Translate this page into:
Chemical composition and antioxidant and antibacterial activity of Platymiscium gracile Benth.: A species threatened by extinction
⁎Corresponding author. dldurango@unal.edu.co (Diego Durango)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Sawdust of Platymiscium gracile Benth. (Fabaceae) was extracted by percolation using n-hexane, dichloromethane, ethyl acetate, and methanol sequentially. Then, fractions were screened for their phenolic contents and antioxidant and antibacterial properties. The phenolic contents ranged from 13,667 ± 773 (ethyl acetate fraction) to 36,350 ± 3049 mg GAE/100 g extract (hexane fraction). In general, all fractions displayed a significant antioxidant activity with values ranging from 20,055 ± 1341 and 194,919 ± 7163 μmol TE/100 g extract, 1785 ± 225 and 32,264 ± 326 mg AAE/100 g extract, 30,081 ± 1618 and 901,706 ± 20,393 μmol TE/100 g extract for the DPPH, FRAP, and ABTS assays, respectively. Eleven compounds were isolated and identified, corresponding to homopterocarpin, scoparone, 8-hydroxyhomopterocarpin, calycosin, 3,4-dimethoxycinnamaldehyde, medicarpin, liquiritigenin, isoliquiritigenin, 3,4,5-trimethoxycinnamaldehyde, 8-methoxyhomopterocarpin, and oleanolic aldehyde acetate. Radical scavenging and antioxidant properties of isolated compounds showed that isoliquiritigenin exhibited the highest activity with values of 1173 ± 61 and 8104 ± 38 μmol TE/g for DPPH radical and ABTS radical cation assays, respectively, and presented 23.6 ± 0.7 mg AAE/g for reducing power assay. In addition, MIC values of isoliquiritigenin for S. aureus, B. cereus, and E. faecalis were 62.5 µg/mL (MIC value for Ampicillin® was 250 µg/mL against B. cereus). The results suggested that P. gracile might be an important source of metabolites with antibacterial and antioxidant activity; therefore, greater efforts should be made for the conservation of this species.
Keywords
Platymiscium gracile Benth.
Total phenolics
DPPH assay
FRAP assay
B. cereus
Isoliquiritigenin
1 Introduction
Platymiscium sp. (Fabaceae), is a neotropical genus of forest trees. It comprises 19 species (29 taxa), distributed from northern Mexico to southern Brazil (Saslis-Lagoudakis et al., 2008). In Colombia, Platymiscium sp. is commonly known as Granadillo or Guayacan Trebol and has great economic and social importance (WWF, 2013; López and Cárdenas, 2002). Its wood has various uses, due to the beauty and resistance to insects and fungi, that includes fine furniture, cabinet making, decorative moldings and veneers, bedroom furniture, musical instruments, and more (WWF, 2013; López and Cárdenas, 2002). In addition, species of Platymiscium have been reported to contain pterocarpans, isoflavonoids and coumarins with antimicrobial and antioxidant properties (Reyes-Chilpa et al., 1998; Falcão et al., 2005).
Unfortunately, the timber exploitation and the intensive use of precious woods have dramatically reduced the species population of Platymiscium sp (Dzib-Castillo et al., 2012). This reality has generated a great pressure on the weak ecosystems of the country and especially on this species with high value. Even though some species of Platymiscium have been listed as threatened regarding the risk of extinction, there are few studies about their chemical composition and biological and pharmacological properties (Veloso et al., 2012; Vila-Nova et al., 2013; Reyes-Chilpa et al., 1998; Falcão et al., 2005). Priority should be given to chemical research on endangered species, since their extinction would lead to the permanent loss of potential drug leads and new uses and chemical entities (Zhou et al., 2013). Also, a deeper knowledge about the properties of the species would stimulate efforts focused on its protection and sustainable use.
P. gracile, an endemic timber species of Peru, has a reduced habitat. In Colombia, some specimens have been found in the Putumayo region; this region concentrates a large part of the forestry activity in the country, which exposes these specimens to a constant risk. In a recent study, it was found that the extracts and the major pure compounds from P. gracile (a species classified as vulnerable on the IUCN (2018) red data list) were effective against the fungi C. acutatum and C. gloeosporioides (Martinez et al., 2017). Additionally, this previous investigation led to the isolation of large quantities of the antifungal compounds homopterocarpin, scoparone, and calycosin from wood sawdust, a waste by-product from timber industry (Martinez et al., 2017). Considering the importance of publicizing the biological properties and chemical composition of threatened species so that efforts are made towards their protection and conservation, in this complementary work is described the potential of P. gracile for obtaining phenolic antioxidants and antibacterial compounds.
