Translate this page into:
Chitosan/silver nanocomposites enhanced the biofilm eradication in biofilm forming Gram positive S. aureus
⁎Corresponding authors at: Lab of Toxicology, Department of Health Sciences, The Graduate School of Dong-A University, 37, Nakdong-Dearo 550 Beon-Gil, Saha-Gu Busan, 49315, South Korea (Dr.M. Maruthupandy); Laboratorio de Nanocelulosa y Biomateriales, Departamento de Ingeniería Química, Biotecnología y Materiales, Facultad de Ciencias Físicas y Matemáticas, Universidad de Chile, Avenida Beauchef 851, 8370456 Santiago, Chile (Dr.G. Rajivgandhi). Rajivgandhimicro@yahoo.com (Govindan Rajivgandhi), maruthupandym@hotmail.com (Muthuchamy Maruthupandy)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Biofilm is the important virulence factors in bacteria to develop multi drug resistant effect against existing bacteria word wide. The eradication of biofilm is the important research recent years due to the increased mortality rate. For this, the current research was focused on inhibition of biofilm producing bacteria S. aureus using chitosan/silver nanocomposites by various invitro experiments. The result of anti-biofilm activity was shown 26 mm zone of inhibition against gram positive S. aureus after treatment with chitosan/silver nanocomposites combination at 24 h incubation. In addition, the biofilm adherent ability in 24-well polystyrene well plate result was exhibited 96 % biofilm degradation and viewed by after crystal violet stain wash. Importantly, biofilm survival rate was decreased to 97 % at 400 µg/mL concentrations, and it confirmed by liquid maintenance method. Furthermore, the complete exopolysaccharide damages were observed at 400 µg/mL concentrations, and confirmed by congo red agar assay. In congo red agar plate, the black color was absent in the biofilm forming S. aureus after 24 h incubation. It conveyed that the synthesized Chitosan/silver nanocomposites was very efficient nanomaterial to eradicate the biofilm formation in gram positive bacteria at concentration dependent manner.
Keywords
Chitosan
Silver nanoaprticles
Nanocomposites
Biofilm inhibition assay
Metabolic survival effect
Exopolysachharide damage
1 Introduction
Chitosan, poly [(1 → 4)-β-linked 2-amino-2-deoxy-d-glucose]is a N-deacetylated form of chitin. Chitin is insoluble while the chitosan is soluble in both organic and inorganic acids. The activity of chitosan is higher than the chitin because of the presence of the free primary amino group in its molecular chain (Ogawa et al., 2004). Chitin and chitosan have reported antibacterial, antifungal, antiviral and many other agriculture uses. Chitosan possess broad spectrum antibacterial activity and less toxicity towards mammalian cells (Liu et al., 2001). There are three functional group in chitosan there are amino group, primary and secondary hydroxyl groups at different positions (Rajivgandhi et al., 2019). Chitosan nanoparticles have various applications in tissue engineering, drug delivery systems, enzyme immobilization support, agriculture, antimicrobial agent, water treatment and cancer therapy. Most of the studies in chitosan loaded silver nanoparticles reported for using it as a medical agent (wound dressing material) because of anticoagulant, antibacterial, antiplatelet and thrombolytic activities (Rajivagndhi et al., 2021; Zhou et al., 2021).
Biofilm can be formed as a well-organized community of microorganisms. In biofilm very most of the part is composed of extracellular materials called as exopolysaccharides (EPS). Biofilm can form in mostly all kind of surface (Rajivagndhi et al., 2020; Kokare et al., 2009;). Biofilm formation is only caused due to the facilitation of survival in microbes. Some Gram negative bacteria like P. aeruginosa, P. fluorescens, some strain of E. coli and V. cholerae can form biofilm (Maruthupandy et al., 2019; Kayalvizhi et al., 2022). Graphene chitosan nanocomposites have antibiofilm activity against P. aeruginosa and K. pneumoniae in very low concentration and more efficiently than graphene alone.
