Translate this page into:
Chemical variability in essential oils isolated from roots, stems, leaves and flowers of three Ruta species growing in Morocco
⁎Corresponding author. med.barbouchi08@gmail.com (Mohammed Barbouchi)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
In this study, the essential oils (EOs) of separated organs (roots, leaves, stems and flowers) of Ruta chalapensis L., Ruta gravealens L., and Ruta montana L. are characterized by GC/MS analysis and the intra-species variations of the chemical composition of essential oils from three Morocco species were investigated using statistical analysis (principal component analysis (PCA) and cluster analysis (CA)). Correlations between the EO chemical compositions, organs and species were discussed. The results obtained show that a mixture of ketones generally characterizes the EOs of the different organs of three species studied, which 2-undecanone reach 74,36%. Nevertheless, it was observed specific significant disparities between species but also within the same species, especially for presence of chalepensin, geijerene and elemol.
Keywords
Ruta
2-Undecanone
Essential oil
GC/MS
1 Introduction
About 700 spontaneous species, in temperate and warm regions, make up the Rutaceae family. The Ruta genus encompasses about sixty species, some of which are found around the Mediterranean (Hammiche and Azzouz, 2013). In Morocco, the Ruta genus is represented by four species: Ruta tuberculata Forsk., Ruta montana L., Ruta gravealens L., and Ruta chalepensis L., they are designated by the same vernacular names fidjel in Arabic and aourmi in Berber (Bellakhdar, 1997). These last three species are the most diffused and are morphologically poorly differentiated and were probably interchangeably used during Antiquity (Pollio et al., 2008). All are perennial plants with yellow flowers, characterized by a strong smell, fetid, nausea, due to an essential oil contained in huge dry pockets. They were already known in ancient Greece and studied by Hippocrates, five centuries before our era, then by Dioscoride, in the first century. The “herb of grace”, which intervened during Catholic rites as a means of spraying holy water, was the first plant introduced into the American continent (Hammiche and Azzouz, 2013).
Essential oils (sometimes called vegetable essence) are generally complex combinations containing many unique compounds, which are produced in all aromatic plants and trees through three pathways (mevalonic acid (MEV), 2-C-methyl-D-erythritol-4-phosphate (MEP) and shikimate pathway). Terpenoids are produced by two biosynthetic pathways: the MEV that occurs in the cytoplasm, and the MEP, which occurs in the plastids. Phenylpropanoids are derived from the shikimate pathway (Luz et al., 2020). Therefore, when referring about constituents of essential oils; there are two distinct chemical classes: terpenoids and phenylpropanoids. Terpenoids are the most common and abundant, in addition, they are extremely variable, showing different carbon skeletons and a wide variety of oxygenated derivatives including alcohols, esters, aldehydes, ketones, ethers, peroxides and phenols. Phenylpropanoids are the least common group of aromatic compounds and when these compounds are present, they impart a specific smell and flavor to plants (Zuzarte and Salgueiro, 2015).
The applications of essential oils are always oriented by its composition. Nowadays in the world intensively developed research with use of essential oils from plants with their healthy effects (Bakkali et al., 2008; Barão Paixão and Freire de Carvalho, 2021; Elshafie and Camele, 2017; Karakaya et al., 2020, 2019; Ložienė et al., 2018; Luz et al., 2020; Matias et al., 2016). Hence, the importance of studying the composition of essential oils because each of its constituents contributes their beneficial or harmful effects (Haddouchi et al., 2013). Beyond the benefit provided by essential oils, we can, however, realize that there were risks which, in some cases, could lead to toxic effects (Barão Paixão and Freire de Carvalho, 2021; Elshafie and Camele, 2017; Posadzki et al., 2012).
Analysis of the composition of EOs of several Ruta species indicates that a mixture of ketones, generally characterizes them; which 2-undécanone, 2-nonanone and 2-dodécanone are its main constituents. Nevertheless, variations and the existence of significant specific disparities can occur between species but also within the same species (Khadhri et al., 2014). 2-undécanone was reported as a promising antifungal and antibacterial compound (Benali et al., 2020).