2 Materials and methods
2.1 General methods
UV spectra were measured on a Jemway 6405 instrument. 1H and 13C NMR spectra were recorded on a Bruker AMX 300 spectrometer. FTIR spectra were obtained in CHCl3 on a Perkin-Elmer RXI. Mass spectra were performed on a Hewlett-Packard 6890 (Agilent Technologies) gas chromatograph with mass selective detector-quadrupole type (HP 5973 MSD). Detention of compounds was carried out by thin layer chromatography (TLC) on silica gel F254 (0.2 mm Merck) plates.
2.2 Plant material and extraction procedure
The pruned branches of P. gracile were obtained from trees found in the municipality of Puerto Asís, department of Putumayo (Colombia). Dr. Jorge Mario Vélez at the Herbarium “Gabriel Gutiérrez Villegas” of the Universidad Nacional de Colombia-Medellín performed the botanical identification. A voucher of specimen (MEDEL#64111) was deposited. The wood was debarked, cut, and ground to a fine powder. Then, the powder (783 g) was extracted successively with n-hexane, dichloromethane, ethyl acetate, and methanol by percolation until completion. Extracts were concentrated at 40 °C under reduced pressure and used for the determination of antimicrobial and antioxidant activities, in addition to total phenolic contents.
2.3 Isolated metabolites
The n-hexane, dichloromethane, and ethyl acetate extracts were submitted to column chromatography (on silica gel) with mixtures of n-hexane and ethyl acetate of different polarity. The fractions were combined according to TLC profile and further chromatographed by size-exclusion chromatography on Sephadex LH 20® column using the mixture n-hexane, methanol, dichloromethane (2:1:1; v/v) to yield eleven compounds. Scoparone: The compound (73.4 mg) was isolated as a white crystalline solid. The spectroscopic data agree with those previously reported (Martínez et al., 2017). Homopterocarpin: It was isolated as a white solid (60.4 mg). The spectroscopic data agree with those previously reported (Martínez et al., 2017). Calycosin: This compound was isolated as a pale yellow crystalline solid (37.9 mg). The spectroscopic data agree with those previously reported (Martínez et al., 2017). Medicarpin: A pale yellow crystalline solid was obtained (12.3 mg). The spectroscopic data agree with those previously reported (Martínez-Sotres et al., 2012). Liquiritigenin: It was isolated as an amorphous yellowish solid (8.5 mg). The spectroscopic data agree with those previously reported (Li et al., 2014). Isoliquiritigenin: An amorphous yellowish solid was isolated (6.3 mg). The spectroscopic data agree with those previously reported (Li et al., 2014). Oleanolic aldehyde acetate: The compound was isolated as a white crystal from n-hexane (37.0 mg). The spectroscopic data agree with those previously reported (Anjum et al., 2007). 8-hydroxyhomopterocarpin: It was obtained as a white crystalline solid (6.1 mg). The spectroscopic data agree with those previously reported (Morimoto et al., 2006). 8-methoxyhomopterocarpin: It was isolated as yellow oil (4.1 mg). The spectroscopic data agree with those previously reported (Morimoto et al., 2006). 3, 4-dimethoxycinnamaldehyde: This compound was isolated as yellow oil (5.1 mg). The spectroscopic data agree with those previously reported (Burgi et al., 1993). 3, 4, 5-trimethoxycinnamaldehyde: This compound was isolated as yellow oil (5.3 mg). The spectroscopic data agree with those previously reported (Mohammad et al., 1985).
2.4 Total phenolic content
Total phenolic content was determined according to the Folin-Ciocalteau colorimetric method (Singleton et al., 1999), using gallic acid as a standard phenolic compound. The mean ± standard deviation (SD) of total phenolic contents of triplicate analyses were expressed as gallic acid equivalent (mg GAE)/100 g extract.
2.5 DPPH free radical scavenging activity
The hydrogen donating ability of some pure compounds and the plant extracts from P. gracile were measured by decolorization of 2,2-diphenyl-2-picryl hydrazyl (DPPH) (Preciado et al., 2016). Results were expressed by the TEAC (Trolox equivalent antioxidant capacity) values (µmol TE)/100 g of sample.
2.6 ABTS radical cation scavenging activity
The antioxidant capacity assay was performed using the ABTS radical cation decolorization method (Preciado et al., 2016). Results were expressed by the TEAC values as µmol TE/100 g of sample.
2.7 Ferric reducing antioxidant power assay (FRAP)
The reducing power of the extracts and pure compounds of P. gracile was determined according to Preciado et al. (2016). Results were expressed by the AEAC (Ascorbic acid equivalent antioxidant capacity) values (mg AAE)/100 g of sample.