2 Materials and method
2.1 Collection and processing
The nanomaterial of chitosan/silver nanocomposites was obtained from Dr. Muthuchamy Maruhtupandy, Laboratorio de Nanocelulosa y Biomateriales, Departamento de Ingeniería Química, Biotecnología y Materiales, Facultad de Ciencias Físicas y Matemáticas, Universidad deChile, Avenida Beauchef 851, 8370456 Santiago, Chile for check the anti-biofilm ability. This material and their characterization with biosensor capacity were already published by Maruthupandy et al. (2019). In this current study and their impact of chitosan/silver nanocomposites against biofilm forming S. aureus procedure was effectively indicated in Fig. 1.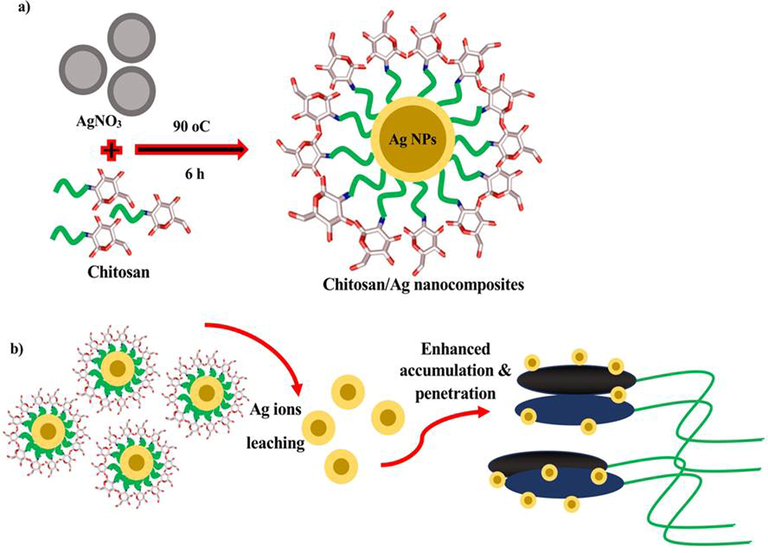
Schematic representation of (a) chitosan/silver nanoparticles preparation and (b) prepared nanocomposites against biofilm forming S. aureus.
2.2 Anti-biofilm evidences against biofilm forming S. aureus
The current study of chitosan/silver nanocomposites was performed against biofilm forming S. aureus using agar well diffusion method. Briefly, 12 h culture of S. aureus was streaked on the well solidified nutrient agar surface properly. After 5 min time interval, the wells were cut into the pathogen streaked agar surface using gel borer and followed by inoculated the chitosan/silver nanocomposites at the concentration of 10–100 µg/mL. Then, the plate was put into the incubator at room temperature for one day. After one day, the zones around the tested wells were measured in diameter.
2.3 Calculate the adherent and non-adherent assay
The result of Palanisamy et al. (2014), was helped to performed this work. In short form, the nutrient broth of 100 µg/mL and 100 µg/mL of 24 h and 48 h S. aureus cultures were taken together into the 96-well plate and then 10–100 µg/mL of inhibition concentrations of chitosan/silver nanocomposites was added into the wells except control well. All the materials of the wells were diluted, mix thoroughly and put on room atmosphere for 1 day. Then, the formed biofilm of adherent cells on the treated and non-treated wells were calculated on 600 nm O.D. Based on the turbidity of adherent cells on the treated and non-treated wells O.Ds were compared each other for detection of biofilm inhibition concentration (BI) used bellowed equation,
2.3.1 Biofilm metabolic property of chitosan/silver nanocomposites
Based on the viability nature of biofilm cells, the complete inhibition effect of chitosan/silver nanocomposites against viability of biofilm cells in various concentration was analyzed by XTT (2,3-bis (2-methoxy-4-nitro-5-sulfophenyl)–2H-tetrazolium-5- carboxanilide) solution based experiment. The 24 h chitosan/silver nanoparticle treated S. aureus culture was used and 1 mL of XTT solution was added into the wells. Then, the plates were kept an incubator 1 h with 37 ℃. After, required amount of menadione acetate solution was taken and added into the wells before filled by 1X PBS. All the mixture of the sample was shaken gradually and put into the incubator after clear mix for 1 h time duration. Finally, the formed and unformed results of the treated and untreated wells were read on UV-spectrometer at 540 nm. Then, the calculation was made after compared the control and tested values.
