Translate this page into:
Chronic ecotoxicity of ciprofloxacin exposure on taxonomic diversity of a meiobenthic nematode community in microcosm experiments
⁎Corresponding author. ahmed.nasri@fsb.u-carthage.tn (Ahmed Nasri)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
A laboratory bioassay was performed to assess the ecotoxicity of ciprofloxacin on a meiobenthic nematodes community from Bizerte lagoon (NE Tunisia). Four ciprofloxacin doses [D1 (50 µg/g), D2 (100 µg/g), D3 (200 µg/g), and D4 (500 µg/g)] were applied to the substrate, and responses were studied after one month. Discernible differences were observed between control assemblages and those populating ciprofloxacin treated substrates. All univariate indices were affected significantly compared to those in the control microcosm with increasing antibiotic concentration. The non-parametric Multi-Dimensional Scaling based on species abundances (MDS) showed significant separation of the control microcosm from the antibiotic-treated populations. The nematode species responses to the fluoroquinolone treatments varied: Odontophora villoti was reduced at all concentrations of ciprofloxacin and was considered “sensitive,” whereas Metoncholaimus pristiurus was affected by moderate concentrations; its abundance increased with the highest dose D4 and was described as “opportunistic.” Paramonohystera pilosa, whose abundance increased with antibiotic doses appeared “resistant.”
Keywords
Meiobenthic nematodes
Susbstrate
Antibiotic
Ciprofloxacin
Exposure
Bioassay
- C
-
Concentration
- H
-
Shannon index
- d
-
Margalef’s species richness
- J
-
Pielou’s evenness
- S
-
species number
Abbreviations
1 Introduction
Antibiotics were recently considered the second highest found category (15%) of drugs in the water plans (Santos et al., 2010). Their loads have been evaluated in hospital and wastewater treatment plant (WWTP) (Santos et al., 2009; Seifrtová et al., 2008), run-off water (Pena et al., 2007), and groundwater (Kummerer, 2003) and ranged between ng/L and μg/L levels, indicating their hard degradation at Sewage Treatment Plants (STPs). In return, their ecotoxicity for non-target taxa have been rarely explored (Santos et al., 2010).
Antibiotics are extensively used for health applications of human beings and animals. Since several antibiotics are not fully metabolized, they are often detected in downstream outfluxes from most of wastewater treatment installations and animal facilities (Kolpin et al., 2002). Antibiotics pose a potential threat to biota and linked environmental processes in relation with the capital role bacteria play in these ecosystems. Data of Golet et al. (2002) and Kolpin et al. (2002) showed that ciprofloxacin could be found in runoff water in the United States of America and Europe at loads ranging from 0.01 to 0.03 mg/L. The acute toxicity of this chemical has been reported too for some aquatic organisms by Robinson et al. (2005). On the contrary, rare publications were devoted to the relationships of antibiotics and cyanobacteria by giving a concentration variety of mg/L (Kolar et al., 2014; Vázquez-Martínez et al., 2004).
Studies focusing on the impact of fluoroquinolone antibiotics on assemblages from Mediterranea are rare. No laboratory bioassay evaluated the effects of such antibiotics on benthic taxa. Free-living marine nematodes represent excellent tools in environmental monitoring programs (Coull and Chandler, 1992). These invertebrates distinguish themselves by their short lifecycles (days to weeks in most cases), extremely high abundances, and absence of planktonic juvenile stages (Austen and McEvoy, 1997). These small invertebrates (length ranged typically from 400 µm to 1 mm), with short generation time could be maintained with little effort in the laboratory and, thus, their ability to assess rapid responses to environmental changes is extremely promising (Mahmoudi et al., 2005; Gyedu-Ababio and Baird, 2006; Semprucci et al., 2015). As result, it is comparatively easy to keep nematodes in laboratory conditions and manipulate natural sediments in simple and non-costly experimental designs (Millward et al., 2004; Schratzberger et al., 2002). The main goals of the current work were to assess the numerical and taxonomic responses of a nematofauna after its exposure to a control and ciprofloxacin treatments.
2 Materials and methods
2.1 Sample collection and ciprofloxacin-enriched sediments
In Bizerte lagoon (NE, Tunisia), sediments and inhabiting meiofauna were collected from an offshore site (37° 13′33″ N; 9° 49′24″ E) using Plexiglas hand-cores with an internal diameter of 3.2 cm (for a depth equal to 10 cm). On sampling day, water depth and salinity were measured (50 cm and 37 PSU, respectively).
