Translate this page into:
Chitosan loaded plant essential oils efficiently eradicate the multi-drug resistant bacterial infection and lung cancer cells
⁎Corresponding authors at: State Key Laboratory of Biocontrol, Guangdong Provincial Key Laboratory of Plant Resources and Southern Marine Science and Engineering Guangdong Laboratory (Zhuhai), School of Life Sciences, Sun Yat-Sen University, Guangzhou 510275, PR China (W.-J. Li); Department of Marine Science, Bharathidasan University, Tiruchirappalli- 620024, Tamil Nadu, India (G. Ramachandran). ramachandhiranmicro@gmail.com (Govindan Ramachandran), liwenjun3@mail.sysu.edu.cn (Wen-Jun Li)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
In present research, of chitosan loaded plant essential oils (CsEOs) were performed against Pseudomonas aeruginosa (P. aeruginosa), Escherichia coli (E.coli), K. pneumoniae and Staphylococci aureus (S. aureus), also the anti-cancer properties were evaluated against A549 lung cancer cells. Consequently, the CsEOs against P. aeruginosa, E.coli, K. pneumoniae and Staphylococci aureus were shown 24 mm, 22 mm, and 26 mm and 22 mm zones of inhibition respectively. In addition, 500 µg/mL of CsEOs exhibited 92, 89, 88 and 94% of inhibition against P. aeruginosa, E.coli, K. pneumoniae and S. aureus were observed respectively using micro broth dilution broth experiment. Further, cytotoxicity ability of CsEOs against A549 human lung cancer cells were monitored by 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide (MTT) assay, and result of 500 µg/mL concentration was exhibited 58% inhibition against A549 lung cancer cells. Furthermore, the microscopic evidence of 500 µg/mL concentrations of CsEOs treated A549 lung cancer cells was showed irregular morphology in phase contrast microscope. Altogether, both the invitro and morphological experiments of anti-bacterial and anti-cancer study results were conveyed that the CsEOs have excellent anti-bacterial and anti-cancer properties. Finally, the entire study was proposed, the chosen CsEOs was excellent bionanomaterial used to deliver the drugs and bioactive compounds in target sites for inhibit the infectious pathogens and cancer cells.
Keywords
Chitosan
Essential oils
Encapsulation
Invitro inhibition study
Anti-microbial activity
Cytotoxicity assay
1 Introduction
Chitin and chitosan are polysaccharides composed of N-acetylglucosamine (GlcNAc) and glucosamine (GlcN). Chitin is one of the most abundant biopolymer which is highly stable in nature (Mourya and Inamdar, 2008; Kanimozhi et al., 2018). Chitosan is a derivative formed by deacetylation of chitin. Initially in chitosan, membrane studies were done because of their properties (Candy and Sharma, 1990). Essential oils (EOs) from plants have been used in folk medicines. EOs primarily consist of terpenoids like monoterpenes, diterpenes, sesquiterpenes and other molecules aldehydes, acids, lactones, sulphur-containing compounds and homologues of phenylpropanoids (Nalini et al., 2019; Kanimozhi et al., 2019). Essential oils can inhibit or reduce the growth of bacteria, moulds and yeast. In addition, it also neutralises the toxic bacterial metabolites (Bakkali et al., 2008; Nazzaro et al., 2013). Essential oils components are convertible by isomerization, oxidation, and dehydrogenation reaction induced chemically or by enzymatically (Kanimozhi et al., 2018). Stability of the essential oils is determined by the presence of several chemicals and edaphic factors. In lemon oils, the amount of geranial, terpinolene will rise in p-cymene is observed (Turek and Stintzing, 2013; Kanimozhi et al., 2019). Satureja montana essential oils exhibited antimicrobial activity against all gram positive and gram negative microbes and fifty-eight clinical oral Candida sp. (Chouhan, Sharma and Guleria, 2017; Kayalvizhi et al., 2022). According to the available data and most of the studies represents that the gram positive bacteria shows easily destroyed bacteria in essential oils treatment (Seow et al., 2014; Wińska et al., 2019). Combination of chitosan and essential oils may provide exponential properties for antimicrobial activity with specificity. Recent report of Zhang et al., 2020 reported with excellent anti-bacterial activity of plant essential oils against some gram negative bacteria (Zhang et al., 2020). The two step nanoencapsulaiton process for preparation of essential oils loaded chitosan nanoparticles are oil-in water emulsification and ionic gelation method (Narmatha Christy et al., 2020; Bus et al., 2020). The synergetic effects of chitosan and essential oils have activity against S. aureus, S. epidermidis, E. coli and P. aeruginosa. Chitosan coating also worked as a reinforcement and improving flexibility of the electrospun membranes containing essential oils (Milanesi et al., 2021). M. piperita essential oil-chitosan nanoparticles showed a down regulation effect on eight genes involved in biofilm and virulence factors causing bacterial adherence (Ashrafi et al., 2019). A Morinda citrifolia essential oil chitosan (MCEO-CHs) nanoparticle exhibits excellent cytotoxicity activity against A549 lung cancer without damaging the red blood cells. Chitosan inclusion increases the cytotoxicity along with the biocompatibility (Rajivgandhi et al., 2020).