2.4 Structural deformation of chitosan/silver nanocomposites
The exopolysaccharides removal or non-removal effect of chitosan/silver nanocomposites was detected with the help of phenol sulfuric acid solution. The biofilm degradation of S. aureus cells using effective BI concentration of chitosan/silver nanocomposites was used to centrifuge at 6,000 rpm for 5 min. Then, the pellet was purified separately form mixture culture and treated by 25 µL of pronase E and vortex the sample properly. Then, the sample was maintained at 50 ℃ for 10 min, and 100 µL of ice precipitated protein treated trichloroacetic acid was used to inoculate into the sample. Then, all the reaction mixture of the samples were recentrifuged at 3,000 rpm for 5 min, and then used 10 mL of cold absolute alcohol in drop wise mode to collect the polysaccharides. Then, 98 % of H2SO4 by distilled water was added into the sample and vortex. Then, the sample was put in water bath 10 min and cooled used ice box. Finally, the mixture sample was analyzed by UV-spectrophotometer at 540 nm, as same as glucose as a standard control. Also, distilled water was acted as an experimental blank and used for comparison (Rajivgandhi et al., 2020). The percentage of exopolysaccharides damages were calculated based on the bellowed formula,
2.5 Confirmation of exopolysaccharides degradation
The antigenicity and pathogenicity variations between the control and chitosan/silver nanocomposites treated S. aureus through exopolysaccharides was confirmed by nutrient agar plate method (Ansari et al., 2014). Briefly, the after complete the process of phenol sulfuric acid solution, the control and treated plates were streaked on the nutrient agar plate, and also congo red agar plate for detection of antigenicity and pathogenicity of S. aureus. Then, all the plates were kept into the 37 ℃ set up incubator for 12 h and 24 h. After 12 h, the antigenicity was confirmed based on the growth of nutrient agar based on the growth, and pathogenicity was confirmed based on the color variation. Weather the black color turned to pink in chitosan/silver nanocomposites treated plate was indicated as result was very effective against S. aureus biofilm formation. Contrary, black color formation of control plate was used to confirm the treated plate result.
3 Result
3.1 Anti-biofilm evidences against biofilm forming S. aureus
In result, the zones were produced around the treated wells of biofilm forming S. aureus plate and it confirmed that the chitosan/silver nanocomposites has excellent anti-bacterial property. The zones of 10 mm, 16 mm and 26 mm were observed against 50 µg/mL, 100 µg/mL and 250 µg/mL concentration of chitosan/silver nanocomposites (Fig. 2a, b). Therefore, the result was more supported to the obtained chitosan/silver nanocomposite material with excellent anti-bacterial activity against biofilm producing S. aureus. In addition, the inhibition ranges were differed based on the concentration of the nanomaterial. When, we use the lowest inhibition of chitosan/silver nanocomposites material, the inhibition effect was also very low. In 250 µg/mL concentration, the excellent zone of inhibition was shown against S. aureus. It was suggested that the chitosan/silver nanocomposites inhibit the biofilm bacteria at concentration dependent inhibition mode. This performance was further confirmed in the adherent 24-well polystyrene plate. Similarly, the chitosan/silver nanocomposites has very efficient anti-bacterial role against various bacteria was reported by Maruthupandy et al. (2020). Recently, Lopez-Carrizales et al. (2020) was agreed the current research and chitosan/silver nanocomposites was very effective against biofilm bacteria. Previously, Ionescu et al. (2015), stated that the chitosan/silver nanocomposites was very efficient due to the nature of the chitosan.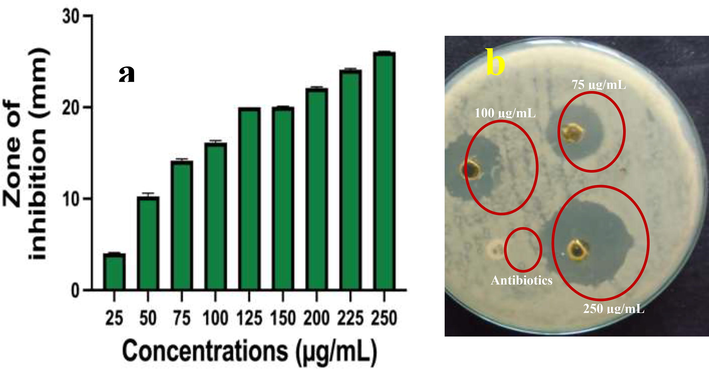
Anti-bacterial activity of chitosan/silver nanocomposites against biofilm forming S. aureus at different concentration (a, b).