Freezing of sediments at −20 °C (12 h) followed by thawing at laboratory temperature (48 h) were repeated three times before being spiked with ciprofloxacin (Schratzberger et al., 2002). Then, large substrate particles (>63 µm) were removed through wet sieving and appropriate amounts of ciprofloxacin were finally added to sediment sub-samples of 100 g (dry weight; dw) to prepare four pre-fixed ciprofloxacin concentrations (in dw), D1 (50 µg/g), D2 (100 µg/g), D3 (200 µg/g), D4 (500 µg/g)], once they are gently mixed with an amount of 200 g of fresh sediment.
2.2 Experimental designs and sample handling
Experiment enclosures consisted of glass bottles of 570 ml. Three replicated five treatments (one control and four enriched with ciprofloxacin) were set up (Schratzberger and Warwick, 1998). All microcosms, enriched or not with ciprofloxacin, were filled with 300 g dw and a litre of pre-filtered (1 µm) seawater collected on the sampling day of meiofauna (Schratzberger and Warwick, 1998).
Technically, each experimental enclosure was connected to an aeration pump during the exposure duration of one month and water parameters (namely, salinity, pH, dissolved oxygen and temperature) were monitored at short regular intervals of two days. At the end of the bioassay, all sediment samples were conserved in 4% neutralized formaldehyde.
Meiobenthic organisms were isolated based on the resuspension–decantation method followed by their coloring with Rose-Bengal (0.2 g/L) according to Wieser (1960). After 2 days, stained nematodes were counted and picked-up using a stereomicroscope to assess the nematode abundance. Pictorial keys (Platt et al., 2015), records and descriptions of taxa to the lowest level acquired from the word database of nematodes ‘NeMys’ maintained by nematologists of Ghent University, Belgium (Vanaverbeke et al., 2015), were used to identify the genus or species of free-living nematodes.
2.3 Data analysis
All data analyses were performed with the software Primer v5.0 as proposed by Clarke (1993) and Clarke et al. (2014). In details, the non-parametric Multi-Dimensional Scaling (MDS) analysis was applied using Bray–Curtis index of similarity (square-root transformed abundance) to detect whether the exposed nematode communities to ciprofloxacin became significantly different from controls in function of the contamination levels.
Discernible differences between assemblages exposed or not to ciprofloxacin, were determined by pairwise ANalysis Of SIMilarities (ANOSIM). The contribution of individual species towards average dissimilarities was deduced from the SIMPER analysis (SIMilarity PERcentages). Five univariate indices, namely, abundance, species number (S), Shannon diversity index (H′), Margalef’s species richness (d), Pielou’s evenness (J′), were computed. Analysis of variance (1-ANOVA) was conducted for global search of differences, and Tukey’s HSD test was finally considered to compare treatment and control groups. An α of 0.05 assesses continuously the significance of differences.
3 Results
Univariate indices showed significant variation compared with controls. Measures of nematode assemblages between controls and treatment groups are shown in a graphical summary (Fig. 1). ANOVA results and multiple pairwise comparisons (Tukey HSD test) revealed discernible differences between control nematode assemblages and those collected from ciprofloxacin amended sediments, practically for all univariate indices considered (p < 0.05). Total nematode abundance, number of species, species richness, and Shannon-Weaver index declined significantly with an intensification in the ciprofloxacin exposure, with the exception of a significant increase in equitability (J′) with the two lower doses, D2 and D3, of ciprofloxacin.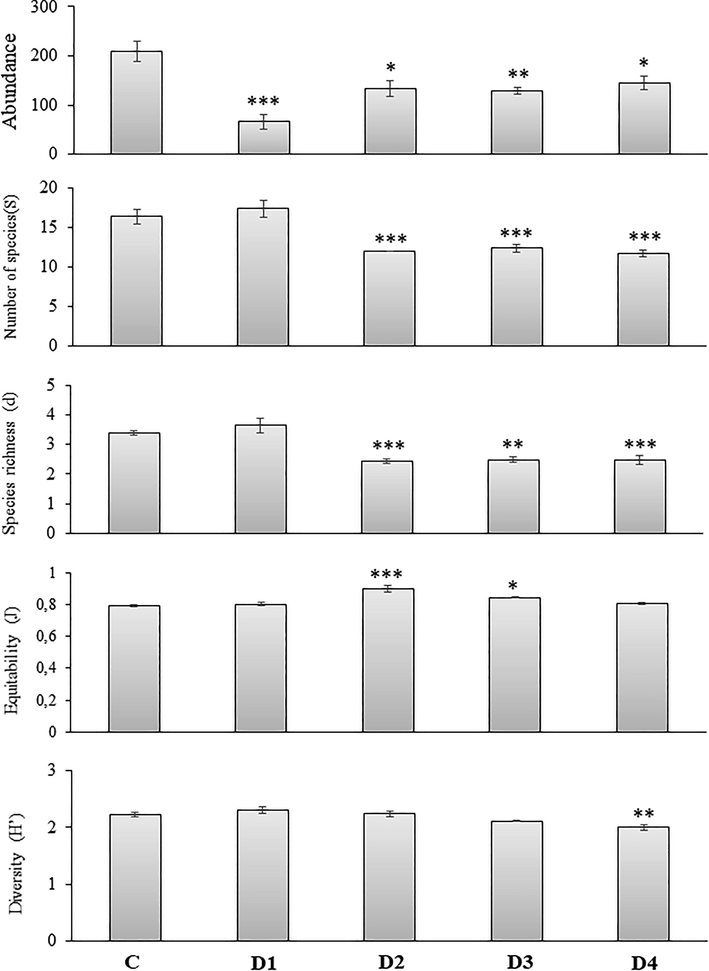