2.8 Hydrophilic oxygen radical absorbance capacity assay (H-ORAC)
Peroxyl radicals was generated using [2,2′-azobis (2-amidinopropane) dihydrochloride] (AAPH). The stock solutions were 10.0 mM sodium fluorescein and 0.6 M AAPH dissolved in phosphate buffer solution (75 mM, pH 7.4). In cells, the solution consisted of fluorescein (21 µL), the pure compound (30 µL), AAPH (50 µL) and phosphate buffer solution (3 µL). Readings were performed with an excitation wavelength of 493 nm (slit of 5 nm) and emission wavelength of 515 nm (slit of 13 nm), using both an attenuator of 1% and without attenuator plate. ORAC values were calculated based on the net area under the curve (AUC) and compared to Trolox standard curve. The antioxidant capacity (ORAC) related to Trolox was estimated as: where AUCsample, AUCblank and AUCTrolox are the area under the curve for: pure compounds, the blank and Trolox respectively, and f is the dilution factor. Results were expressed in µmol TE/g of sample (Preciado et al., 2016).
2.9 Antibacterial activity assay
Four food-borne bacterial strains were used in this study. The strain included Bacillus cereus (ATCC 10876; Gram “+“), Staphylococcus aureus (ATCC 29223; Gram “+“), Escherichia coli (ATCC 25922; Gram “−“), and Enterobacter faecalis (ATCC 29912; Gram “+“). Antibacterial activity was evaluated using two methods: disc diffusion (Bhuyan et al., 2017) and microdilution (Costa et al., 2017).
Disc diffusion method. Filter paper discs (6 mm diameter) were prepared with 20 μL of 1, 0.1 and 0.01% solution in water (<5% ethanol) for all extracts, in addition to the pure compounds. The discs were placed in Petri dishes containing Mueller Hinton agar (MHA) inoculated with a bacteria suspension of 1.5 × 108 CFU (0.5 McFarland density). After 24 h incubation at 35 °C, inhibition zones were measured (diameters). Discs impregnated with Ampicillin (Haupt Pharma Latina, Italy) and water–ethanol (<5%) were used as positive and negative controls, respectively.
Microdilution method. The minimum inhibitory concentration (MIC) of the samples was assessed using the microdilution method. A stock solution of 8000 µg/mL extract (and 2000 µg/mL pure compound) was prepared in nutritious broth. Then, serial double dilutions were prepared in the range of 8000 to 7.8 µg/mL for extracts and 2000 to 2.0 µg/mL for pure compounds. Next, 40 µL of each dilution and 140 µL of nutritious broth were deposited in the wells of a microtiter plate. As negative control, 40 µL of solvent and 140 µL of nutritious broth was used. After, 20 µL of bacterial suspension (0.5 McFarland standard) was incorporated to the wells. The microtiter plates were incubated at 35 °C during 24 h. Subsequently, 20 µL of 5 mg/mL TTC (2,3,5-triphenyl tetrazolium chloride) was aggregated to the wells and the microtiter plate incubated at 35 °C and re-examined after 30 min. Ampicillin (Haupt Pharma Latina, Italy) was used as the positive control. The MIC was considered as the lowest concentration of antimicrobial agent that prevented the microbial growth after 24 h incubation (Balouiri et al., 2016). For the determination of the MBC (minimum bactericidal concentration), 5 µL from the well that exhibited no growth (with concentrations equal or greater than the MIC) was transferred to Petri dishes and incubated at 35 °C for 24 h. The MBC corresponds to the lowest concentration that showed no visible bacterial growth after sub-culturing.
2.10 Statistical analysis
Each experiment was performed three times and the results were reported as mean ± SD. Mean values were compared with the Fisher’s least significant differences (LSD) at the 0.05 probability level.
3 Results
The phenolic content and antioxidant activity of different polarity extracts of P. gracile were evaluated respectively using the Folin-Ciocalteu, and DPPH, ABTS, and FRAP methods. Results are presented in Table 1. Notes: a–d, means within a column with different letters are significantly different (p = 0.05). n.d.: not determined.
Sample
DPPH (µmol TE/100 g Extract)
ABTS (µmol TE/100 g Extract)
FRAP (mg AAE/100 g Extract)
Phenolic content (mg GAE/100 g Extract)
HEX
20,055 ± 1341a
30,081 ± 1618a
n.d.
36,350 ± 3049c
DCM
71,755 ± 2247b
901,706 ± 20,393d
32,264 ± 326c
25,383 ± 1541b
EA
194,919 ± 7163c
458,989 ± 45,720c
13,787 ± 1183b
26,056 ± 1488b
ME
74,090 ± 386b
75,335 ± 2172b
1785 ± 225a
13,667 ± 773a
3.1 DPPH free radical scavenging activity
The DPPH assay is the most used chemical method for determining free radical scavenging activity by antioxidant agents. A higher TEAC value implies greater protective action against free radicals. According to the results, the DPPH radical scavenging activity ranged from 20,055 to 194,919 µmol TE/100 g. Ethyl acetate extract was the most efficient free radical scavenger with the highest TEAC value of 194,919 ± 7163 µmol/100 g, followed by the methanol (74,090 ± 386 µmol TE/100 g) and dichloromethane (71,755 ± 2247 µmol TE/100 g) extract. The n-hexane extract was the least active of all samples (20,055 ± 1341 µmol TE/100 g).