3.2 Calculate the adherent and non-adherent assay
Based on the chitosan efficiency, the silver nanoparticles was delivered high efficiency into the bacterial cells and deactivates the virulence factors in bacteria. After careful comparison between the control and treated O.D values, the chitosan/silver nanocomposites started their inhibition at 25 µg/mL concentration. In this concentration, the bacteria were lost their cell wall production and organelles (Shah Izhar, et al., 2018; Hernández-Rangel et al., 2019). Mechanistically, the electrostatic bond between the bacteria and chitosan/silver nanoparticles were bind each other, and chitosan/silver nanocomposites entered and acted easily within the bacterial cells. Consecutively, the cells were damaged and lost their cells cycle process, it leads to death. In addition, the positive charges of the chitosan/silver nanocomposites easily attracted by negatively charged bacterial surface, after clumped each other, the chitosan/silver degrade the cell wall and disconnect the contact between the groups of organisms. All the virulence factors were inactivated and bacteria undergone to decline phase and lead to complete death. In addition, the inhibition percentage of 250 µg/mL was very perfect against S. aureus biofilm formation and shown 96 % cell death. Evidently, the various concentration and their increased inhibition abilities were effectively shown in Fig. 3. This result was agreed by anti-bacterial activity result and 250 µg/mL concentrations were fixed as a biofilm inhibition (BI) concentration. This statement was agreed by dos Santos et al. (2021), and chitosan/silver nanocomposite was very efficient. Recent reports of Aguayo, et al. (2020); Regiel-Futyra et al. (2017) published that the chitosan has very efficient biodegradable ability for any drug molecules and it may delivered the silver nanoparticles highly. The bioavailability, excellent surface area, biodegradation efficiency of chitosan is suitable carrier molecules for any drug, antibiotics, nanoparticles, plant materials and others (Maruthupandy et al., 2020) Biofilm metabolic property of chitosan/silver nanocomposites.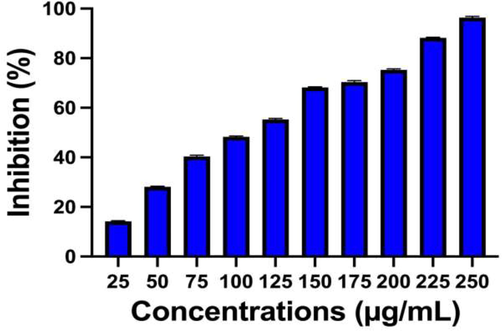
Find out the anti-biofilm inhibition concentration using adherent plate assay method against biofilm forming S. aureus at different concentration of chitosan/silver nanocomposites.
After seen in the 96-well plate, the more turbidity was shown in chitosan/silver nanocomposites treated wells when compared with untreated control wells. In biofilm degradation, the detection of metabolic activity is one of the important factors, because it is targeting the virulence factors. In this step only, the bacteria maintain their log phase for produced responsible enzymes, more signaling molecules production, ROS level variation, exopolysaccharide production, induced or suppressor cells activation. In log phase, the chitosan/silver nanocomposites acted very efficiently, and stopped bacterial virulence’s and regenerative molecules in inside of the bacteria. As same as the present work was highly suitable for biofilm inhibition using chitosan/silver nanocomposites. In this study, the selected material was stopped the bacterial survival in high rate, the rate was shown 97 % at 400 µg/mL concentrations. The survival rate was started at 75 µg/mL only. Because, the bacteria was developed more different layers around the biofilms, and it was lost their biofilm forming ability at increasing concentration. The various concentrations and their survival inhibition effect of biofilm formation were shown in Fig. 4. The current evidence was accordance with recently published report of Zienkiewicz-Strzałka et al. (2020), and biofilm metabolic activity is the important step in biofilm forming bacteria. The reported result good agreement with previously published result of biofilm metabolic activity using chitosan/silver nanocomposites (Ansari et al., 2014). In addition, Rajivagndhi et al. (2020) reported that the use of XTT solution in the experiment for detection of biofilm degradation, survival rate decrease was very important because of liquid media. Therefore, the current result of chitosan/silver nanocomposites against biofilm forming S. aureus was more suitable.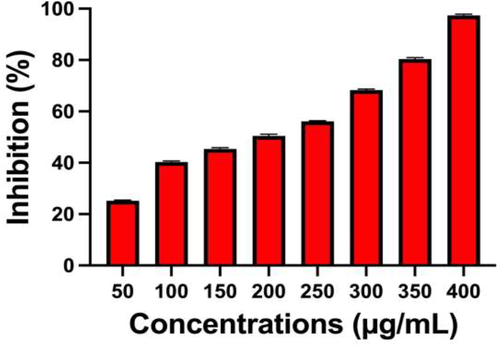
Find out the survival of biofilm forming S. aureus by XTT solution of biofilm metabolic activity assay using chitosan/silver nanocomposites.