Graphical summary of means and 95% pooled confidence intervals of univariate indices for nematode assemblages from each microcosm. Index of Shannon–Weaver (H′), species richness = Margalef’s (d), evenness = Pielou’s (J), no. species = number of species (S). Asterisk indicates a significant difference (p < 0.05) of the univariate measure in the contaminated microcosm when compared to the control.
The MDS ordination showed that all treated microcosm replicates, except those contaminated with the lowest dose D1, were distinct from the controls; those of the highest ciprofloxacin dose D4 were placed at the extreme limit of the 2D-plot (Fig. 2). This pattern was confirmed by results of SIMPER analysis where an enhance in average dissimilarity was recorded between nematode communities inhabiting microcosms gradually enriched with ciprofloxacin in comparison with the control ones, even though ANOSIM probabilities did not reached the significance level (p ≥ 0.05). The maximum average divergence in terms of community structure (44.06%) was noted between control replicates and those spiked with the uppermost concentration of ciprofloxacin (D4) (Table 1).![Non-metric MDS ordination of square-root transformed nematode species abundance data from uncontaminated sediment control microcosm (C) and ciprofloxacin amended sediment treatments [D1, D2, D3, and D4]](/content/185/2020/32/2/img/10.1016_j.jksus.2019.11.044-fig2.png)
Non-metric MDS ordination of square-root transformed nematode species abundance data from uncontaminated sediment control microcosm (C) and ciprofloxacin amended sediment treatments [D1, D2, D3, and D4]
Groups
R value
Average Dissimilarity (%)
Significance level
C-D1
0.856
16.4
0.1
C-D2
0.856
31.32
0.1
C-D3
0.856
27.45
0.1
C-D4
0.856
44.06
0.1
Seven nematode species were dominant in the control samples (C), and included Odontophora villoti (23.71%), Paracomesoma dubium (21.49%), Metoncholaimus pristiurus (18.15%), Terschellengia longicaudata (11.85%), Paramonohystera pilosa (7.78%), Synonchiella edax (3.70%), and Daptonema trabeculosum (3.70%). The replicates related to the lowest concentration of ciprofloxacin, D1, were populated by Paracomesoma dubium (20.35%), Metoncholaimus pristiurus (19.03%), Odontophora villoti (18.14%), Paramonohystera pilosa (12.83%), Terschellengia longicaudata (12.39%), Daptonema trabeculosum (4.43%), and Synonchiella edax (3.1%). At D2, 8 species including Paracomesoma dubium (18.29%), Terschellengia longicaudata (17.9%), Metoncholaimus pristiurus (15.73%), Paramonohystera pilosa (13.09%), Synonchiella edax (8.86%), Daptonema trabeculosum (8.46%), Odontophora villoti (5.55%), and Metalinhomoeus torosus (5.51%) dominated the microcosm. Eight taxa were remarkably present into microcosms spiked with D3, Paramonohystera pilosa (19.43%), Paracomesoma dubium (19.43%), Terschellengia longicaudata (18.27%), Metalinhomoeus torosus (12.95%), Odontophora villoti (9.72%), Synonchiella edax (5.64%), Daptonema trabeculosum (3.64%), and Anticoma acuminata (364%). Regarding replicates highly contaminated with ciprofloxacin D4, 7 taxa were regularly inventoried, namely, Metoncholaimus pristiurus (39%), Paramonohystera pilosa (19.51%), Synonchiella edax (11.32%), Daptonema trabeculosum (8.17%), Paracomesoma dubium (6.29%), Metalinhomoeus torosus (4.4%), and Steineria pilosa (3.77%).
