Translate this page into:
Chemical components of Choerospondias axillaris wood by TD-GC/MS, Py-GC/MS, and TG
⁎Corresponding author. tingwang126@126.com (Ting Wang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Choerospondias axillaris is a better fast-growing species. Its bark and fruit have the functions of anti-inflammation, detoxification, hemostasis and treatment of external burns. However, due to the lack of systematic and in-depth analysis of the chemical composition of Choerospondias axillaris, it is difficult to develop high value-added products, resulting in low processing efficiency and even direct abandonment. In order to improve the application value of Choerospondias axillaris and excavate its application in many fields, this paper takes Choerospondias axillaris as the research object to reveal the characteristics of volatile organic compounds and the variation rule of molecular components of jujube before and after extraction of Choerospondias axillaris. The extracts of Choerospondias axillaris were detected and identified by GC-MS and FTIR. The pyrolysis process of Choerospondias axillaris was characterized by TGA-DTG and Py-GC-MS. The volatile organic compounds in the ethanol extracts of Choerospondias axillaris were mainly alcohols, petroleum ether extracts were mainly alkanes and organic acids, and phenylethanol extracts were mainly esters. Among the three kinds of jujube extracts, nonanal, beta-caryophyllene, humus and caryophyllene oxides and other bioactive VOCs were observed. The total content of VOCs from high to low was petroleum ether extract, ethanol extract and benzene/ethanol extract. Choerospondias axillaris has three distinct stages of heat loss treatment: the first stage is 30–50°C, the second stage is 50–200°C, and the third stage is 200–247°C. During the heat loss treatment, three critical temperature turning points (50, 200 and 237) were observed, accompanied by significant chemical changes such as pyrolysis of macromolecules into small volatile molecules. By pyrolysis of the extracts and residues of Choerospondias axillaris, a large number of new components have been produced, which can be used in other industries and provide a new way for sour jujube to become a high-grade application resource.
Keywords
Choerospondias axillaris
Volatile organic component
Py-GC/MS
Component characteristics
1 Introduction
The core of Choerospondias axillaris is large and very hard. Since ancient times, it has been a symbol of “wufu rimmon” (Hua et al., 2008). Choerospondias axillaris is one of the growth in several provinces in south China edible wild fruits, and it appropriate chooses an elevation of 300–800 m (He et al., 2003). The fruit is sweet, sour and delicious, and can be eaten directly. It can also be used to process wine and sour jujube cake; the nucleus can be activated carbon raw material; leaves can do green manure; Bark can also be used as a raw material for tanning and tannin extracts (Doanh et al., 1996; Joshi et al., 2015). But Choerospondias axillaris flavour, acidity, and after harvest perishable fermentation, lose a lot of nutrients, is not conducive to fresh jujube fresh-keeping and circulation, thus has often been processing for south SuanZaoGao, wild jujube juice, zizyphus jujube wine and other products, especially south SuanZaoGao has always been the most popular food in the market (Mi et al., 2019).
Choerospondias axillaris products unique flavor, favored deeply by consumers at home and abroad, affected by food safety concerns more green food becomes the inevitable pursuit of people (Qing et al., 2005; Wang et al., 2014). However, the development of jujube is not very good due to the lack of leading processing enterprises and the lack of breeding and efficient supporting technologies (Li et al., 2016; Mi et al., 2015). Therefore, we should give full play to all aspects of the Choerospondias axillaris resource value, efforts to achieve the comprehensive utilization of resources sustainability, at the same time, the resource advantage into economic advantage, effective way of innovation, and actively develop the green industry, through thinking to guide the industrialization of ecological construction, conversion of forest products processing, extend the industrial chain. In this experiment, the analysis of TG, Py-GC/MS, TD-GC/MS and FTIR are used to analyze the molecular active components, cracking rules and cleavage products of Choerospondias axillaris, and to help maximize the utilization of Choerospondias axillaris.