3.3 Structural deformation of chitosan/silver nanocomposites
In bacteria, exopolysachharide is the physical barrier and it helped to form the biofilm perfectly (Badawy et al., 2019). Therefore, the target site of exopolysaccharide in biofilm producing bacteria is the better choice to complete eradication of biofilm formation (Aguayo et al., 2020). In addition, when the foreign particles enter into the bacteria through cell wall, the exopolysaccharide layers were neutralized the foreign particles and deactivated efficiently. The colloidal nature of the bacterial surface material did not allow the foreign particles in intracellular level of the bacteria body. It covered the bacteria like a fence type and the bacterial virulence factors production was continuously synthesis, and other polymeric substances were also synthesized highly. So, targeted in the exopolysaccharide is the excellent choice in biofilm inhibition research, recent reports were published based on the exopolysaccharide inhibition. In our study, the chitosan/silver nanocomposites was efficiently targeted the exopolysaccharide layer and cleaved that layers efficiently. After cleaved, the production of amino acids, proteins, nucleic acids, enzymes and other bacterial growth factors were stopped. For this, we have found more membrane damages; intracellular granule leakages and other microbial materials leakages were observed. Then, the inhibition rate was very high compared with untreated control. The inhibition rate was started on the concentration of 100 µg/mL only. It was depends on the effect of polysaccharide content of the bacteria. Because, exopolysaccharide has more polysaccharide in their molecules and leads to thick and rigid fence layer in and around the nucleus, and also DNA of the bacteria. Previously, the researcher of Hemmati et al. (2020), reported that the chitosan/silver nanocomposites exhibited more inhibition against exopolysaccharide. Based on this approach, the current study result was exhibited that the chitosan/silver nanocomposites has more inhibition ability against exopolysaccharide production. The inhibition percentage was shown at 500 µg/mL concentration with 98 % inhibition against biofilm forming S. aureus. The result was most accordance with previous result of Ansari et al. (2014), and chitosan/silver nanocomposites is an efficient material to inhibit the exopolysaccharide and arrested the flagella movement and slime production particles. All the inhibition percentages based on the increasing concentration was shown in Fig. 5.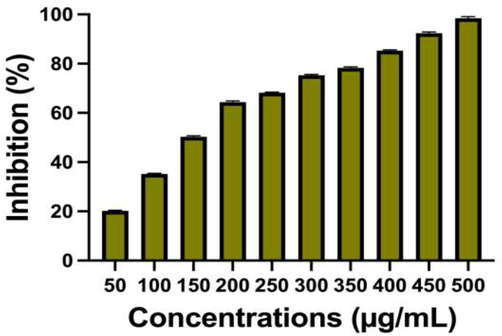
Decrease the exopolysaccharide production in biofilm forming S. aureus by chitosan/silver nanocomposites at different concentration.
3.4 Confirmation of exopolysaccharide degradation
Based on the inhibition effect of chitosan/silver nanocomposites efficiency against biofilm producing S. aureus through liquid inhibition assay, biofilm metabolic activity and exopolysaccharide degradation, the present method was clearly supported whether the above said results were true or not. In this method, the antigenicity of the biofilm producing bacteria was proved by nutrient agar medium, and pathogenicity was confirmed by congo red agar medium assay. In result of nutrient agar assay, the streaked liquid medium of XTT solution treated wells shown absence of growth at 250 µg/mL concentration (Fig. 6b). In contrast, the control well of the solution streaked nutrient agar plate shown with excellent growth (Fig. 6a). It was clearly confirmed that the growth was arrested by chitosan/silver nanocomposites.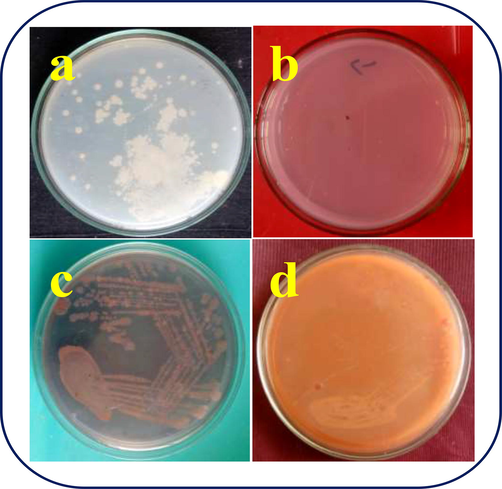
Confirmation of biofilm destruction of S. aureus by nutrient agar and congo red agar plate method using chitosan/silver nanocomposites.