Above said advantages, this research was highly motivated with CsEOs to treat multi drug resistant bacteria and cancer cells through invitro analysis. The shematic representation of chitosan loaded palnt essential oils interaction between the MDRs bacteria and cancer cells (Fig. 1).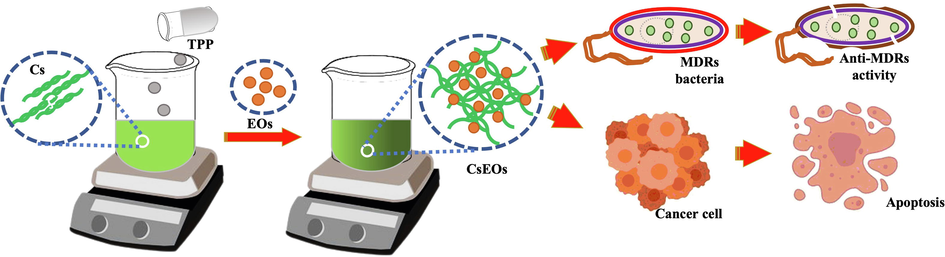
Structural arrangement of chitosan nanoparticles loaded plant essential oils and their biomedical applications.
2 Materials and methods
2.1 Antimicrobial properties against various pathogens
Previously, the chitosan nanoparticle synthesis, essential oils extraction from the medicinal plant of Solanum nigrum, and their spectroscopic characterizations were effectively published in our previous reports (Khaled et al., 2021). Based on the previous reports, the CsEOs has more biofilm eradication property against biofilm producing P. mirabilis. For this advantage, CsEOs sample was obtained to complete inhibition of bacterial growth. The procedure of De La Cruz Sanchez et al. (2019) was also helped to this experiment, and followed by the protocol of agar well diffusion method using with spread the 48 h old cultures of P. aeruginosa, E.coli, P. aeruginosa and S. aureus solidified nutrient agar media containing plate. Then, cut the 4 wells for add different concentration of CsEOs. For the antimicrobial resistant behavior of selected pathogens were confirmed by third generation antibiotic disc ceftazidime. All the plates were monitored under ordinary room temperature using 12–24 h time interval. After this time duration, the zones formed wells on the agar surface of the plates were noted clearly and noted.