Significant differences characterized comparisons of controls and treatments with ciprofloxacin, as given in Table 2, principally resulted from abundance changes for the dominant taxa. The difference between structure of the control assemblage and that exposed to D1 was caused by the numerical increase in the proportion of Paramonohystera pilosa, and decrease of those of Odontophora villoti, Paracomesoma dubium, Terschellengia longicaudata, Metoncholaimus pristiurus and Synonchiella edax. The nematofauna collected from the treatment D2 was more distinct from controls. This resulted from an increase in the input of Paramonohystera pilosa, Terschellengia longicaudata, and Synonchiella edax, and the reduction of Odontophora villoti, Paracomesoma dubium, and Metoncholaimus pristiurus. The average dissimilarity between controls and treatment D3 resulted from the increased contribution of Paramonohystera pilosa, Paracomesoma dubium, Terschellengia longicaudata, and Synonchiella edax. Lastly, the difference between the control replicates and treatment with the highest concentration D4 was the consequence of the increased impact of Paramonohystera pilosa, Metoncholaimus pristiurus, and Synonchiella edax, and the reduction of Odontophora villoti, Paracomesoma dubium, and Terschellengia longicaudata. + More abundant, − less abundant species. Species accounting for ∼50% of the overall dissimilarity between treatment groups are ranked in the order of importance with respect to their contribution to this dissimilarity.
D1
D2
D3
D4
Odontophora villoti (−)
Odontophora villoti (−)
Odontophora villoti (−)
Odontophora villoti (−)
Paramonohystera pilosa (+)
Paramonohystera pilosa (+)
Paramonohystera pilosa (+)
Paramonohystera pilosa (+)
Paracomesoma dubium (−)
Paracomesoma dubium (−)
Paracomesoma dubium (+)
Paracomesoma dubium (−)
Terschellengia longicaudata (−)
Terschellengia longicaudata (+)
Terschellengia longicaudata (+)
Terschellengia longicaudata (−)
Metoncholaimus pristiurus (−)
Metoncholaimus pristiurus (−)
Metoncholaimus pristiurus (−)
Metoncholaimus pristiurus (+)
Synonchiella edax (−)
Synonchiella edax (+)
Synonchiella edax (+)
Synonchiella edax (+)
A discernible difference was observed between ciprofloxacin-spiked and control substrates (p < 0.05) after multivariate analysis indicating that changes in abundance of the meiobenthic nematode taxa exposed to ciprofloxacin were related to the intensity of this fluoroquinolone antibiotic treatment. All contaminated microcosms were different from control replicates as shown in the MDS ordination (Fig. 2). The highest dose D4 of this pharmaceutical appeared as the most distinct from the control treatment in comparison with the others. Differences in the degree of sensitivity of the exposed taxa to the introduced antibiotic in the culture medium contribute to these modifications. Thus, the species Odontophora villoti, which was disadvantaged by all ciprofloxacin doses applied, may be classified as “ciprofloxacin-sensitive.” Metoncholaimus pristiurus was impacted by moderate concentrations and was increased in abundance at the highest dose D4; this species can thus be described as “opportunistic.” Paramonohystera pilosa, whose presence increased with increasing of the antibiotic concentration, appeared to be “ciprofloxacin-resistant” (Table 2).
4 Discussion
The negative response of Bizerte lagoon nematodes to experimental ciprofloxacin-enriched microcosms was in accord with ecotoxicological effects; practically all univariate indices considered discernibly decreased with ciprofloxacin treatment except for equitability (J′), which showed a significant enhance after the exposure to the intermediate concentrations applied, D2 and D3 (Fig. 1).