In addition, the congo red agar plate of control shown black color colonies were shown in streaked plate after 24 h incubation (Fig. 6c). In addition, after biofilm damage, the growth was shown pink color colonies, that confirmed that the pathogenicity was lost, but antigenicity was remain withstand (Fig. 6d). For this, we taken the sample of exopolysaccharide treated solution treated solution. In 500 µg/mL, the entire bacteria and biofilm formation was arrested, and it conveyed that the chitosan/silver nanocomposites was efficient nanomaterial to inhibit the biofilm formation.
Mechanistically, exopolysaccharide is the prime factor in the biofilm formation, because it was acted as a protective layer that is constituents of extracellular biofilm matrix. It was helped to develop the biofilm organelles within the bacteria, and helped to the anchoring the microbes to surface. Covering the biofilm within the exopolysaccharide is very safe and protect. Due to this reason, any foreign particles, drugs and antibiotics entered into the outer layer, the exopolysaccharide acted as a barrier and weakened the antibiotic efficiency. In addition, antibiotic was inactive and absence of inhibition ability against biofilm forming bacteria. Therefore, the inhibition of exopolysaccharide in biofilm forming bacteria is the important target to eradicate the biofilm formation in bacteria. In biofilm formation, the exopolysaccharide produced genes were deactivated the all the inhibition factors. In congo red agar plate, if the antibiotics or drugs or nanoparticles inhibited the biofilm producing cells within the exopolysaccharide, the black color producing biofilm cells were absent. So, we could conclude that the antibiotics were disrupted the exopolysaccharide and killed the biofilm forming bacteria. In addition, the non-biofilm forming bacteria produced the pink color colonies in congo red agar due to the absence of exopolysaccharide presence. Above mentioned reason, the detection of exopolysaccharide inhibition suing antibiotics, nanoparticles or drugs by congo red agar assay is more important study in biofilm inhibition.
4 Conclusion
Chitosan is an excellent natural polymer to help as a carrier molecules for many sources like, antibiotics, bioactive compounds, essential oils and nanoparticles. In this research, the silver nanoparticle was loaded into the chitosan for inhibit the biofilm forming S. aureus. As exhibited result was very favor to the selected material and it inhibited the biofilm forming bacteria at lower concentration. In increasing concentration of chitosan/silver nanocomposite, the bacterial growth damage, survival arrest, membrane deformation, exopolysachharide alterations were observed. In addition, the selected nanomaterial was effectively interfering the biofilm forming S. aureus by antigenicity and pathogenicity mode of action. Therefore, the current result was confirmed that the selected nanomaterial of chitosan/silver nanocomposites was more effective nanomaterial against biofilm forming S. aureus.
Acknowledgement
G.Rajivagndhi and Franck Quero acknowledge the financial support from ANID-FONDECYT (Chile) under the Postdoctoral Fellowship No. 3220019. The authors extend their appreciation to the Researchers Supporting Project number (RSP2023R70), King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Antimicrobial and antibiofilm capacity of chitosan nanoparticles against wild type strain of pseudomonas sp. isolated from milk of cows diagnosed with bovine mastitis. Antibiotics. 2020;9(551):1-15.
- [Google Scholar]
- Antimicrobial and antibiofilm capacity of chitosan nanoparticles against wild type strain of Pseudomonas sp. isolated from milk of cows diagnosed with bovine mastitis. Antibiotics. 2020;9(551):1-15.
- [Google Scholar]
- Gum arabic capped-silver nanoparticles inhibit biofilm formation by multi-drug resistant strains of Pseudomonas aeruginosa. J. Basic Microbiol.. 2014;54:688-699.
- [Google Scholar]
- Preparation and antibacterial activity of chitosan-silver nanoparticles for application in preservation of minced meat. Bull. Nat. Res. Centre. 2019;43(83):1-14.
