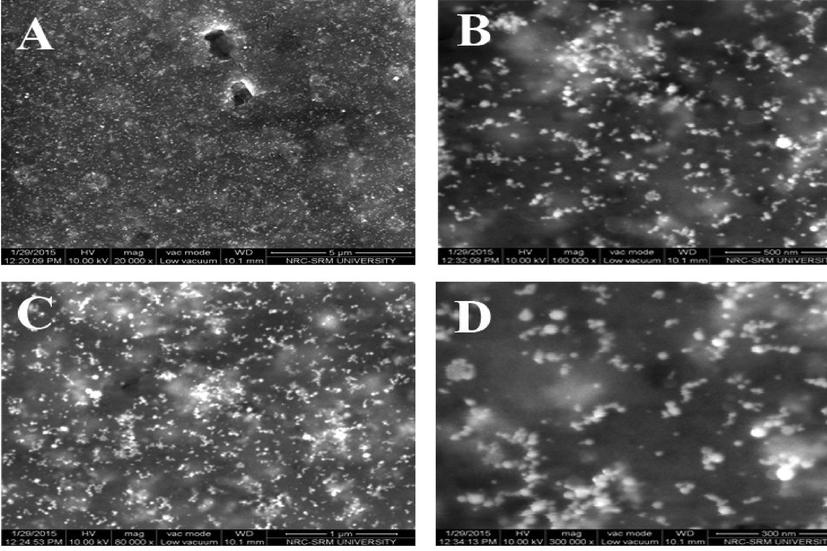
FESEM images of green synthesized gold nanoparticles. (A) 5 µm scale. (B) 500 nm scale. (C) 1 µm scale. (D) 300 nm scale.
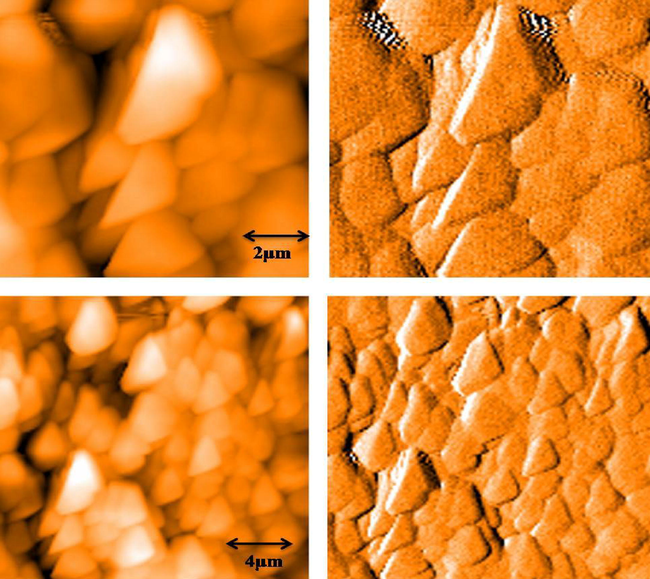
AFM images of green synthesized gold nanoparticles.
3.5 Antioxidant activity
Antioxidant activity (free radicals scavenging activity) of green prepared gold nanoparticles was examined by a series of assays. DPPH is a stable synthetic free radical that is being applied for the assessment of antioxidant activity of various materials (Medhe et al., 2014). This is a fast, easy and reliable method. This method is based on the principal that DPPH can easily receive an electron from antioxidant molecules to become a stable diamagnetic molecule (Soares et al., 1997). In this study, DPPH radical scavenging activity of gold nanoparticles was analyzed at different concentration (20–100 μg/ml) to identify the percentage inhibitory concentration. Results showed that gold nanoparticles exhibited significant DPPH scavenging activity in a dose-dependent manner (Fig. 6A). The 50 % DPPH radical scavenging activity was observed at 51.84 μg/ml concentration of gold nanoparticles.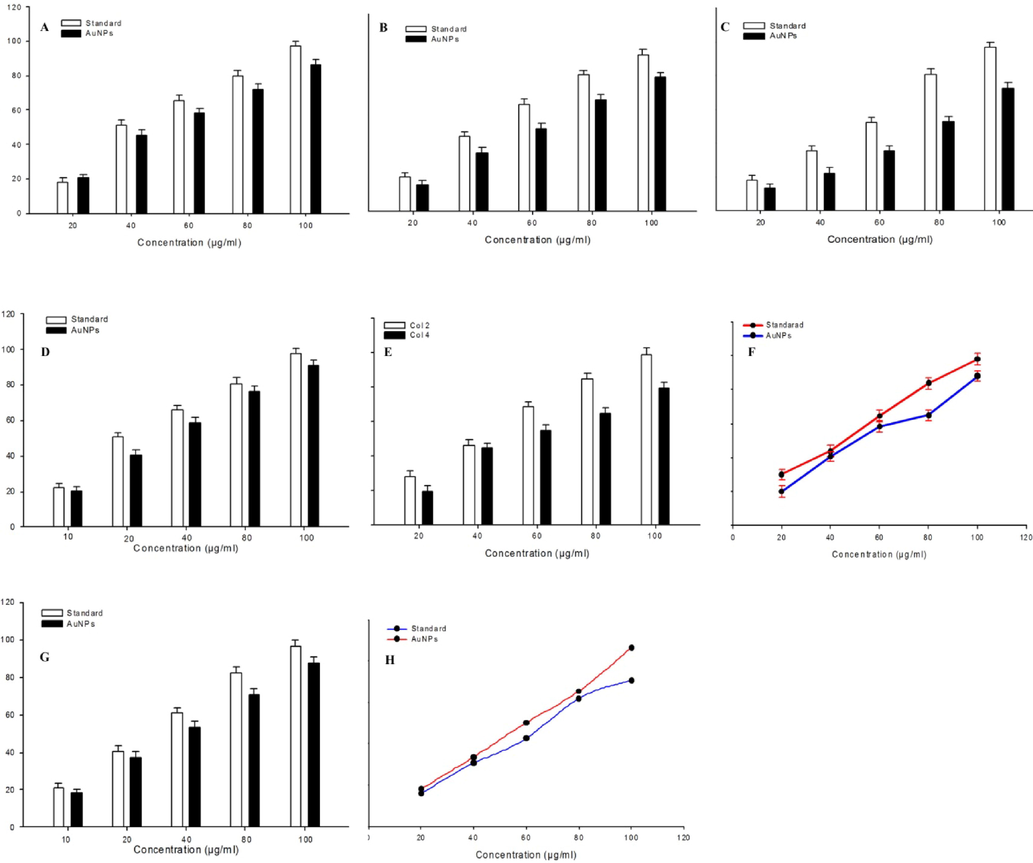
A series of assays demonstrating the antioxidant activity of green synthesized gold nanoparticles. (A) DPPH radical scavenging activity. (B) Hydroxyl radical scavenging activity. (C) Nitric oxide radical scavenging activity. (D) Hydrogen peroxide scavenging activity. (E) Superoxide anion scavenging activity. (F) Lipid peroxidation inhibition activity. (G) ABTS scavenging activity. (H) Reducing power. Data are presented as the % of mean ± SD of triplicate experiments (n = 3).
As we can see in Fig. 6B, hydroxyl radical scavenging activity of gold nanoparticles was observed in a concentration-dependent manner. The IC50 value of gold nanoparticles and standard (vitamin E) were 45.15 μg/ml and 35.5 μg/ml, respectively.