In our experiment, the impact of the antibiotic presence into the experimental enclosure on nematode species seems to occur through the easily uptake of surrounding ciprofloxacin from the sediment over their cuticles as well as the direct feeding of the part of the pollutant adsorbed on substrate particles (Boonsaner and Hawker, 2013; Li et al., 2012). Ciprofloxacin treatment caused a reduce in abundance and diversity, by deleting the sensitive taxa. Ciprofloxacin gathered on sediment particle surfaces shows a 3.5-fold increase in potency and has an affinity for adsorption (Halling-Sørensen et al., 2003; Tolls, 2001). Ciprofloxacin is reported to adsorb to sludge, sediments, and clay (Cardoza et al., 2005; Lindberg et al., 2005).
In addition, ciprofloxacin is not readily biodegradable (Kümmerer et al., 2000). It also strongly adsorbs to soil and sediments (Uslu et al., 2008), mostly by cation exchange (Vasudevan et al., 2009). Therefore, grain surfaces of these matrices may constitute a gathering areas for antibiotics (Rooklidge, 2004). In a previous study (Lillenberg et al., 2010), the concentration of ciprofloxacin was found to remain unchanged in these matrices during exposure to various doses (10, 50, 200 and 500 µg/g) during 28 days in the laboratory.
Ciprofloxacin is one of the fluoroquinolone antibiotics, and is very effective against various pathogenic bacteria including a wide range of gram-negative and a number of gram-positive organisms (Naora et al., 1999). The mode of action of ciprofloxacin involves inhibition of the bacterial enzymes, DNA gyrase and topoisomerase IV, which are required for replication and transcription in prokaryotic cells (Fisher et al., 1989; Hooper et al., 1987; Robinson et al., 2005). Quinolone antibiotics interact differently with the eukaryotic enzyme topoisomerase II, primarily because of differences in DNA structure, therefore the potential of genotoxic effects in eukaryotes is considerably lower compared to those in prokaryotic organisms (Toolaram et al., 2016). Several studies have shown that ciprofloxacin can modify the microbial community structure (i.e. abundance and diversity) in water, sediments, and soil (Cui et al., 2014; Girardi et al., 2011; Gonzalez-Martinez et al., 2014) from concentrations of 200 μg/L and 0.1 μg/kg (Näslund et al., 2008).
In our work, the density of marine free-living nematodes was significantly decreased with all tested doses, suggesting that this antibiotic by its antibacterial action, eliminated the bacteria that form the main food source of certain nematological trophic groups. Many studies have shown that the presence of antibiotics reduces microbial biodiversity. Moreover, they can influence the growth and enzyme activities of bacterial communities and ultimately ecological functions such as biomass production and nutrient transformation, leading to loss of functional stability (Pallecchi et al., 2008; Pauwels and Verstraete, 2006).
The taxonomic composition of aquatic bacterial communities was found to be changed significantly after exposure to 1 μg/L of ciprofloxacin (Kraupner et al., 2018). In synthetic wastewater, ciprofloxacin reduced nitrification, denitrification, and phosphorus uptake at concentrations of 200–350 μg/L. This was accomplished by a reduction in ammonium oxidizing bacteria, denitrifying bacteria, and polyphosphate-accumulating organism (Gonzalez-Martinez et al., 2014; Yi et al., 2017). In a wetland mesocosm (planted with Phragmites australis seeded with activated sludge from a WWTP) exposed to 2000 μg/L of ciprofloxacin (Weber et al., 2011), a temporary decrease in the activity and overall catabolic capabilities was observed in the bacterial communities along with decreased overall diversity of bacterial operational taxonomic units.
In aquatic organisms, principally in fish, scientific research has shown that ciprofloxacin (1000 μg/L) exerts adverse effects such as significant alteration in body weight and length at the early life stage of Cyprinus carpio (Zivna et al., 2016). Further, a greater hatching rate was observed at all concentrations (1–3000 μg/L), with reduced development in some larval stages at 1–500 μg/L, and accelerated development at 1000–3000 μg/L (Zivna et al., 2016). In zebrafish (Danio rerio), no effects on growth were observed at concentrations up to 3000 μg/L (Plhalova et al., 2014). Furthermore, dispersed results were obtained for some oxidative stress markers and enzyme activity in fish, suggesting that these effects were not always dose-response related (Zivna et al., 2016; Plhalova et al., 2014).