In folk medicine, Ruta species represents several therapeutic properties for the treatment of many diseases involved in public health problems, such as infections, neurological diseases, cardiovascular and reproductive system disorders, menstrual disorders, skin inflammations, cramps, earache and headache (Coimbra et al., 2020; Orlanda and Nascimento, 2015; Pollio et al., 2008). Pharmacological trials have proven their as anthelmintic, antioxidants, antiparasitics, anti-inflammatory, and-rheumatic, antifebrile, antiulcer, anti-diabetic, antidepressants, anti-diarrheic, and antimicrobial properties (Attia et al., 2018; Haddouchi et al., 2013; Khlifi et al., 2013; Orlanda and Nascimento, 2015).
To the best of our knowledge the chemical composition of EOs of separated organs (roots, leaves, stems and flowers) from Ruta chalapensis L., Ruta gravealens L., and Ruta montana L. has not been studied previously, see all cited references of results and discussion section, also (Al-shuneigat et al., 2015; Bennaoum et al., 2017; Ghazghazi et al., 2015; Haddouchi et al., 2013; Hammami et al., 2015; Semerdjieva et al., 2019; Yosra et al., 2019). Therefore, in this case, the investigation seems to be an interesting case of study. In this study, the EOs of separated organs (roots, leaves, stems and flowers) from three Moroccan species (Ruta chalapensis L., Ruta gravealens L., and Ruta montana L.) were characterized by GC/MS analysis and the intra-species variations of the chemical composition of EOs were investigated using statistical analysis PCA and CA.
2 Material and methods
2.1 Plant material
The roots, stems, leaves and flowers of Ruta species (Rutaceae family) were collected in the morning between 9:00–11:00 am (during the flowering stage: Mai) from two different regions of Morocco. Ruta chalepensis and Ruta montana were collected from Moulay Idriss Zerhoun (33° 50′ 50,4636′' N, 5° 19′ 0,3972′' W) and Ruta gravealens from Meknes (33° 51′ 57,6468′' N, 5° 32′ 05,8164′' W). Botanical identification was carried out by Prof. Nadia Belahbib, Laboratory of Botany, Biotechnology and Plant Protection, Faculty of Sciences, University Ibn Tofail, BP 133, 14,000 Kenitra, Morocco. Plant materials were air-dried in the shade at room temperature for 10 days and then extracted separately using hydrodistillation method for 4 h in Clevenger type apparatus. EOs were stored in the dark vials and stored frozen at 4 °C, until analysed.
The extracted EOs were weighed in order to calculate their extraction yield and evaluated from 3 extractions. It is expressed as a percentage and calculated by the following formula:
With: W1: Weight of the dry matter of the plant in g
W2: Weight of EO in g.
2.2 Gas chromatographic-Mass spectral analysis
The analysis process of each essential oil was carried out using Perkin Elmer Clarus SQ 8C Gas chromatograph coupled with a mass spectrometer (GC/MS), equipped with a Rxi-5MS capillary column (30 m × 0.25 mm, film thickness 0.25 µm). The carrier gas was helium (He: 1 mL/min. Temperature program: For the first 2 min the oven temperature was kept at 40 °C and then increased at a rate of 4 °C/min until reached a temperature of 180 °C and from 180 to 300 at a rate of 20 °C/min and then held isothermally at 300 °C for 2 min. As regards the split injection was conducted with a ratio split of 1/20, the injected volume: 1 µL. Injector and detector temperature were held at 220 °C. Ion source temperature: 200 °C; energy ionization: 70 eV; electron ionization mass spectra were acquired oven the mass ranges 40–450 Da. The chemical components of Ruta species essential oils were identified by their Kovats retention indexes, as well as the mass spectra with those on the stored in NIST library-version 2014. For the Kovats index were estimated using to the retention time of the homologous series of n-alkanes (C8-C20) under the same operating conditions.
2.3 Statistical studies
Statistical analyses of the chemical compositions were performed using the XLSTAT (version 2015) statistical software; Principal Component Analysis (PCA) and Cluster Analysis (CA) were used to evaluate the chemical composition variability between the EOs obtained from different plant organs and the three Ruta species.