3.2 ABTS radical cation scavenging activity
The ABTS assay is frequently used to determinate the antioxidant activity of plant extracts. The ABTS radical cation scavenging activity ranged from 30,081 to 901,706 µmol TE/100 g. The ABTS assay showed that maximum scavenging activity in the samples was observed in the dichloromethane extract (901,706 ± 20,393 µmol TE/100 g), being about two-fold higher than the ethyl acetate (458,989 ± 45,720 µmol TE/100 g) extract. The weakest antioxidant capacity was exhibited by the methanol and n-hexane extract. As can be seen, ABTS assay values were higher than those of DPPH assays.
3.3 Reducing power assay (FRAP)
The results showed that FRAP values were higher in dichloromethane (32,264 ± 326 µmol AAE/100 g) and ethyl acetate (13,787 ± 1183 µmol AAE/100 g) extracts as compared to methanol (1785 ± 225 µmol AAE/100 g) extraction. FRAP values were not determined for n-hexane extract, due to difficulties in solubility.
3.4 Phenolic content
Phenolic substances are recognized for imparting antioxidant activity to vegetal extracts. According to Table 1, the phenolic contents ranged from 3235 to 36,350 mg GAE/100 g extract. HEX extract of P. gracile had the highest phenolic content (36,350 ± 3049 mg GAE/100 g), followed by the EA and DCM extracts. The ME extract presented the lowest phenolic content. The results showed that the n-hexane, dichloromethane, and ethyl acetate extraction was more efficient in extracting antioxidant compounds in P. gracile as compared to methanol.
3.5 Antibacterial activity assay
3.5.1 Agar disc-diffusion method
The antimicrobial activity of the extracts of P. gracile was studied in different concentrations (1, 0.1, and 0.01%). The results are presented in Table 2. The extracts exhibited inhibition zones ranging from 7 to 12 mm. The antibacterial activity of the extracts augmented with increase in concentration. The highest antibacterial activity was found in the HEX extract, followed by the DCM extract. The results revealed that E. coli was more sensitive as compared with S. aureus, B. cereus, and E. faecalis. In general, all extracts presented low antibacterial activity compared to Ampicillin (18–23 mm). Zone size includes 6-mm disk. Values are mean of three parallel measurements.
Extract
Concentration (%)
E. coli
S. aureus
B. cereus
E. faecalis
HEX
1
11
12
9
7
0.1
10
9
8
7
0.01
9
8
8
7
DCM
1
10
9
8
7
0.1
9
8
7
7
0.01
8
8
7
7
EA
1
8
7
9
8
0.1
8
7
8
7
0.01
7
7
7
7
ME
1
9
9
9
8
0.1
9
9
9
7
0.01
8
8
8
7
Negative control
1
6
6
6
6
3.5.2 Microdilution method
MIC values of extracts from P. gracile are reported in Table 3. Results showed that MICs ranged from 500 to 8000 µg/mL; extracts DCM and EA presented the highest activities (MICs 500 µg/mL against S. aureus and B. cereus, respectively). The bacteria most inhibited by the extracts were S. aureus and B. cereus. The HEX extract was not active against S. aureus and E. coli. –: No antibacterial activity at 8000 µg/mL and below.
Extract
E. coli
S. aureus
B. cereus
E. faecalis
HEX
–
MIC: 8000
–
MIC: 8000
MBC: 8000
MBC: –
DCM
MIC: 4000
MIC: 500
MIC: 1000
MIC: 4000
MBC: 4000
MBC: 4000
MBC: –
MBC: 8000
EA
MIC: 8000
MIC: 1000
MIC: 500
MIC: 8000
MBC: 8000
MBC: 8000
MBC: –
MBC: –
ME
MIC: 8000
MIC: 1000
MIC: 1000
MIC: 4000
MBC: 8000
MBC: 8000
MBC: –
MBC: 8000
Ampicillin®
MIC: 31.2
MIC: 15.6
MIC: 250
MIC: 15.6
For E. coli, MBC values were exactly identical to MIC values, ranging from 4000 to 8000 µg/mL, while DCM extract was the most active. Results showed that all extracts inhibited bacterial growth after sub-culturing of S. aureus. In contrast, all extracts failed to inhibit the growth of B. cereus. MBC values for E. faecalis were approximately 8000 µg/mL for the DCM and ME extracts. Compared to ampicillin, all extracts displayed low antibacterial activity.