In invertebrates, ciprofloxacin showed no effects on the growth of two species Gammarus spp. and Lepidostoma liba (macroinvertebrates), upon exposure to 0.9 and 90 μg/L in experimental microcosms, respectively, for 45 days (Maul et al., 2006). In another study investigating the effects on Amphibia (Rhinella arenarum) exposed to 1, 10, 100 and 1000 μg/L for 4 days, ciprofloxacin reduced the larval length at 10 μg/L. A significant development inhibition greater than 10% was observed for the concentrations of 100 and 1000 μg/L, and additionally, GST levels were increased at 1000 μg/L (Peltzer et al., 2017). For meiofauna, marine nematodes have especially shown that the beta-lactam antibiotic, penicillin G, significantly decreases the univariate index including species number, Shannon index, Margalef’s richness and Pielou’s equitability after experimental treatment during 30 days (Nasri et al., 2015). Ciprofloxacin also restructured the trophic diversity of free-living nematodes in marine sediments experimentally enriched with increasing doses of the antibiotic (Nasri et al., 2014, 2016). These findings are in accord with our work.
Our study supported that ciprofloxacin has a negative effect on Mediterranean natural communities, specifically on the taxonomic diversity on meiobenthic nematofauna. These invertebrates showed clearly a significant response in function of the intensity of the antibiotic added. These results support the use of these particular organisms as bioindicative models toward antibiotics in biomonitoring programs of aquatic ecosystems.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Special thanks, to Pr. Patricia Aïssa, Professor of Zoology and specialist of meiobenthic nematology (Faculty of Science of Bizerte, University of Carthage), for work follow-up of this paper. This study was supported by Scientists Supporting Project number RSP-2019/17 (King Saud University, Riyadh (Saudi Arabia)).
References
- The use of offshore meiobenthic communities in laboratory microcosm experiments: response to heavy metal contamination. J. Exp. Mar. Biol. Ecol.. 1997;211(2):247-261.
- [CrossRef] [Google Scholar]
- Evaluation of food chain transfer of the antibiotic oxytetracycline and human risk assessment. Chemosphere. 2013;93(6):1009-1014.
- [CrossRef] [Google Scholar]
- Factors affecting the fate of ciprofloxacin in aquatic field systems. Water Air Soil Pollut.. 2005;161(1–4):383-398.
- [CrossRef] [Google Scholar]
- Non-parametric multivariate analyses of changes in community structure. Aust. J. Ecol.. 1993;18(1):117-143.
- [CrossRef] [Google Scholar]
- Change in Marine Communities: An Approach to Statistical Analysis and Interpretation (third ed.). Plymouth UK: PRIMER-E; 2014.
- Pollution and meiofauna: field, laboratory, and mesocosm studies. Oceanogr. Mar. Biol.. 1992;30:191-271.
- [Google Scholar]
- Influence of ciprofloxacin on microbial community structure and function in soils. Biol. Fertil. Soils. 2014;39(3):1333-1341.
- [CrossRef] [Google Scholar]
- Ciprofloxacin and the fluoroquinolones. New concepts on the mechanism of action and resistance. Am. J. Med.. 1989;87(5A):2S-8S.
- [CrossRef] [Google Scholar]
- Biodegradation of ciprofloxacin in water and soil and its effects on the microbial communities. J. Hazard. Mater.. 2011;198:22-30.
- [CrossRef] [Google Scholar]
- Environmental exposure and risk assessment of fluoroquinolone antibacterial agents in wastewater and river water of the Glatt Valley watershed, Switzerland. Environ. Sci. Technol.. 2002;36(17):3645-3651.
- [CrossRef] [Google Scholar]
- Effect of ciprofloxacin antibiotic on the partial-nitritation process and bacterial community structure of a submerged biofilter. Sci. Total Environ.. 2014;476–477:276-287.
- [CrossRef] [Google Scholar]
- Response of meiofauna and nematode communities to increased levels of contaminants in a laboratory microcosm experiment. Ecotoxicol. Environ. Saf.. 2006;63(3):443-450.
- [CrossRef] [Google Scholar]
- Reduced antimicrobial potencies of oxytetracycline, tylosin, sulfadiazin, streptomycin, ciprofloxacin, and olaquindox due to environmental processes. Arch. Environ. Contam. Toxicol.. 2003;44(1):7-16.