Indeed, the PCA provides the data for the diagrams in which the objects (EO samples) and variables (EO components) are plotted while canonical analysis informs a classification tree in which the objects (the sampling species) are gathered. The CA produced a dendrogram (tree) using Ward's method of Hierarchical Clustering, based on the Euclidean distance between pairs of EO samples.
3 Results and discussion
3.1 Yields of essential oils from Ruta plants
The essential oil yields from the Moroccan samples shown difference between analyzed plant organs and species. In the organs from the Ruta chalapensis, the yield ranged from 0.06% in roots, 0.22% in stems, 3.37% in leaves and 3.86% in flowers. As regards to the Ruta gravealens EOs, the yield was 0.07% in roots, 0.17% in stems, 0.84% in leaves and 1.13% in flowers, while in the Ruta montana EOs, the yield was 0.08% in roots, 0.09% in stems, 0.85% in leaves and 2.24% in flowers.
It is apparent through observing the extraction yields presented in Table 1, they differ significantly (p < 0.05) on the one hand, according to the organs, on the other hand, the species. The flower EOs gives the height yield an average of 2.41%, while the root EOs gives the lowest yield (0.07% on average).
Yields% (w/w)
Roots
Stems
Leaves
Flowers
Ruta chalapensis
0,06 ± 0.00c
0,22 ± 0.01a
3,37 ± 0.04a
3,86 ± 0.02a
Ruta gravealens
0,07 ± 0.00b
0,17 ± 0.02a
0,84 ± 0.02b
1,13 ± 0.04c
Ruta montana
0,08 ± 0.00a
0,09 ± 0.00b
0,85 ± 0.00b
2,24 ± 0.02b
Means Yields%
0,07 ± 0.00
0,16 ± 0.00
1,86 ± 0.01
2,41 ± 0.01
Previous work has shown that the yield of Ruta plants differs depending from which plant organs the EOs were extracted, the species and the origin of the plant population. For example, from a Tunisian population the leaf and flower EOs of Ruta chalapensis yielded 0.25% and 1.75% (Akkari et al., 2020), while the leaf EO from Palestinian yield ranged between 0.66%−1.6% to (Jaradat et al., 2017). The yield of Ruta graveolens leaf and flower EOs obtained from Egypt was 0.36% and 0.21%, respectively (Attia et al., 2018). The essential oil yield from Ruta gravealens leaf was 0.1% v/w. The leaf and stem EOs of Ruta montana from Tunisia (Khadhri et al., 2014) showed a same yield 0.66%.
3.2 Chemical composition of essential oils
The chemical composition of the EOs from different organs of Ruta chalapensis, Ruta gravealens, and Ruta montana was reported in Table 2. In total, 27 volatile components were identified and listed based on their Kovats indices. The number of identified components varies and depends both on the plant organ and species. R: Roots; S: Stems; L: Leaves; F: Flowers; tr: trace (<0.2); –: Not detected.
Compounds
IK
Ruta chalepensis
Ruta gravealens
Ruta montana
R
S
L
F
R
S
L
F
R
S
L
F
1
α-Pinene
930
2,78
3,39
1,66
1,67
–
0,92
–
–
–
tr
0,68
–
2
β-Terpinene
969
0,47
–
0,46
–
–
–
–
–
tr
–
–
–
3
β-Myrcene
988
0,32
–
tr
–
–
1,28
0,44
2,18
–
–
–
tr
4
p-Cymene
1020
0,85
–
tr
–
–
–
–
–
–
–
–
–
5
Limonene
1024
1,92
–
1,63
1,00
–
0,62
–
–
–
tr
–
–
6
Eucalyptol
1027
2,07
3,29
5,97
3,64
–
–
–
–
–
–
–
–
7
2-Nonanone
1089
0,04
4,58
5,21
5,01
tr
8,60
13,94
24,00
–
–
8,64
1,77
8
Linanool
1096
0,60
tr
0,73
0,80
–
–
–
–
–
–
–
–
9
2-Nonanol
1098
–
–
–
–
–
2,03