- [Google Scholar]
- Silver nanoparticles–chitosan composites activity against resistant bacteria: tolerance and bioflm inhibition. J. Nanopart. Res.. 2021;23:196.
- [Google Scholar]
- Fabrication and in vitro behavior of dual-function chitosan/silver nanocomposites for potential wound dressing applications. Mater. Sci. Eng.: C. 2019;94:750-765.
- [Google Scholar]
- Silver–polysaccharide antimicrobial nanocomposite coating for methacrylic surfaces reduces Streptococcus mutans biofilm formation in vitro. J. Dent.. 2015;43:1483-1490.
- [Google Scholar]
- Kayalvizhi, K., Alhaji, N,M,I., Saravanak kumar, D., Beer Mohamed, S., Kaviyarasu, K., Ayeshamariam, A., Amal, M.Al-M., Mohamed Ragab, A.G., Mohamed, S.E., Adsorption of copper and nickel by using sawdust chitosan nanocomposite beads – A kinetic and thermodynamic study, Environmental Research, 203, 2022, 111814.
- Antibacterial action of chitosan and carboxymethylated chitosan. J. Appl. Polym. Sci.. 2001;79:1324-1335.
- [Google Scholar]
- Characterization, antibiofilm and biocompatibility properties of chitosan hydrogels loaded with silver nanoparticles and ampicillin: an alternative protection to central. Colloids Surf. B: Biointerfaces. 2020;196:111292
- [Google Scholar]
- M. Maruthupandy, G. Rajivgandhi, S. Kadaikunnan, T. Veeramani, N.S. Alharbi, T. Muneeswaran, J.M. Khaled, W.J. Li, K.F. Alanzi, Anti-biofilm investigation of graphene/chitosan nanocomposites against biofilm producing P. aeruginosa and K. pneumonia, Carbohydrate Polymers, 230, 2020, 115646.
- Chitosan/silver nanocomposites for colorimetric detection of glucose molecules. Int. J. Biol. Macromol.. 2019;121:822-828.
- [Google Scholar]
- Antibiofilm properties of chemically synthesized silver nanoparticles found against Pseudomonas aeruginosa. J. Nanobiotechnol.. 2014;12:1-7.
- [Google Scholar]
- G. Rajivgandhi, G. Ramachandran, M. Maruthupandy, N. Manoharan, N.S. Alharbi, S. Kadaikunnan, J.M. Khaled, T.N. Almanaa, W-J. Li, Anti-oxidant, anti-bacterial and anti-biofilm activity of biosynthesized silver nanoparticles using Gracilaria corticata against biofilm producing K. pneumonia, Colloids and Surfaces A: Physicochemical and Engineering Aspects, 600, 2020, 124830.
- Anti-ESBL investigation of chitosan/silver nanocomposites against carbapenem resistant Pseudomonas aeruginosa. Int. J. Biol. Macromol.. 2019;132:1221-1234.
- [Google Scholar]
- Enhanced anti-cancer activity of chitosan loaded Morinda citrifolia essential oil against A549 human lung cancer cells. Int. J. Biol. Macromol.. 2020;164:4010-4021.
- [Google Scholar]
- Anti-oxidant, anti-bacterial and anti-biofilm activity of biosynthesized silver nanoparticles using Gracilaria corticata against biofilm producing K. pneumonia. Colloids Surf. A. 2020;600:124830
- [Google Scholar]
- Physiochemical characterization and anti-carbapenemase activity of chitosan nanoparticles loaded Aegle marmelos essential oil against K. pneumoniae through DNA fragmentation assay. Surfaces Interfaces. 2021;23:100932
- [Google Scholar]
- Development of noncytotoxic silver–chitosan nanocomposites for efficient control of biofilm forming microbes. RSC Adv.. 2017;7:52398.
- [Google Scholar]
- Chemical synthesis and characterization of chitosan/silver nanocomposites films and their potential antibacterial activity. Int. J. Biol. Macromol.. 2018;116:520-529.
- [Google Scholar]
- Antibacterial and wound healing–promoting effect of sponge-like chitosan-loaded silver nanoparticles biosynthesized by iturin. Int. J. Biol. Macromol.. 2021;181:1183-1195.
- [Google Scholar]
- Silver nanoparticles on chitosan/silica nanofibers: characterization and antibacterial activity. Int. J. Mol. Sci.. 2020;21:166.
- [Google Scholar]