- [CrossRef] [Google Scholar]
- Mechanisms of action of and resistance to ciprofloxacin. Am. J. Med.. 1987;82(4A):12-20.
- [Google Scholar]
- The toxic effect of oxytetracycline and trimethoprim in the aquatic environment. Chemosphere. 2014;115:75-80.
- [CrossRef] [Google Scholar]
- Pharmaceuticals, hormones, and other organic wastewater contaminants in U.S. streams, 1999–2000: a national reconnaissance. Environ. Sci. Technol.. 2002;36(6):1202-1211.
- [CrossRef] [Google Scholar]
- Selective concentration for ciprofloxacin resistance in Escherichia coli grown in complex aquatic bacterial biofilms. Environ. Int.. 2018;116:255-268.
- [CrossRef] [Google Scholar]
- Significance of antibiotics in the environment. J. Antimicrob. Chemother.. 2003;52(1):5-7.
- [CrossRef] [Google Scholar]
- Biodegradability of some antibiotics, elimination of the genotoxicity and affection of wastewater bacteria in a simple test. Chemosphere. 2000;40(7):701-710.
- [CrossRef] [Google Scholar]
- Occurrence of antibiotics in water, sediments, aquatic plants, and animals from Baiyangdian Lake in North China. Chemosphere. 2012;89(11):1307-1315.
- [CrossRef] [Google Scholar]
- Enrofloxacin and ciprofloxacin uptake by plants from soil. Agron. Res.. 2010;8(1):807-814.
- [Google Scholar]
- Screening of human antibiotic substances and determination of weekly mass flows in five sewage treatment plants in Sweden. Environ. Sci. Technol.. 2005;39(10):3421-3429.
- [CrossRef] [Google Scholar]
- Effects of hydrocarbon contamination on a free living marine nematode community: results from microcosm experiments. Mar. Pollut. Bull.. 2005;50(11):1197-1204.
- [CrossRef] [Google Scholar]
- Effects of the antibiotic ciprofloxacin on stream microbial communities and detritivorous macroinvertebrates. Environ. Toxicol. Chem.. 2006;25(6):1598-1606.
- [CrossRef] [Google Scholar]
- Mixtures of metals and hydrocarbons elicit complex responses by a benthic invertebrate community. J. Exp. Mar. Biol. Ecol.. 2004;310(1):115-130.
- [CrossRef] [Google Scholar]
- Distribution of ciprofloxacin into the central nervous system in rats with acute renal or hepatic failure. J. Pharm. Pharmacol.. 1999;51(5):609-616.
- [CrossRef] [Google Scholar]
- Effects of the antibiotic ciprofloxacin on the bacterial community structure and degradation of pyrene in marine sediment. Aquat. Toxicol.. 2008;90(3):223-227.
- [CrossRef] [Google Scholar]
- Impact of penicillin G on the trophic diversity (Moens & Vincx, 1997) of marine nematode community: results from microcosm experiments. Cah. Biol. Mar.. 2014;56:65-72.
- [Google Scholar]
- Effects of increasing levels of pharmaceutical penicillin G contamination on structure of free living nematode communities in experimental microcosms. Environ. Toxicol. Pharmacol.. 2015;40(1):215-219.
- [CrossRef] [Google Scholar]
- Trophic restructuring (Wieser 1953) of free-living nematode in marine sediment experimentally enriched to increasing doses of pharmaceutical penicillin G. Ecotoxicology. 2016;25:1160-1169.
- [CrossRef] [Google Scholar]
- Antibiotic resistance in the absence of antimicrobial use: mechanisms and implications. Expert Rev. Anti. Infect. Ther.. 2008;6(5):725-732.
- [CrossRef] [Google Scholar]
- The treatment of hospital wastewater: an appraisal. J. Water Health. 2006;4(4):405-416.
- [CrossRef] [Google Scholar]
- Ecotoxicity of veterinary enrofloxacin and ciprofloxacin antibiotics on anuran amphibian larvae. Environ. Toxicol. Pharmacol.. 2017;51:114-123.
- [CrossRef] [Google Scholar]
- Determination of fluoroquinolone antibiotics in surface waters from Mondego River by high performance liquid chromatography using a monolithic column. J. Sep. Sci.. 2007;30(17):2924-2928.