1,43
–
tr
–
–
–
10
Nonanal
1100
0,22
3,28
2,41
2,75
–
tr
–
–
–
–
tr
–
11
Geijerene
1140
12,21
3,91
–
–
8,90
9,90
3,72
–
2,22
–
–
tr
12
2-Decanone
1191
2,28
10,36
16,20
13,39
–
8,27
9,23
10,74
tr
5,91
7,74
2,96
13
Nonan-2-yl, acetate
1235
–
–
–
–
–
6,81
10,12
2,81
tr
–
–
–
14
2-Undecanone
1294
10,76
32,42
30,04
28,60
18,43
26,54
22,44
31,07
4,29
48,64
74,36
40,61
15
2-Undecanol
1296
1,15
3,19
2,35
2,76
tr
1,99
tr
–
tr
14,15
4,72
20,38
16
Undecanal-2-methyl-
1352
0,95
9,63
8,15
8,13
–
8,42
7,47
8,43
–
–
–
–
17
2-Dodecanone
1377
1,94
15,09
16,17
11,17
tr
7,60
6,97
5,53
–
3,27
2,76
–
18
Caryophellene
1408
–
–
–
–
–
–
–
–
–
3,46
–
2,89
19
Undecan-2-yl acetate
1417
9,54
–
tr
1,49
8,39
0,27
8,61
tr
6,34
9,96
tr
22,16
20
trans-β-Ionone
1486
tr
–
–
–
6,17
–
–
–
9,46
–
–
–
21
2-Tridecanone
1494
7,27
6,79
7,81
15,64
–
6,24
5,36
8,31
–
2,77
–
3,32
22
Elemol
1546
6,79
tr
–
–
20,61
3,20
1,41
tr
17,19
tr
–
tr
23
Tridecane-2,4-dione
1577
8,94
–
–
–
tr
tr
–
–
4,13
5,97
–
4,13
24
Pentadecane-2,4-dione
1770
13,59
–
–
–
0,26
1,63
–
–
25,12
–
–
–
25
Palmitic acid
1959
1,87
–
–
–
–
–
–
–
–
2,09
–
–
26
(Z)-Phytol
2116
–
–
–
tr
–
–
2,05
–
–
–
–
0,91
27
Chalepensin
2198
11,59
2,99
0,75
1,23
36,41
4,48
5,80
6,02
29,78
2,81
tr
–
Total identified (%)
99,06
98,91
99,10
99,09
99,18
98,77
98,99
99,10
98,52
99,04
98,90
99,11
The most abundant compound in the EOs from stems, leaves and flowers of Ruta chalapensis were 2-undecanone, which ranged from 28.69 to 32.42%, 2-dodecanone 11.17–16.17% and 2-decanone 10.36–16.20%, while the pentadecane-2,4-dione was the most abundant compound found in the root EO, with levels of 13.59%, followed by geijerene (12,21%), chalapensin (11.59%) and 2-undecanone (10.76%).
For Ruta gravealens, the main constituents of the root EO are chalepensin (36.41%), elemol (20.61%) and 2-undecanone (18.43%), whereas its stem EO is characterized by the dominance of 2-undecanone (26.54%) while the leaf and fruit EOs showed a predominance of 2-undecanone (22.44% and 31.07%, respectively) and 2-nonanone (13.94% and 24%).
As regards to the root EO of Ruta montana, the chalepensin, pentadecane-2,4-dione and elemol are the dominant compounds (29.78%, 25.12% and 17.19%, respectively), the major components detected in the stem EO were 2-undecanone (48.64%) and 2-undecanol (14.15%), the 2-undecanone was the major compound of the leaf and fruit EOs (74,36% and 40,61%, respectively), we also note the abundant of undecan-2-yl acetate (22.16%) and 2-undecanol (20.38%).
It can be concluded from the results obtained that a mixture of ketones generally characterizes the essential oils of the different parts of three species studied, namely: 2-undecanone, 2-dodecanone, 2-decanone, pentadecane-2,4-dione and 2-nonanone. Nevertheless, there are specific significant disparities between species but also within the same species, such as compounds elemol, geijerene and chalepensin.
Previous studies have studied the chemical composition of the EOs obtained from the aerial parts of Ruta chalapensis, Ruta gravealens and Ruta montana. All these studies clearly showed that the EOs from of three species characterized by high contents of 2-undecanone compound. Table 3 describes the aforementioned results in detail.