2.2 Micro broth dilution method
The antimicrobial abilities of the CsEOs used to cross check, and confirmed their inhibition role against tested pathogens was monitored under liquid medium treatment process by 96-well plate method (Feng et al., 2020). In this method, 100 µl, 10 µl and 100–500 µl of muller hinton broth, 24 h pathogens and CsEOs were taken one by one in 96-well plate. The plate was gently shake and should not maintain the leakage of the solutions. Then, the sample was put into 37 ℃ maintaining incubator and monitored from 6 h to 24 h. After 6–24 h time interval, the turbidity levels were checked every time and noted. Finally, how much content of turbidity was identified in treated plates wells and also untreated plates wells. Based on the UV-spectroscopy analysis of treated result was compared with control result in percentage mode at 640 nm and calculated the percentage using bellowed formula,
2.3 Cytotoxicity effect against lung cancer cells using essential oils
The cytotoxicity nature of CsEOs and their ability against A549 lung cancer cells were detected by dimethylthiazol-diphenyltetrazolium bromide assay followed by effective methodology of Govindan et al. (2020). Initially, the amount of ∼ 2 × 104 cells of A549 was taken in previously filled DMEM medium of 96-well plate and monitored in room temperature with one day under the CO2 influence at 5% and 95% humidity. Consecutively, add different concentration 50–500 µg/mL of CsEOs into all the wells except control well. In room atmospheric temperature of 37 ℃ was set in CO2 incubator and stored in same incubator 24 h for detachment process. After detachment treatment process, the 25 µl of MTT solution was added gradually in all the wells and then diluted by PBS. Next, covered by aluminum foil and maintained under room atmosphere for 4–5 h. Then, the plates were taken and cooled for find the crystal formazan present in the treated well or not. The untreated control and treated wells were clearly monitored in naked eye and confirmed the result based on the turbidity variation. After, the clear color intensity variations of the plates were confirmed by spectrometer analysis using 540 nm O.D values. Based on the interpretation, the IC50 value was identified and noted,
The untreated control well was considered as 100% due to the well grown culture, and interpret the result with control result. Last, IC50 value of inhibition effect in the Morinda citrifolia extract was found based on the equation result.
3 Result
3.1 Bacterial inhibition by well diffusion method
After one day, CsEOs was performed against various pathogens, the zones were clearly formed around some of the bacteria and varied zones were also formed in some of the bacteria. In result, the 24 mm, 22 mm and 26 mm zones around the wells of chitosan nanoparticle loaded nanoparticles against P. aeruginosa (Fig. 2a), E. coli (Fig. 2b) and K. pneumoniae (Fig. 2c) were observed in respective plates at 500 µg/mL concentration. In addition, 18 mm zone of inhibition against S. aureus of the extract was also found after 24 h (Fig. 2d). Also, the inhibition zone based on the different concentration result was also exhibited in Fig. 2d. Therefore, the biological properties of the CsEOs were evidently suggested, bacteria were entirely killed. It also favored for MDRs bacteria and closely related to similarly reported researchers (Soltanzadeh et al. (2021); Soltanzadeh et al. (2021). As it has the inhibition ability against both GPB and GNB, the CsEOs is the important molecule in drug discovery studies. The evident of Hassan et al. (2021) was more similar to this result. Also, the CsEOs is the important research work against MDRs pathogens and it can be used as a future drug discovery candidate molecule. Based