Nitric oxide (NO) is a reactive free radical generated from sodium nitroprusside in aqueous solution and reacts with oxygen to form nitrite. It is well known that NO radical plays an important role in various inflammatory processes such as carcinomas, juvenile diabetes, multiple sclerosis, arthritis, and ulcerative colitis (Hazra et al., 2008). In this study, NO radical scavenging activity of green synthesized gold nanoparticles increases with increasing the concentration of nanoparticles (Fig. 6C). The IC50 value of gold nanoparticles for NO scavenging activity was 59.39 μg/ml.
The scavenging of hydrogen peroxide (H2O2) by gold nanoparticles is shown in Fig. 6D. Gold nanoparticles scavenge the H2O2 radical dose-dependently. The 50 % H2O2 scavenging activity was observed at 56.58 μg/ml concentration of gold nanoparticles. Gold nanoparticles contain phenolic groups that donate electron to H2O2 and thus neutralizing H2O2 into water (Mathew and Abraham, 2006). Superoxide anion is a highly toxic radical that generate oxidative damage to biological molecules (Ahamed et al., 2021b). Superoxide anion scavenging activity of green gold nanoparticles is presented in Fig. 6E. Decrease of absorbance at 560 nm with the increment of gold nanoparticles concentrations indicates dose-dependent superoxide anion scavenging efficacy. The IC50 value of gold nanoparticles in superoxide anion scavenging activity was 74.25 μg/ml.
Fig. 6F shows lipid peroxidation inhibition activity of gold nanoparticles. Results showed that gold nanoparticles effectively inhibit the lipid peroxidation reaction. Lipid peroxidation inhibition activity was increases with increasing the concentration of gold nanoparticles (20–100 μg/ml). The IC50 value of gold nanoparticles was 39.64 μg/ml.