3.6 Isolated metabolites
After separation and purification procedures on the ethyl acetate, dichloromethane and n-hexane extracts, eleven known compounds were obtained. From n-hexane extract, three compounds were isolated corresponding to scoparone (1) (6,7-dimethoxycoumarin), homopterocarpin (2), and oleanolic aldehyde acetate (3) (Fig. 1). In addition, five other known compounds were isolated from dichloromethane extract, and the spectra data were in agreement with calycosin (4) and medicarpin (5) 8-hydroxyhomopterocarpin (6), 8-methoxyhomopterocarpin (7), 3,4-dimethoxycinnamaldehyde (8) and 3,4,5-trimethoxycinnamaldehyde (9). Additionally, compounds liquiritigenin (10) and isoliquiritigenin (11) were obtained from ethyl acetate extract. In review of the literature, this is the first report on the isolation of compounds (3), (5), (6), (7), (8), (9), (10) and (11) from P. gracile. Surprisingly, P. gracile is a source of a wide variety of compounds that includes coumarin, chalcone, flavanone, triterpene, cinnamic aldehyde derivatives, and pterocarpans. Some of these compounds are present in high quantities (Martinez et al., 2017).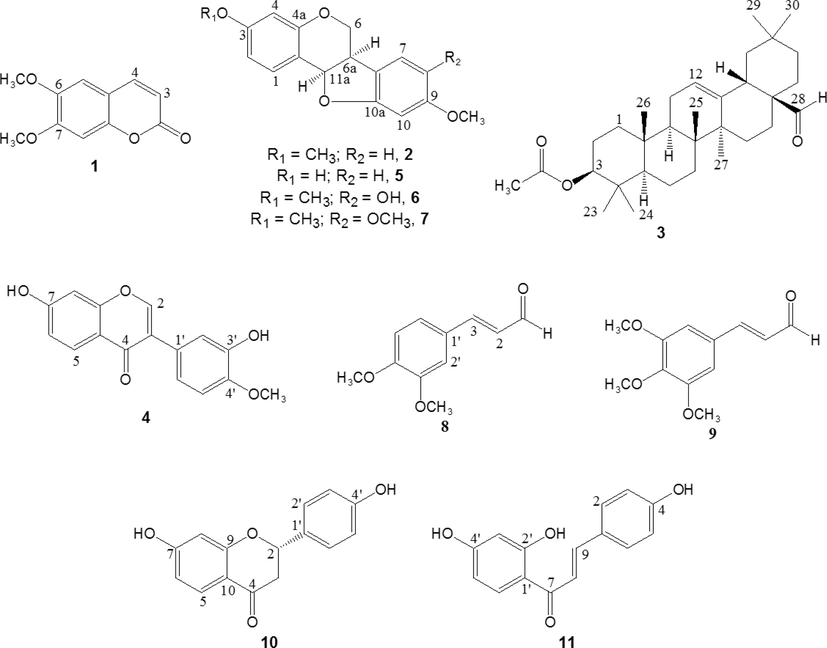
Structure of compounds isolated from P. gracile.
3.7 Antioxidant and antimicrobial activity of isolated compounds
Table 4 presents the results of the antioxidant activity of some of the isolated compounds. Only phenolic compounds were included, with the exception of compound (6) which was not isolated in sufficient quantity to be tested. Compounds (5) and (11) were found to possess significant antioxidant properties. Compound (11) (a chalcone) displayed the strongest antioxidant activity in all assays applied. The activity of (11) was followed by that of pterocarpan (5) in the ABTS, FRAP, and ORAC assays. For these same assays, the lowest activity was found for the isoflavone (4). Notes: a–d, means within a column with different letters are significantly different (p = 0.05). Compounds: 4, calycosin (isoflavone); 5, medicarpin (pterocarpan); 10, liquiritigenin (flavanone); 11, isoliquiritigenin (chalcone).
Sample
DPPH (µmol TE/g Sample)
ABTS (µmol TE/g Sample)
FRAP (mg AAE/g Sample)
ORAC (µmol TE/g Sample)
4
413 ± 14c
17 ± 1a
1.5 ± 0.1a
263 ± 5a
5
105 ± 6b
4378 ± 118c
18.1 ± 1.4c
5928 ± 430c
10
54 ± 2a
367 ± 21b
1.8 ± 0.1b
577 ± 40b
11
1173 ± 61d
8104 ± 38d
23.6 ± 0.7d
9601 ± 613d
Antibacterial activity of (1), (2), and (3) using the disc diffusion method are summarized in Table 5. Compounds (6, 7, 8 and 9) were excluded of assay, due to their insufficient quantities. The highest antibacterial activity against the Gram-negative bacterium was found for (3). Both (1) and (2) were ineffective. On the other hand, (2) and (3) were slightly active against B. cereus, S. aureus, and E. faecalis. Notes: Compounds: 1, scoparone (coumarin); 2, homopterocarpin (pterocarpan); 3, oleanolic aldehyde acetate (triterpene).