on the recent reports, CsEOshave excellent anti-microbial activity and anticancer activities. Because, the plant extracts, secondary metabolites, hormones and various other contents were involved to inhibit the infectious pathogens (Nidal et al., 2020; Anita et al., 2022). Also, chitosan acted as an effective polymer used to improve the efficiency of EOs against pathogenic bacteria and lung cancer cells (Xiqiang et al., 2023; Anush et al., 2023). Also, chitosan as an excellent drug candidate for various biomedical applications, they are bactericidal (Rajivgandhi et al., 2021), anti-biofilm (Yang et al., 2020; Khaled et al., 2021) and anti-cancer activities (Rajivagndhi et al., 2020)). Chitosan itself has excellent anti-bacterial and anti-cancer properties against various diseases. Therefore, CsEOsas an excellent alternative drug to eradicate bacterial infections and lung cancer diseases (Ramachandran et al., 2020; Lin et al., 2019).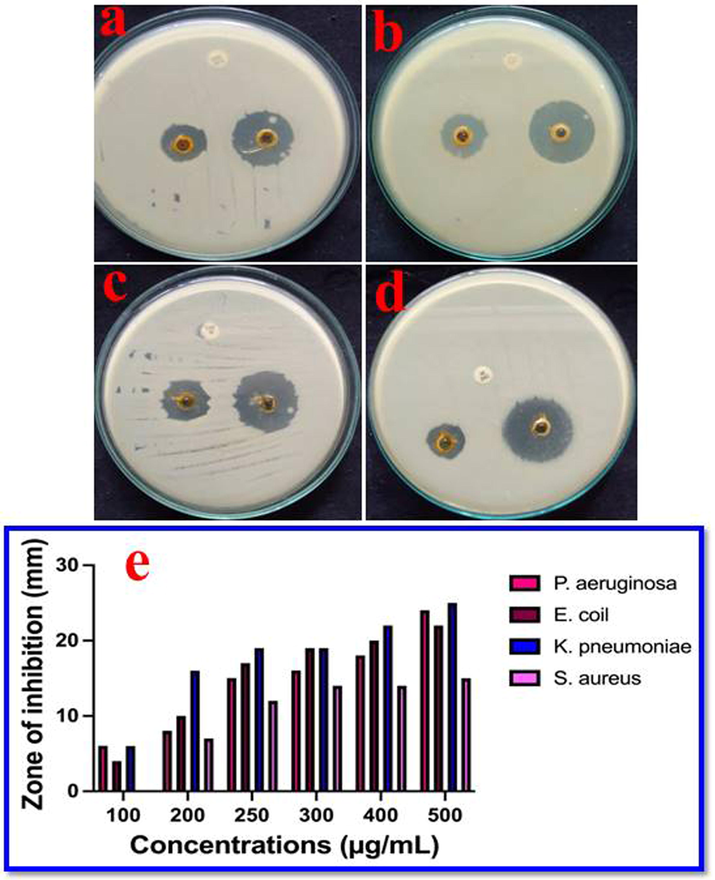
Anti-microbial activity of chitosan nanoparticles loaded plant essential oils against various bacteria at increasing concentration. The zone of inhibition results against P. aeruginosa (a), E. coli (b), K. pneumoniae (c), S. aureus (d) and differentiation of increasing concentration of chitosan nanoparticles loaded plant essential oils against tested bacteria was available (e).
3.2 Micro broth dilution method
The consecutive biological properties identification of CsEOs and their effective nature was confirmed by microbroth dilution method. Based on the O.D value of this study proved, EOs as an effective antimicrobial agent for pathogenic bacteria. It was effectively confirmed by various time intervals. The time interval of 6 h exhibited 50% death cells in 500 µg/mL CsEOs. In addition, the 300 µg/mL of EOs effectively inhibit the pathogens with percentages of 65, 71, 60 and 58%. The initial inhibition was started at 100 µg/mL concentration only. Interestingly, 12 h and 24 h of the time interval is more relevant to this study and inhibit the bacterial growth stage by stage. At 12 h incubation, the complete inhibition of growth was observed in 500 µg/mL concentration. The range of inhibition percentages were 66, 65, 60 and 58 at 250 µg/mL concentration. As same as, at the time interval of 24 h, the inhibition was started at 25 µg/ml concentration and half inhibition percentage was started at 200 µg/mL concentration. Surprisingly, the highest inhibition rate of 92, 89, 88 and 94% of death cells were observed at 500 µg/mL. Current result of CsEOs treatment was influenced bacterial growth, and the growth went undergone the decline phase. Based on the microbroth dilution result, highest number of cell death with decreased dose of CsEOs was shown at 250 µg/mL (Fig. 3). Hence, the minimum inhibition concentration was fixed at 250 µg/mL concentration, and this concentration can be used efficiently in future study. The result was highly correlated with previous report of CsEOs study result. Recent researchers of Barani et al., 2014; Rajivagndhu et al. (2021); Junfeng et al. (2016) also agreed this statement and confirmed CsEOs, and the EOs rich plant of Solanum nigrum is the important phyto plant to active against various infectious diseases. More number of essential oils were present in the selected plant, and these all available in previously published report (Khaled et al., 2021), notably cubenol, a-pinene, a-terpineol, P-aucubin, a-terpinolene, a-pinene, lscopoletin, Β-farnesene, a-terpenyl, Β-asarone, sabinene, a-terpineol, bpinene are present in the plant.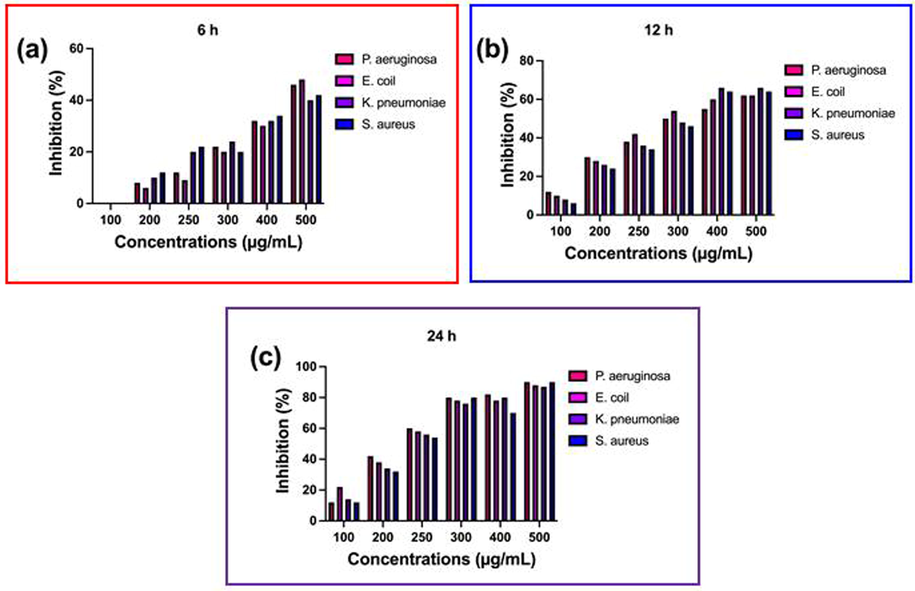
Minimum inhibition concentration of chitosan nanoparticles loaded plant essential oils against selected multi drug resistant pathogens at different concentration.