2,2′‐Azinobis (3‐ethylbenzothiazolin‐6‐sulphonic acid) (ABTS) radicals scavenging activity of gold nanoparticles was increases in a dose–response manner from 28.21 % to 79.77 % from the concentration of 20 μg/ml to 100 μg/ml (Fig. 6G). The IC50 value for the gold nanoparticles was found to be 54.39 μg/ml. This result shows that gold nanoparticles have the ability to scavenge the free ABTS radicals generated by the reaction ABTS molecule and potassium persulfate (Venkatesh et al., 2014). It prevents ABTS radicals through chain‐breaking reaction. The reducing power of gold nanoparticles was analyzed due to its electron donating ability and therefore, may serve as a strong antioxidant agent. Fig. 6H shows the reducing potential of gold nanoparticles along with standard butylated hydroxyl toluene. The reducing power of gold nanoparticles increases with increasing the amount of prepared sample. These results suggested the antioxidant activity of gold nanoparticles synthesized from fruit lime peel extract.
3.6 Catalytic activity
Transition metal nanoparticles prepared via green route have shown potential in catalytic reduction of organic pollutants because of their unique physicochemical properties (Gao et al., 2014). The catalytic conversion of 4-nitrophenol to 4-aminophenol by gold nanoparticles is presented in Fig. 7. The characteristic absorption peak (400 nm) of 4-nitrophenol decreases over the time and when the final product 4-aminophenol is produced, obtained the peak shifts to 300 nm. The shifting of absorption peak from 400 nm to 300 nm was due to the catalytic reduction of 4-nitrophenol to 4-aminophenol by gold nanoparticles. The catalytic activity of green-prepared gold nanoparticles is also supported by earlier investigations (Dash et al., 2014).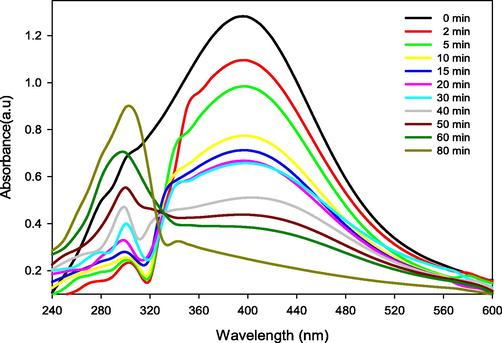
Catalytic activity of green synthesized gold nanoparticles.
4 Conclusion
A simple, economical, and environmentally-safe method was applied to synthesize gold nanoparticles using peel extract of fruit sweet lime (Citrus limetta Risso). Phytochemicals present in fruit peel extract act as reducing and stabilizing agents for gold nanoparticle preparation from chloroauric acid. The appearance of a characteristic absorption peak at 530 by UV–vis spectroscopy confirms the presence of gold nanoparticles in colloidal suspension. Green synthesized gold nanoparticles were further characterized by XRD, FSEM, AFM, and FTIR. Gold nanoparticles demonstrated excellent antioxidant activity indicated by inhibition/scavenging of DPPH, hydroxyl radical, nitric oxide, hydrogen peroxide, superoxide anion, lipid peroxidation, and ABTS radicals. Green prepared gold nanoparticles also exhibited catalytic activity evident by the reduction of 4-nitrophenol into 4-aminophenol. This study indicated the significance of bio-waste (e.g. fruit peel) in the green synthesis of noble metal nanoparticles for biomedical and environmental research.
Acknowledgement
The authors extend their sincere appreciation to the Researchers Supporting Project (RSP-2021/129) at the King Saud University, Riyadh, Saudi Arabia. The authors thank Mr. A.S.T. Jeyaraj, Lab Assistant (Nanotechnology lab), Centre for Nanotechnology Research, VIT, Vellore-14, India for technical support.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Ag-doping regulates the cytotoxicity of TiO2 nanoparticles via oxidative stress in human cancer cells. Sci. Rep.. 2017;7:17662.
- [CrossRef] [Google Scholar]
- Oxidative stress mediated cytotoxicity and apoptosis response of bismuth oxide (Bi2O3) nanoparticles in human breast cancer (MCF-7) cells. Chemosphere. 2019;216:823-831.
- [Google Scholar]
- A novel green preparation of Ag/RGO nanocomposites with highly effective anticancer performance. Polymers. 2021;13:3350.
- [CrossRef] [Google Scholar]
- SnO2-doped ZnO/reduced graphene oxide nanocomposites: synthesis, characterization, and improved anticancer activity via oxidative stress pathway. Int. J. Nanomed.. 2021;16:89-104.
- [CrossRef] [Google Scholar]
- Facile green synthesis of ZnO-RGO nanocomposites with enhanced anticancer efficacy. Methods. 2022;199:28-36.
- [CrossRef] [Google Scholar]
- Influence of nanotechnology on herbal drugs: A review. J. Adv. Pharm. Technol. Res.. 2012;3:142-146.
- [CrossRef] [Google Scholar]
- Biosynthesis and characterization of gold nanoparticles using Brazilian red propolis and evaluation of its antimicrobial and anticancer activities. Sci. Rep.. 2021;11:1974.