Ruta Plants
Organes
Predominant compounds
Countries
References
Ruta chalepensis
Aerial parts
2-undecanone (36.42%), 2-acetoxytridecane (25.42%)
Algeria
(Terkmane et al., 2017)
Ruta chalepensis
2-nonanone (41.7%)
2-undecanone (40.1%).Chile
(Tampe et al., 2016)
Ruta graveolens
2-undecanone (43.66%), 2-nonanone (16.09%)
India
(Reddy and Al-rajab, 2016)
Ruta graveolens
2-undecanone (47.21%)
2-nonanone (39.17%).Brazil
(Orlanda and Nascimento, 2015)
Ruta graveolens
2-undecanone (56.92%)
2-nonanone (23.62%)Tunis
(Yosra et al., 2019)
Ruta montana
2-undecanone (63.97%)
Morocco
(Benali et al., 2020)
Ruta montana
2-undecanone (67%)
2-decanone (9%)Algeria
(Drioueche et al., 2020)
Ruta montana
2-undecanone (27.2–81.7%) 2-nonanone (1.9–39.5%)
Algeria (7 locations)
(Mohammedi et al., 2020)
Ruta montana
2-undecanone (86.77%)
Tunis
(Yosra et al., 2019)
However, studies on the EOs from different organs (leaf, stem, fruit, or flower) of the three species have also shown that there is a qualitative and quantitative difference in their chemical composition. Thus, it should be noted that the 2-undecanone compound is always present with high contents in all the EOs studied.
According to (Krayni et al., 2018), the chemical composition of the EOs obtained from the leaves, stems and fruits of Ruta chalepensis from Tunisia show that the 2-undecanone is the most predominant compound (23.0–58.4%) and 2-nonanone (16.7–23.3%). In another study conducted by (Akkari et al., 2020), were found that the leaf and flower EOs of this species are dominated by 2-undecanone with high contents (85.94% and 89.89%, respectively). As shown to (Jaradat et al., 2017), 2-undecanone (44.31%) and 2-nonanone (43.02%) were found to be the main chemical group in the leaf EO from Palestinian (Jenin region), while the leaf EOs from the regions of Hebron and Jerusalem were characterized by the dominance of linalyl acetate 29.51 34.21%, followed by β-linalool 26.78–31.78%, 2-undecanone 14.99–7.66% and 2-nonanone 14.29–8.15%. Ruta graveolens leaf and flower EOs obtained from Egypt (Attia et al., 2018), the major identified compounds, were 2-undecanone (50.06% and 74.80%, respectively) and 2-nonanone (25.14% and 7.44%). The leaf and stem EOs of Ruta montana from Tunisia (Khadhri et al., 2014) showed a predominance of 2-undecanone (52.20% and 44.9%, respectively) and 2-nonanone (13.50% and 5.80%).
3.3 Chemical variability
PCA and CA were used to identify the possible relationships between EO compounds and the three species of the Ruta plants. The CA (Fig. 1) for organ essential oils (Roots (R), Stems (S), Leaves (L), and flowers (F)) makes it possible to distinguish 3 groups of samples. The first group (I) consists of six EO samples, the second group (II) contains three EO samples and the third group (III) three EO samples.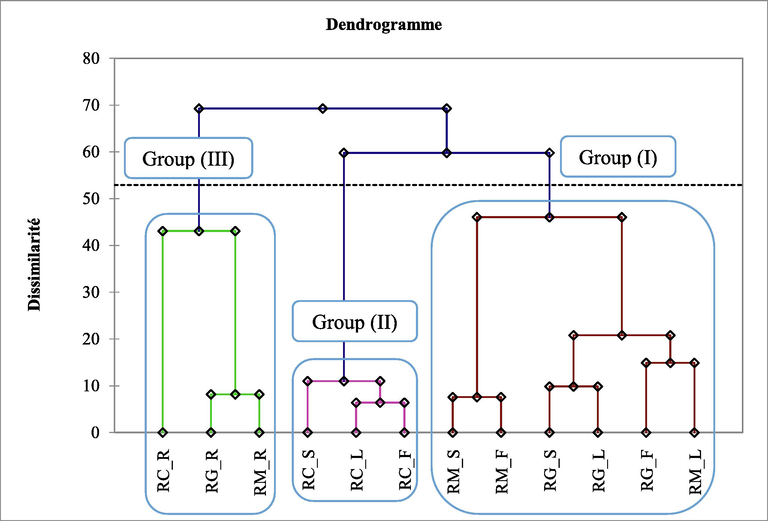
Cluster Analysis of chemical compositions of Ruta species from Morocco. RG: Ruta gravelons; RM: Ruta montana; RC: Ruta chalepensis; R: Roots, S: Stems; L: Leaves; F: Flowers.