3.3 Inactivation of A549 lung cancer cells
Based on the observation of MTT assay, the highest concentration of 500 µg/mL was excellent dose against A549 cells. The most confluent layer was formed in treated wells, especially at 250 µg/mL concentration, the IC50 concentration was nearly 56% (Fig. 4). The clear cytocompatibility and decreased growth of A549 lung cancer cells were observed in increasing concentration of CsEOs. In addition, the proliferation effect was very high compared with any other plant EOs (Suthagar Pillai, et al., 2012). Recent evidence result produced by Hesami et al., (2022), and it correlated with present result. EOs of ɑ-norenone, L-scopoletin, nordamnacanthal and morindadiol may influence the high level of apoptosis through ROS production and mitochondrial damages. Previously, Campos et al. (2017); Jaya Kumar et al. (2012) also evidenced that the bioactive compounds of Morinda citriflia was influenced the necrosis process and lead to increase the programmed cells death. Zielińska et al. (2018) also agreed the current documented evidence of CsEOs as an excellent alternative drug molecule for treat cancer cells. After treatment and incubation with 24 h, the more formazan production was observed when addition of MTT solution, it may happened through reduction of cellular enzymes. This process was also conveyed information about Solanum nigrum essential oils has the ability of anti-cancer role by formation of a blue color in treated wells. Previously reported evidences of Salehi et al. (2020); Alper Ozturk et al. (2020) were more supported to this research and CsEOs is the important material to use future drug discovery process of anti-cancer study.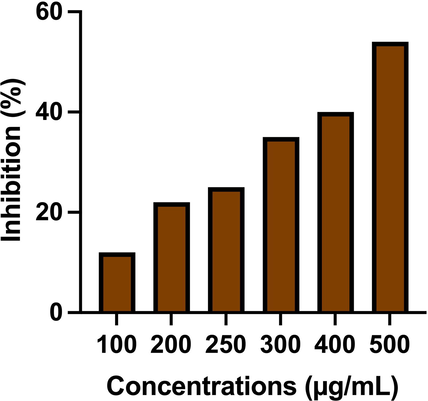
Discovery of anti-cancer property of chitosan nanoparticles loaded plant essential oils against A549 lung cancer cells using cytotoxicity assay.
3.4 Morphological variation by phase contrast microscope
The Fig. 5 of morphological evidences was exhibited with high condensed morphology of A549 lung cancer cells. In addition, obtained evidence of CsEOs may chance to produce bundle of apoptosis process within the cells. More cells were shown irregular shape compared with original morphology of A549 lung cancer cells. Also, the rough, attached uniform cells, high vegetative formed cells were shown in control (Fig. 5a), whereas the complete morphology alteration, smooth, inability to produce and excess level production of ROS formed structures were clearly shown (Fig. 5b). The morphological differentiation result of Fig. 5a and Fig. 5b were clearly indicates, received nanomaterial of CsEOs acted as an excellent anti-cancer module, and it can be used in future anti-cancer drugs after some toxicity evaluation. Previously, Amiri et al. (2020); Kavaz et al. (2019) also reported similar result of the plant essential oils against cancer cells, and shown increased anti-cancer activities.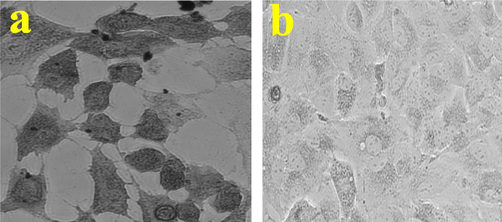
Cytotoxicity effect of chitosan nanoparticles loaded plant essential oils against A549 lung cancer cells was morphologically confirmed by phase contrast microscope.