- [Google Scholar]
- Saraca indica bark extract mediated green synthesis of polyshaped gold nanoparticles and its application in catalytic reduction. Appl. Nanosci.. 2014;4(4):485-490.
- [Google Scholar]
- Natural products and their derivatives: promising modulators of tumor immunotherapy. J. Leukocyte Biol.. 2020;108:493-508.
- [CrossRef] [Google Scholar]
- Antimicrobial, antioxidant and anticancer activities of gold nanoparticles green synthesized using Mangifera indica seed aqueous extract. Artif. Cells Nanomed. Biotechnol.. 2020;48(1):1315-1325.
- [Google Scholar]
- Antioxidant and free radical scavenging activity of H. officinalis L. var. angustifolius, V. odorata, B. hyrcana and C. speciosum. Pak. J. Pharm. Sci.. 2010;23:29-34.
- [Google Scholar]
- Glucomannan-mediated facile synthesis of gold nanoparticles for catalytic reduction of 4-nitrophenol. Nanoscale Res. Lett.. 2014;9:404.
- [Google Scholar]
- Formation of thiobarbituric-acid-reactive substances from deoxyribose in the presence of iron salts. The role of superoxide and hydroxyl radicals. FEBS Lett.. 1981;128:347-352.
- [Google Scholar]
- Antioxidant and free radicals scavenging activity of Spondias pinnata. BMC Complement. Altern. Med.. 2008;8:63.
- [Google Scholar]
- Multifunctional gold nanoparticles: A novel nanomaterial for various medical applications and biological activities. Front. Bioeng. Biotechnol.. 2020;8:990.
- [CrossRef] [Google Scholar]
- In vitro antioxidant activities and scavenging effects of Cinnamomum verum leaf extract assayed by different methodologies. J. Food Chem. Toxicol.. 2006;44:198-206.
- [Google Scholar]
- Enhanced antioxidant activity of gold nanoparticle embedded 3, 6-dihydroxyflavone: a combinational study. Appl. Nanosci.. 2014;4(2):153-161.
- [Google Scholar]
- Antioxidative action of indole compounds during the autoxidation of linoleic acid. Eiyo to Shokuryo. 1966;19(3):210-214.
- [Google Scholar]
- Determination of antioxidant activity, phenol and flavonoids content of Parrotia persica Mey. Pharmacologyonline. 2008;2:560-567.
- [Google Scholar]
- Guinea pigs possess a highly mutated gene for L-gulono-gamma-lactone oxidase, the key enzyme for L-ascorbic acid biosynthesis missing in this species. J. Biol. Chem.. 1992;267(30):21967-21972.
- [Google Scholar]
- The Scherrer formula for X-ray particle size determination. Phys. Rev.. 1939;56(10):978-982.
- [CrossRef] [Google Scholar]
- Waste fruit peel-Mediated green synthesis of biocompatible gold nanoparticles. J. Mater. Res. Technol.. 2021;14:2982-2991.
- [Google Scholar]
- Antioxidant, antimicrobial and cytotoxic activities of silver and gold nanoparticles synthesized using Plumbago zeylanica bark. J. Nanostruct. Chem.. 2016;6(3):247-260.
- [Google Scholar]
- Antioxidative properties of xanthone on the auto oxidation of soybean in cylcodextrin emulsion. J. Agric. Food Chem.. 1992;40:945-948.
- [Google Scholar]
- Black pepper assisted biomimetic synthesis of silver nanoparticles. J. Alloys Comp.. 2010;507(1):L13-L16.
- [Google Scholar]
- Studies on antioxidant activities of Mucuna seed (Mucuna pruriens var. utilis) extracts and certain non-protein amino acids through in vitro models. J. Sci. Food Agric.. 2003;83:1517-1524.
- [Google Scholar]
- Copper oxide nanoparticles induced mitochondria mediated apoptosis in human hepatocellular carcinoma cells. PLOS ONE. 2013;8:e69534.
- [CrossRef] [Google Scholar]
- Antioxidant activities of some extracts of Thymus zygi. Free Radical Res.. 1997;26:469-478.
- [Google Scholar]
- Analysis of phytochemicals and free radical scavenging activity of Solanum villosum (Mill)-A traditional medicinal plant in southern India. World J. Pharm. Pharm. Sci.. 2014;3:741-755.
- [Google Scholar]
- Study of green synthesis of ultrasmall gold nanoparticles using Citrus Sinensis peel. Appl. Sci.. 2019;9:2423.
- [Google Scholar]
- Antioxidant activity of various tea extracts in relation to their antimutagenicity. J. Agric. Food Chem.. 1995;43(1):27-32.
- [Google Scholar]
- Redox signaling at the crossroads of human health and disease. MedComm. 2022;3:e127.
- [Google Scholar]