- [CrossRef] [Google Scholar]
- Free-living marine nematodes. Part 1 British Enoplids. South African. J. Zool.. 2015;20(3):177.
- [CrossRef] [Google Scholar]
- The effects of subchronic exposure to ciprofloxacin on zebrafish (Danio rerio) Neuroendocrinol. Lett.. 2014;35(Suppl 2):64-70.
- [Google Scholar]
- Toxicity of fluoroquinolone antibiotics to aquatic organisms. Environ. Toxicol. Chem.. 2005;24(2):423-430.
- [CrossRef] [Google Scholar]
- Environmental antimicrobial contamination from terraccumulation and diffuse pollution pathways. Sci. Total Environ.. 2004;325(1–3):1-13.
- [CrossRef] [Google Scholar]
- Occurrence of pharmaceutically active compounds during 1-year period in wastewaters from four wastewater treatment plants in Seville (Spain) J. Hazard. Mater.. 2009;164(2–3):1509-1516.
- [CrossRef] [Google Scholar]
- Ecotoxicological aspects related to the presence of pharmaceuticals in the aquatic environment. J. Hazard. Mater.. 2010;175(1–3):45-95.
- [CrossRef] [Google Scholar]
- Effects of paint-derived tributyltin on structure of estuarine nematode assemblages in experimental microcosms. J. Exp. Mar. Bio. Ecol.. 2002;272(2):217-235.
- [CrossRef] [Google Scholar]
- Effects of the intensity and frequency of organic enrichment on two estuarine nematode communities. Marine Ecol. Prog. Ser.. 1998;164:83-94.
- [Google Scholar]
- Determination of fluoroquinolone antibiotics in hospital and municipal wastewaters in Coimbra by liquid chromatography with a monolithic column and fluorescence detection. Anal. Bioanal. Chem.. 2008;391(3):799-805.
- [CrossRef] [Google Scholar]
- A review of Italian research on free-living marine nematodes and the future perspectives on their use as Ecological Indicators (EcoInds) Mediterr. Mar. Sci.. 2015;16(2):352-365.
- [CrossRef] [Google Scholar]
- Sorption of veterinary pharmaceuticals in soils: a review. Environ. Sci. Technol.. 2001;35(17):3397-3406.
- [CrossRef] [Google Scholar]
- Initial hazard screening for genotoxicity of photo-transformation products of ciprofloxacin by applying a combination of experimental and in-silico testing. Environ. Pollut.. 2016;211:148-156.
- [CrossRef] [Google Scholar]
- Analysis and sorption behavior of fluoroquinolones in solid matrices. Water Air Soil Pollut.. 2008;1–4:55-63.
- [CrossRef] [Google Scholar]
- Vanaverbeke, J., Bezerra, T.N., Braeckman, U., De Groote, A., De Meester, N., Deprez,T., Derycke, S., Gilarte, P., Guilini, K., Hauquier, F., Lins, L., Maria, T., Moens, T.,Pape, E., Smol, N., Taheri, M., Van Campenhout, J., Vanreusel, A., Wu, X., Vincx, M., 2015. NeMys: World database of free-living marine nematodes.
- pH-dependent ciprofloxacin sorption to soils: interaction mechanisms and soil factors influencing sorption. Geoderma. 2009;3–4:68-76.
- [CrossRef] [Google Scholar]
- Strategy to obtain axenic cultures from field-collected samples of the cyanobacterium Phormidium animalis. J. Microbiol. Methods. 2004;57(1):115-121.
- [CrossRef] [Google Scholar]
- Effect of ciprofloxacin on microbiological development in wetland mesocosms. Water Res.. 2011;45(10):3185-3196.
- [CrossRef] [Google Scholar]
- Benthic studies in buzzards bay II. The Meiofauna. Limnol. Oceanogr.. 1960;5(2):121-137.
- [CrossRef] [Google Scholar]
- Effect of ciprofloxacin on biological nitrogen and phosphorus removal from wastewater. Sci. Total Environ.. 2017;605-606:368-375.
- [CrossRef] [Google Scholar]
- The effects of ciprofloxacin on early life stages of common carp (Cyprinus carpio) Environ. Toxicol. Chem.. 2016;35(7):1733-1740.
- [CrossRef] [Google Scholar]