4 Conclusion
All the invitro experiments were purely accepted the obtained CsEOs has excellent biomedical properties. In addition, the biological properties of CsEOs were shown more inhibition against bacterial growth, and it confirmed by minimum inhibition concentration. Based on the evidences, it clearly evidence, the CsEOs was played an important roles in inactivation of bacterial virulence, and increased the apoptosis in cancer cells. Further, the previous reports of bioactive metabolites producing medicinal plant Solanum nigrum was also more supported to anti-cancer ability against A549 human lung cancer cells. Finally, the result was clearly conveyed that the obtained CsEOs as excellent bio-material for lung cancer cells, and excellent capacity to inhibit the A549 lung cancer cells.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
G. Rajivagndhi and Franck Quero acknowledge the financial support from ANID-FONDECYT (Chile) under the Postdoctoral Fellowship No. 3220019. The authors extend their appreciation to the Researchers Supporting Project number (RSPD2023R696), King Saud University, Riyadh, Saudi Arabia.
References
- Treatment of oxidative stress-induced pain and inflammation with dexketoprofen trometamol loaded different molecular weight chitosan nanoparticles: formulation, characterization and anti-inflammatory activity by using in vivo HET-CAM assay. Microvasc. Res.. 2020;128:103961
- [Google Scholar]
- Fabrication of cumin loaded-chitosan particles: characterized by molecular, morphological, thermal, antioxidant and anticancer properties as well as its utilization in food system. Food Chem.. 2020;310:125821
- [Google Scholar]
- Functionalized graphene oxide based nanocarrier for enhanced cytotoxicity of Juniperus squamata root essential oil against breast cancer cells. J. Drug Delivery Sci. Technol.. 2022;72:103370
- [Google Scholar]
- Tumor targeted delivery of mycobacterial adjuvant encapsulated chitosan nanoparticles showed potential anti-cancer activity and immune, cell activation in tumor microenvironment. Int. Immunopharmacol.. 2023;114:109463
- [Google Scholar]
- Mentha piperita essential oils loaded in a chitosan nanogel with inhibitory effect on biofilm formation against S. mutans on the dental surface. Carbohydr. Polym.. 2019;212:142-149.
- [Google Scholar]
- Biological effects of essential oils - a review. Food Chem. Toxicol.. 2008;46:446-475.
- [Google Scholar]
- Antifungal activity of Morinda citrifolia (noni) extracts against Candida albicans: an in vitro study. Ind. J. Dent. Res.. 2014;25:188-190.
- [Google Scholar]
- Chrysin-loaded chitosan ananoparticles potentiates antibiofilm activity against Staphylococcus aureus. Pathogens. 2020;9:115.
- [Google Scholar]
- Morinda citrifolia lipid transfer protein 1 exhibits anti-inflammatory activity by modulation of pro- and anti-inflammatory cytokines. Int. J. Biolog. Macromol.. 2017;103:1121-1129.
- [Google Scholar]
- Antimicrobial activity of some essential oils—present status and future perspectives. Medicines. 2017;4(3):58.
- [Google Scholar]
- Antibacterial activity of Morinda citrifolia Linneo seeds against Methicillin-Resistant Staphylococcus spp. Microb. Pathog.. 2019;128:347-353.
- [Google Scholar]
- Anti-bacterial activity of chitosan loaded plant essential oil against multi drug resistant K. pneumonia. Saudi J. Biol. Sci.. 2020;27:3449-3455.
- [Google Scholar]
- Anti-cancer activity of Chitosan loaded Morinda citrafolia essential oil against A549 lung cancer cells through cell cycle arrest/mitochondrial damage. Int. J. Biol. Macromol.. 2020;164:4010-4021.