The PCA (Fig. 2) confirms the results of the CA and highlights the main quantitative differences between the 3 groups of chemical composition. The two-dimensional axial system of analysis identified three groups of Ruta species based on the chemical composition of their essential oils (Fig. 2). The first two principal axes represented 51.11% of the total variance, the first axis (29.72% of the total variance) and the second axis (21.39% of the total variance).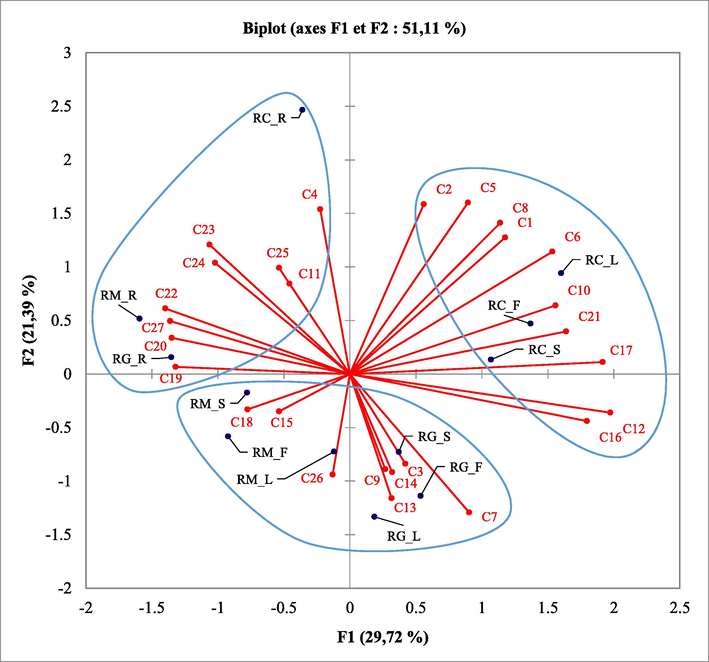
PCA analysis performed on all essential oil compounds of the analyzed Ruta species. Mainly correlated compounds: α-pinene (C1), β-terpinene (C2), β-myrcene (C3), p-cymene (4), limonene (C5), eucalyptol (C6), 2-nonanone (C7), linanool (C8), nonanol (C9), nonanal (C10), 2-geijerene (C11), 2-decanone (C12), nonan-2-yl, acetate (C13), 2-undecanone (C14), 2-undecanol (C15), undecanal-2-methyl- (C16), 2-dodecanone (C17), caryophellene (C18), undecan-2-yl acetate (C19), trans-β-ionone (C20), 2-tridecanone (C21), elemol (C22), tridecane-2,4-dione (C23), pentadecane-2,4-dione (C24), palmitic acid (C25), (Z)-phytol (C26), and chalepensin (C27). RG: Ruta gravelons; RM: Ruta montana; RC: Ruta chalepensis; R: Roots, S: Stems; L: Leaves; F: Flowers.
The discriminating compounds for the first group are α-Pinene (C1), β-Terpinene (C2), Limonene (C5), Eucalyptol (C6), Linanool (C8), Nonanal (C10), 2-Decanone (C12), Undecanal-2-methyl- (C16), 2-Dodecanone (C17), and 2-Tridecanone (C21). The discriminating compounds for the second group are β-Myrcene (C3), 2-Nonanone (C7), 2-Nonanol (C9), Nonan-2-yl, acetate (C13), 2-Undecanone (C14), 2-Undecanol (C15), Caryophellene (C18), and (Z)-Phytol (C26). However, the discriminating compounds for the third group are p-Cymene (4), Geijerene (C11), Undecan-2-yl acetate (C19), trans-β-Ionone (C20), Elemol (C22), Tridecane-2,4-dione (C23), Pentadecane-2,4-dione (C24), Palmitic acid (C25), and Chalepensin (C27).