Compound
Concentration (%)
E. coli
S. aureus
B. cereus
E. faecalis
1
1
6
6
7
7
0.1
6
6
6
6
0.01
6
6
6
6
2
1
6
8
7
7
0.1
6
8
7
8
0.01
6
8
7
7
3
1
10
7
8
8
0.1
8
7
7
7
0.01
8
6
6
7
Negative control
1
6
6
6
6
Due to the low amount of the chalcone (11), antibacterial activity was determined using the microdilution method. In addition, (1) was analyzed in the same way. Results are reported in Table 6. Under these conditions, antibacterial activity of (1) was weak, which is in according to the disc diffusion method. Notes: Compounds: 1, scoparone (coumarin); 11, isoliquiritigenin (chalcone).
Extract
E. coli
S. aureus
B. cereus
E. faecalis
1
–
–
MIC: 2000
–
11
–
MIC: 62.5
MIC: 62.5
MIC: 62.5
MBC: –
MBC: –
MBC: 1000
Ampicillin®
MIC: 31.2
MIC: 15.6
MIC: 250
MIC: 15.6
From Table 6, it becomes clear that (11) was most effective in controlling the growth of Gram-positive bacteria. Compound (11) exhibited strong inhibition of the growth of S. aureus, B. cereus, and E. faecalis with MIC values of 62.5 µg/mL. It is noteworthy that the MIC value for (11) against B. cereus was lower than for the reference antibiotic Ampicillin.
4 Discussion
Some species of the genus Platymiscium are in a vulnerable situation due to forest exploitation. In this study the chemical composition and antioxidant and antimicrobial activity of P. gracile was established. The results (Table 1) show that the highest scavenging capacity of DPPH radical was found to be the ethyl acetate extract, being almost three times greater than for extracts in methanol and dichloromethane. For the ABTS and FRAP assays, the greatest activity was displayed by the dichloromethane extract, followed by the ethyl acetate extract. The phenolic contents data (Table 1) revealed that the order of concentration was n-hexane > dichloromethane = ethyl acetate > methanol. These results indicated a possible effect of the extraction solvent on the phenolic content and antioxidant activity, which is influenced by the polarity and solubility of the phenolic compounds in the solvent used for the extraction (Babbar et al., 2014). In general, the highest antioxidant activity was found in the dichloromethane and ethyl acetate extract, depending on the method used. These differences result from the fact that each method is based on the generation and use of different radicals and species which are involved in the oxidative process through diverse mechanisms. For the n-hexane extract, it was not possible to determine the FRAP activity as a result of the low solubility of the extract in the medium. In the present study, a correlation between phenolic content and antioxidant activity was not evident. Thus, while the n-hexane extract showed the highest phenolic content, its antioxidant activity was lower than that of the dichloromethane and ethyl acetate extracts. The above is possible owing to the presence of some factors. First, the antioxidant capacity found for the dichloromethane and ethyl acetate extracts could be not only due to the phenolic compounds, but also to other phytochemicals (i.e. ascorbic acid or pigments) or synergistic effects among them (Sengul et al., 2009). Second, the antioxidant activity depends on the structure. Therefore, it is possible that extracts containing different classes of phenolic constituents have different antioxidant capacities (Sengul et al., 2009). Antimicrobial activity of extracts revealed a weak activity against bacteria (Tables 2 and 3). According to the agar disc-diffusion method, n-hexane and dichloromethane extracts showed highest degree of inhibition, followed closely by the methanol and ethyl acetate extracts. In the n-hexane extract, the maximum inhibition zone diameter was obtained in E. coli and S. aureus. In general, the differences in the inhibition zone diameter between extracts were less than 5 mm. In contrast, the microdilution method showed that MIC values were the lowest for the dichloromethane (500 µg/mL for S. aureus) and ethyl acetate (500 µg/mL for B. cereus) extracts. The lowest MBC value (4000 µg/mL) was observed in the dichloromethane extract against E. coli and S. aureus. The fact that the ethyl acetate extract was determined to be active using the microdilution method and inactive with the agar disc-diffusion method may be due to differences in physical properties of the constituents, such as volatility, solubility, and diffusion in agar. Compounds having high solubility or diffusion coefficient but low antimicrobial capacity can rapidly be transferred to the agar, even in small amounts, and give inhibitions similar to those that could present the active compounds with poor penetration (Scorzoni et al., 2007). In addition, the inhibition zone size can be influenced by the size and adsorption of the disc, the amount of compound added to the disc, the type and volume of agar, and microbial strains used, among others (Pauli, 2006). Generally, the agar disc-diffusion method has been considered insufficiently sensitive for many extracts (Sengul et al., 2009). In contrast, each extract (or compound) in the microdilution method is combined with an appropriate medium previously inoculated. However, antibacterial activity of all extracts was low compared to ampicillin in both methods.