- [Google Scholar]
- Co-delivery of imidazolium Zn(II)salen and Origanum Syriacum essential oil by shrimp chitosan nanoparticles for antimicrobial applications. Carbohydr. Polym.. 2021;260:117834
- [Google Scholar]
- Synthesis and characterization of chitosan nanoparticles loaded with greater celandine (Chelidonium majus L.) essential oil as an anticancer agent on MCF-7 cell line. Int. J. Biol. Macromol.. 2022;194:974-981.
- [Google Scholar]
- Antioxidant and cytotoxic effects of hexane extract of Morinda pubescens leaves in human liver cancer cell line. Asian Pac. J Trop. Med.. 2012;5:362-366.
- [Google Scholar]
- Two new anthraquinones with antiviral activities from the barks of Morinda citrifolia (Noni) Phytochem. Let.. 2016;15:13-15.
- [Google Scholar]
- Development of biomimetic hybrid porous scaffold of chitosan/polyvinyl alcohol/carboxymethyl cellulose by freeze-dried and salt leached technique. J. Nanosci. Nanotechnol.. 2018;18:4916-4922.
- [Google Scholar]
- In vitro cytocompatibility of chitosan/PVA/methylcellulose – nanocellulose nanocomposites scaffolds using L929 fibroblast cells. Appl. Surf. Sci.. 2018;449:574-583.
- [Google Scholar]
- Development and characterization of sodium alginate/poly(vinyl alcohol) blend scaffold with ciprofloxacin loaded in controlled drug delivery system. J. Nanosci. Nanotechnol.. 2019;19:2493-2500.
- [Google Scholar]
- Salt leaching synthesis, characterization and in vitro cytocompatibility of chitosan/poly(vinyl alcohol)/methylcellulose – ZnO nanocomposites scaffolds using L929 fibroblast cells. J. Nanosci. Nanotechnol.. 2019;19:4447-4457.
- [Google Scholar]
- Physiochemical characterization, antioxidative, anticancer cells proliferation and food pathogens antibacterial activity of chitosan nanoparticles loaded with Cyperus articulatus rhizome essential oils. Int. J. Biol. Macromol.. 2019;123:837-845.
- [Google Scholar]
- Kayalvizhi, K., Alhaji, N,M,I., Saravanak kumar, D., Beer Mohamed, S., Kaviyarasu, K., Ayeshamariam, A., Amal, M.Al-M., Mohamed Ragab, A.G., Mohamed, S.E., Adsorption of copper and nickel by using sawdust chitosan nanocomposite beads – A kinetic and thermodynamic study, Environmental Research, 203, 2022, 111814.
- Anti-biofilm activity of LC-MS based Solanum nigrum essential oils against multi drug resistant biofilm forming P. mirabilis. Saudi J. Biol. Sci.. 2021;28:302-309.
- [Google Scholar]
- Antibacterial properties of nanofibers containing chrysanthemum essential oil and their application as beef packaging. Int. J. Food Microbiol.. 2019;292:21-30.
- [Google Scholar]
- Chitosan-coated poly(Lactic acid) nanofibres loaded with essential oils for wound healing. Polymers. 2021;13:1-16.
- [Google Scholar]
- Chitosan-modifications and applications: opportunities galore. React. Funct. Polym.. 2008;68:1013-1051.
- [Google Scholar]
- Nalini, T., Khaleel Basha, S., Majeeth, M.S., A., Sugantha Kumarid, V., Kaviyarasu, K., Development and characterization of alginate/chitosan nanoparticulate system for hydrophobic drug encapsulation, Journal of Drug Delivery Science and Technology, 52, 2019, 65-72
- Narmatha Christy, P., Khaleel Basha, s., Sugantha Kumari, V., Bashir, A.K.H., Maaza, M., Kaviyarasu, K., Valan Arasu, M., Naif A.Al-D., Ignacimuthu, S., Biopolymeric nanocomposite scaffolds for bone tissue engineering applications – A review, Journal of Drug Delivery Science and Technology, 55, 2020, 101452.
- Effect of essential oils on pathogenic bacteria. Pharmaceuticals. 2013;6(12):1451-1474.