4 Conclusions
In this work, we study the chemical composition of the three Ruta species essential oils growing in Morocco such as Ruta chalpensis, Ruta gravelons and Ruta montana. Results showed differences in the composition and yields according to the part used and species. Different parts of three species are rich in ketones. The use of ketone oils are very beneficial therapeutically, but they should be handled with great care. Hence, the importance of studying the composition of essential oils because each of its constituents contributes their beneficial or harmful effects. However, in spite of considerable data on the several therapeutic properties of essential oils and their constituents of Ruta species. Surprisingly few therapeutic and products based on plant essential oils have appeared in the market place. Further studies are required to determine the cost, applicability, safety and phytotoxicity of these essential oils as therapeutic agents, before any application in the pharmaceutical industry.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Chemical composition, insecticidal and in vitro anthelmintic activities of Ruta chalepensis (Rutaceae) essential oil. Ind. Crop. Prod.. 2020;74:745-751.
- [CrossRef] [Google Scholar]
- The chemical composition and the antibacterial properties of Ruta graveolens L. essential oil grown in Northern Jordan. Jordan J. Biol. Sci.. 2015;8:139-143.
- [Google Scholar]
- Chemical composition and antimicrobial activities of essential oils of Ruta graveolens plants treated with salicylic acid under drought stress conditions. Futur. J. Pharm. Sci.. 2018;4(2):254-264.
- [CrossRef] [Google Scholar]
- Biological effects of essential oils - A review. Food Chem. Toxicol.. 2008;46(2):446-475.
- [CrossRef] [Google Scholar]
- Essential oil therapy in rheumatic diseases: A systematic review. Complement. Ther. Clin. Pract.. 2021;43:101391.
- [CrossRef] [Google Scholar]
- Bellakhdar, J., 1997. La pharmacopée marocaine traditionnelle; Médecine arabe ancienne et savoirs populaires, Le Fennec,. ed. Paris.
- Chemical variability in essential oils from Ruta species among seasons, and its taxonomic and ecological significance. Nat. Prod. Res.. 2017;31(19):2329-2334.
- [CrossRef] [Google Scholar]
- Genus Ruta: A natural source of high value products with biological and pharmacological properties. J. Ethnopharmacol.. 2020;260:113076.
- [CrossRef] [Google Scholar]
- The Performance of the Ruta Montana L. Essential Oil Bisulfite Adduct as Mixed Natural Emulsifier and a Comparison with Single Tailed Surfactant. J. Dispers. Sci. Technol. 2020;41:2159-2168.
- [CrossRef] [Google Scholar]
- An overview of the biological effects of some mediterranean essential oils on human health. Biomed Res. Int.. 2017;2017:1-14.
- [CrossRef] [Google Scholar]
- Chemical composition of Ruta chalepensis leaves essential oil and variation in biological activities between leaves, stems and roots methanolic extracts. J. Essent. Oil-Bearing Plants. 2015;18(3):570-581.
- [CrossRef] [Google Scholar]
- Chemical composition and antimicrobial activity of the essential oils from four Ruta species growing in Algeria. Food Chem.. 2013;141(1):253-258.
- [CrossRef] [Google Scholar]
- Ruta montana L. leaf essential oil and extracts: characterization of bioactive compounds and suppression of crown gall disease. Excli. J.. 2015;14:83-94.
- [Google Scholar]
- Les rues: ethnobotanique, phytopharmacologie et toxicité. Phytothérapie. 2013;22–30
- [CrossRef] [Google Scholar]
- Variability of chemical compositions and antimicrobial and antioxidant activities of Ruta chalepensis leaf essential oils from three Palestinian regions. Biomed Res. Int.. 2017;2017:1-9.