The phytochemical investigation of the active extracts (n-hexane, dichloromethane, and ethyl acetate) from Platymiscium gracile sawdust led to identification of eleven known compounds (one chalcone, one flavanone, one triterpene, two cinnamic aldehyde derivatives, one isoflavone and three pterocarpans). From n-hexane extract, compounds (1), (2), and (3) were isolated. Compounds (4), (5), (6), (7), (8), and (9) were obtained from dichloromethane extract. Compounds (10) and (11) were isolated from ethyl acetate. The above shows that the composition of P. gracile is chemically very diverse. The phenolic compounds (4), (5), (10) and (11) were analyzed for their antioxidant activity using DPPH, ABTS, FRAP, and ORAC methods (Table 4). DPPH free radical-scavenging activity presented the following order: (11) > (4) > (5) > (10). In general, there was a good correlation between ABTS scavenging, FRAP, and ORAC activity, showing the highest antioxidant activity with (11), followed by (5). Furthermore, antibacterial activity of isolated compounds was assessed by agar disc-diffusion and microdilution methods (Tables 5 and 6). Compounds (1), (2), and (3), isolated from n-hexane extract, were evaluated using the agar disc-diffusion disc. Extracts from n-hexane proved to be the most active against bacteria in the agar disc-diffusion method. Their constituents exhibited a moderate antibacterial activity, the triterpene (3) being the most active and followed by (2). According to the microdilution assays, the antibacterial effect was greatest for (11). MIC values for S. aureus, B. cereus, and E. faecalis were 62.5 µg/mL. Remarkably, the inhibition of the growth of B. cereus using (11) was higher than for the reference antibiotic ampicillin. According to results, (11) showed a strong antioxidant and antibacterial activity. Compound (11), a flavonoid with a chalcone structure, has been reported to display a wide range of biological and pharmacological properties, including strong antioxidative and antimicrobial effects (Oldoni et al., 2011; Pu et al., 2015). In accordance with Pu et al. (2015), (11) displayed noticeable antioxidant activity, as compared to the synthetic antioxidants BHA and TBHQ.
5 Conclusions
In conclusion, this is the first time that a comprehensive study about the phytochemicals and antibacterial and antioxidant capacity of P. gracile sawdust was carried out. In general, the highest activity was found in the dichloromethane and ethyl acetate extracts. Then, eleven compounds (scoparone, homopterocarpin, oleanolic aldehyde acetate, calycosin, medicarpin, 8-hydroxyhomopterocarpin, 8-methoxyhomopterocarpin, 3,4-dimethoxycinnamaldehyde, 3,4,5-trimethoxycinnamaldehyde, liquiritigenin and isoliquiritigenin) were isolated, eight of which were identified for the first time from this species. It is important to highlight the wide chemical diversity of compounds present in P. gracile. In addition, it was found that isoliquiritigenin, medicarpin, and oleanolic aldehyde acetate displayed a promising antioxidant and antimicrobial activity. So, P. gracile is an important natural resource of bioactive metabolites that may be used as nutraceuticals and bio-pharmaceuticals, reason that merits greater efforts for its protection, conservation and sustainable use.
Authors’ contribution
J.E.C. and J.M. contributed in carrying out the laboratory work, plant material extraction, compound isolation and antibacterial evaluation. BR conducted the antioxidant assays. JHG performed the interpretation of spectroscopic data and contributed to the structuring and reading of the manuscript. DD designed the study and supervised the laboratory work. The manuscript was read and approved for submission for all authors.
Acknowledgements
The authors would like to thank the Universidad Nacional de Colombia (Grant number 34609). We are grateful to Michael James Stablein (University of Illinois, Urbana-Champaign) for the critical review of the manuscript. The authors are grateful to Dr. W. Quiñones (Universidad de Antioquia) for NMR measurements.
Conflicts of interest
The authors declare no conflicts of interest.
References
- Antimicrobial constituents from Fagonia cretica. J. Chem. Soc. Pak.. 2007;29:634-639.
- [Google Scholar]
- Influence of different solvents in extraction of phenolic compounds from vegetables residues and their evaluation as natural sources of antioxidants. J. Food Sci. Tech.. 2014;51:2568-2575.
- [Google Scholar]
- Methods for in vitro evaluating antimicrobial activity: a review. J. Pharmaceut. Anal.. 2016;6:71-79.
- [Google Scholar]
- Phytochemical, antibacterial and antifungal properties of an aqueous extract of Eucalyptus microcorys leaves. S. Afr. J. Bot.. 2017;112:180-185.