- [Google Scholar]
- Nidal, J., Nawaf, Al-M., Samer, A., Ramzi, S., Ahmad, M., Abeer, Q., Nepeta curviflora essential oil: Phytochemical composition, antioxidant, anti-proliferative and anti-migratory efficacy against cervical cancer cells, and α-glucosidase, α-amylase and porcine pancreatic lipase inhibitory activities, Industrial Crops and Products, 158, 2020, 112946.
- Enhanced anti-cancer activity of chitosan loaded Morinda citrifolia essential oil against A549 human lung cancer cells. Int. J. Biol. Macromol.. 2020;164:4010-4021.
- [Google Scholar]
- G. Rajivgandhi, A. Stalin, C. Chenthis Kanisha, G. Ramachandran, N. Manoharan, N.S. Alharbi, S. Kadaikunnan, J.M. Khaled, K.F. Alanzi, W-J. Li, Physiochemical characterization and anti-carbapenemase activity of chitosan nanoparticles loaded Aegle marmelos essential oil against K. pneumoniae through DNA fragmentation assay, Surfaces and Interfaces, 23, 2021, 100932.
- Physiochemical characterization and anti-carbapenemase activity of chitosan nanoparticles loaded Aegle marmelos essential oil against K. pneumoniae through DNA fragmentation assay. Surf. Interfaces. 2021;23:100932
- [Google Scholar]
- G. Ramachandran, G. Rajivgandhi*, S. Murugan, N.S. Alharbi, S. Kadaikunnan, J.M. Khaled,T.N. Almanaa, N. Manoharan, Wen-Jun, L., Anti-carbapenamase activity of Camellia japonica essential oil against isolated carbapenem resistant klebsiella pneumoniae (MN396685), Saudi Journal of Biological Sciences, 27, 2020, 2269–2279
- Incorporation of Zataria multiflora essential oil into chitosan biopolymer nanoparticles: a nanoemulsion based delivery system to improve the in-vitro efficacy, stability and anticancer activity of ZEO against breast cancer cells. Int. J. Biol. Macromol.. 2020;143:382-392.
- [Google Scholar]
- Plant essential oils as active antimicrobial agents. Crit. Rev. Food Sci. Nutr.. 2014;54:625-644.
- [Google Scholar]
- Chitosan nanoparticles encapsulating lemongrass (Cymbopogon commutatus) essential oil: physicochemical, structural, antimicrobial and in-vitro release properties. Int. J. Biol. Macromol.. 2021;192:1084-1097.
- [Google Scholar]
- Chemical composition, antioxidant and cytotoxicity activities of the essential oils of Myristica fragrans and Morinda citrifolia. J. Sci. Food. Agricult.. 2012;92:593-597.
- [Google Scholar]
- Stability of essential oils: a review. Compr. Rev. Food Sci. Food Saf.. 2013;12:40-53.
- [Google Scholar]
- Wińska, K. et al. (2019) ‘Essential oils as antimicrobial agents—myth or r1. Wińska K, Mączka W, Łyczko J, Grabarczyk M, Czubaszek A, Szumny A. Essential oils as antimicrobial agents—myth or real alternative? Molecules. 2019, 24, 1–21.
- Cinnamon cassia oil chitosan nanoparticles: Physicochemical properties and anti-breast cancer activity. Int. J. Biol. Macromol.. 2023;224:1065-1078.
- [Google Scholar]
- X. Yang, G. Rajivgandhi*, G. Ramachandran, N.S. Alharbi, S. Kadaikunnan, J.M. Khaled, T.N. Almanaa, N. Manoharan, R. Viji, Preparative HPLC fraction of Hibiscus rosa-sinensis essential oil against biofilm forming Klebsiella pneumonia, Saudi Journal of Biological Sciences 27 (2020) 2853–2862
- Zhang, F. et al. (2020) ‘Anti-bacterial activity of chitosan loaded plant essential oil against multi drug resistant K. pneumoniae’, Saudi Journal of Biological Sciences. The Author(s), 27, 3449–3455
- Anti-inflammatory and anti-cancer activity of citral: optimization of citral-loaded solid lipid nanoparticles (SLN) using experimental factorial design and LUMiSizer®. Int. J. Pharm.. 2018;553:428-440.
- [Google Scholar]