- [Google Scholar]
- Identification of non-alkaloid natural compounds of Angelica purpurascens (Avé-Lall.) Gilli. (Apiaceae) with cholinesterase and carbonic anhydrase inhibition potential. Saudi Pharm. J.. 2020;28(1):1-14.
- [CrossRef] [Google Scholar]
- Screening of non-alkaloid acetylcholinesterase inhibitors from extracts and essential oils of Anthriscus nemorosa (M.Bieb.) Spreng. (Apiaceae) South Afr. J. Bot.. 2019;125:261-269.
- [CrossRef] [Google Scholar]
- Chemical variability of two essential oils of Tunisian Rue: Ruta montana and Ruta chalepensis. J. Essent. Oil Bear. Plants. 2014;17:445-451.
- [CrossRef] [Google Scholar]
- Composition and anti-oxidant, anti-cancer and anti-inflammatory activities of Artemisia herba-alba, Ruta chalpensis L. and Peganum harmala L. Food Chem. Toxicol.. 2013;55:202-208.
- [CrossRef] [Google Scholar]
- Fruits of Ruta chalepensis L. (Rutaceae) as a source of 2-undecanone. J. Essent. Oil Bear. Plants. 2018;21(3):789-795.
- [CrossRef] [Google Scholar]
- Influence of plant origin natural α-pinene with different enantiomeric composition on bacteria, yeasts and fungi. Fitoterapia. 2018;127:20-24.
- [CrossRef] [Google Scholar]
- Essential oils and their chemical constituents against Aedes aegypti L. (Diptera: Culicidae) larvae. Acta Trop.. 2020;212:105705.
- [CrossRef] [Google Scholar]
- Seasonal variation, chemical composition and biological activity of the essential oil of Cordia verbenacea DC (Boraginaceae) and the sabinene. Ind. Crops Prod.. 2016;87:45-53.
- [CrossRef] [Google Scholar]
- Variability in essential oil composition, antioxidant and antimicrobial activities of Ruta montana L. collected from different geographical regions in Algeria. J. Essent. Oil Res.. 2020;32:23-36.
- [CrossRef] [Google Scholar]
- Chemical composition and antibacterial activity of Ruta graveolens L. (Rutaceae) volatile oils, from São Luís, Maranhão. South African J. Bot.. 2015;99:103-106.
- [CrossRef] [Google Scholar]
- Continuity and change in the Mediterranean medical tradition : Ruta spp. (rutaceae) in Hippocratic medicine and present practices. J. Ethnopharmacol.. 2008;116:469-482.
- [CrossRef] [Google Scholar]
- Adverse effects of aromatherapy: A systematic review of case reports and case series. Int. J. Risk Saf. Med.. 2012;24:147-161.
- [CrossRef] [Google Scholar]
- Chemical composition, antibacterial and antifungal activities of Ruta graveolens L . volatile oils. Cogent Chem.. 2016;2:1220055.
- [CrossRef] [Google Scholar]
- Essential oil composition of Ruta graveolens L. Fruits and Hyssopus officinalis Subsp. aristatus (Godr.) nyman biomass as a function of hydrodistillation time. Molecules. 2019;24(22):4047.
- [CrossRef] [Google Scholar]
- Potential repellent activity of the essential oil of Ruta chalepensis (Linnaeus) from Chile against Aegorhinus superciliosus (Guérin) (Coleoptera: Curculionidae) J. Soil Sci. Plant Nutr.. 2016;16(1):48-59.
- [CrossRef] [Google Scholar]
- Chemical Composition, Antioxidant, and Anticancer Effect of Ruta chalepensis’s Extracts against Human Leukemic Cells. Phytothérapie 2017:1-12.
- [CrossRef] [Google Scholar]
- Biological Study from Ruta Plants Extracts Growing in Tunisia. Iran. J. Chem. Chem. Eng.. 2019;38:85-89.
- [Google Scholar]
- Essential oils chemistry. In: Damião, Pergentino de Sousa, eds. Bioactive Essential Oils and Cancer. Switzerland: Springer International Publishing; 2015. p. :19-61. https://doi.org/10.1007/978-3-319-19144-7
- [Google Scholar]