- [Google Scholar]
- Synthese von Alkylphenolen und -pyrocatecholen aus Plectranthus albidus (Labiatae) Helv. Chim. Acta. 1993;76:1901-1915.
- [Google Scholar]
- In vitro antibacterial effects of Zanthoxylum tingoassuiba root bark extracts and two of its alkaloids against multiresistant Staphylococcus aureus. Rev. Bras. Farmacogn.. 2017;27:195-198.
- [Google Scholar]
- Emergence of seedlings of native timber trees of Yucatan peninsula. Rev. Mex. Cien. For.. 2012;3:77-87.
- [Google Scholar]
- Cytotoxic flavonoids from Platymiscium floribundum. J. Nat. Prod.. 2005;68:423-426.
- [Google Scholar]
- IUCN. (2018). Red List of Threatened Species. http://www.iucnredlist.org/details/36791/0. (Accessed 2 August 2018).
- Flavonoids from Astragalus membranaceus and their inhibitory effects on LPS-stimulated pro-inflammatory cytokine production in bone marrow-derived dendritic cells. Arch. Pharm. Res.. 2014;37:186-192.
- [Google Scholar]
- López, R., Cárdenas, D., 2002. Manual de identificación de especies maderables objeto de comercio en la Amazonia colombiana. Instituto Amazónico de Investigaciones Científicas, SINCHI.
- Antifungal activity against Colletotrichum acutatum and Colletotrichum gloeosporioides of the major constituents from wood sawdust of Platymiscium gracile Benth. B. Latinoam. Caribe Pl.. 2017;16:14-25.
- [Google Scholar]
- Medicarpin, an antifungal compound identified in hexane extract of Dalbergia congestiflora Pittier heartwood. Int. Biodeterioration Biodegrad.. 2012;69:38-40.
- [Google Scholar]
- Chemistry in the Annonaceae, XVII. Phenylpropenes from Uvarzodendron connzvens seeds. J. Nat. Prod.. 1985;48:328-329.
- [Google Scholar]
- Insect antifeedants, pterocarpans and pterocarpol, in heartwood of Pterocarpus macrocarpus Cruz. Biosci. Biotechnol. Biochem.. 2006;70:1864-1868.
- [Google Scholar]
- Isolation and analysis of bioactive isoflavonoides and chalcone from a new type of Brazilian propolis. Sep. Purif. Technol.. 2011;77:208-213.
- [Google Scholar]
- Anticandidal low molecular compounds from higher plants with special reference to compounds from essential oils. Med. Res. Rev.. 2006;26:223-268.
- [Google Scholar]
- Antioxidants from three Swietenia Species (Meliaceae) J. Med. Plant Res.. 2016;10:8-17.
- [Google Scholar]
- Structural characterization and evaluation of the antioxidant activity of phenolic compounds from Astragalus taipaishanensis and their structure-activity relationship. Sci. Rep.. 2015;5(13914):1-11.
- [Google Scholar]
- Flavonoids and isoflavonoids with antifungal properties from Platymiscium yucatanum Heartwood. Holzforschung. 1998;52:459-462.
- [Google Scholar]
- Phylogenetics of neotropical platymiscium (leguminosae: dalbergieae): systematics, divergence times, and biogeography inferred from nuclear ribosomal and plastid DNA sequence data. Am. J. Bot.. 2008;95:1270-1286.
- [Google Scholar]
- Comparative study of disk diffusion and microdilution methods for evaluation of antifungal activity of natural compounds against medical yeasts Candida spp and Cryptococcus sp. Rev. Cienc. Farm. Basica Apl.. 2007;28:25-34.
- [Google Scholar]
- Total phenolic content, antioxidant and antimicrobial activities of some medicinal plants. Pak. J. Pharm. Sci.. 2009;22:102-106.
- [Google Scholar]
- Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Method. Enzymol.. 1999;299:152-178.
- [Google Scholar]
- New Flavonoids and Coumarins from Platymiscium floribundum Vogel. J. Braz. Chem. Soc.. 2012;23:1239-1243.
- [Google Scholar]
- Different susceptibilities of Leishmaniaspp. promastigotes to the Annona muricataacetogenins annonacinone and corossolone, and the Platymiscium floribundum coumarin scoparone. Exp. Parasitol.. 2013;133:334-338.
- [Google Scholar]
- WWF-World Wild Fund for Nature (Colombia). 2013. Woods of Colombia. http://d2ouvy59p0dg6k.cloudfront.net/downloads/maderas_de_colombia_15_version_aprobada.pdf. (Accessed 2 August 2018).
- Phytochemical Studies of Korean Endangered Plants: A New Flavone from Rhododendron brachycarpum G.Don. Bull. Korean Chem. Soc.. 2013;34:2535-2538.
- [Google Scholar]