2 Materials and methods
2.1 Materials and reagents
Choerospondias axillaris was provided from the woods in front of YiFu building of Central South University of Forestry and Technology. After working under natural conditions (40 °C), the camellia seed cake identification smashed into powder by FZ102 crusher is suitable for the plant (cape Taisite Ins).
2.2 Methods
2.2.1 Choerospondias axillaris extraction by solvent
Choerospondias axillaris samples were mixed with benzene/ethanol (2:1) with the solid–liquid ratio of 1:20. After immersing at room temperature for 12 h, the mixed solution were fully extracted by automatic FOSS Soxhlet Extracted apparatus (Agilent, USA) at 70 °C for 5 h.
2.2.2 TD-GC/MS annlysis for Choerospondias axillaris
TD conditions: the initial temperature was 30 °C, retained for 1 min, raised to 100 °C at a rate of 10 °C/min, retained for 5 min, and then increased to 200 °C at a rate of 10 °C/min, not retained. The transmission line temperature is 230 °C.
GC conditions: capillary column (30 m × 250 μm × 0.25 μm). The initial temperature was 50 °C, kept 1 min, in succession, increased to 150 °C at 5 °C/min, kept 10 min, then to 250 °C at 8 °C/min, stayed 2 min; injection volume of 1 μL; no a split mode; vaporization chamber temperature was 280 °C. MS detection conditions: the program of MS was scanned over the 35–600 amu (m/z).
2.2.3 Choerospondias axillaris and its extracted residue analysis by FTIR
The FT-IR spectra of the extracted samples were obtained with a FT-IR spectrophotometer (Thermo Fisher Scientific iS10) using KBr discs containing a 1.00% finely ground sample.
2.2.4 Choerospondias axillarisThermostability by TG
Thermogravimetric analysis (TGA) was conducted using a thermogravimetric analyzer (TGA Q50 V20.8 Build 34, USA), and the temperature program of TA started at 30 °C, and increased 600 °C at 10 °C/min; carrier gas is high purity nitrogen, with a flow rate of 40 mL/min.
2.2.5 Extractives and residues analysis by Py-GC/MS
PY-2020iS (Frontier Co) was used to pyrolyze 0.1 mg of residue in helium atmospheres at 50, 100, 200, and 250°Celsius (residual at 50 and 250°Celsius), respectively. Pyrolysis products (pyrolyzate) were analyzed by on-line connected GC/MS. GC/MS analysis was carried out on Agilent 5975C/6890 N (Agilent Corp., USA) using an elastic quartz capillary column DB-5MS coated with neutral phase (30 m × 0.25 mm × 0.25 um). The temperature of the inlet is 250 °C. The temperature program of GC starts at 50 °C. The temperature rises to 300 °C at the rate of 10 °C/min, and then sprays at a ratio of 30:1. MS program scans on 35–550 amu (m/z) with an ionization voltage of 70 eV and an ionization current of 150 mu A (EI).
3 Results and analysis
3.1 VOC components analysis of extractive from Choerospondias axillaris
The TD-GC/MS chromatograms of Choerospondias axillaris in the range of 50 °C to 250 °C were shown in Fig. 1. It can be seen that the extractives had three obvious peaks at 12.237 min, 19.610 min and 25.231 min. From Table 1, it can be found that this material is a kind of Ethanol, 2-butoxyethoxy at 12.23 min. The molecular weight of C18H32O4 and the molecular weight is 312.44, and the molecular structure. The study of the properties of this property is still unknown. Also, from Table1, it can be know that the substance present at 19.61 min was 1,2-Bezenedicarboxylic acid, bis(2-methylpropyl) ester, the chemical formula wasC16H22O4, and molecular structure . It’s colorless transparent oily liquid, not volatile, slightly aromatic smell, flammable and toxic. Mainly as plasticizer, it can make the products have good softness, but the volatile and water extraction is bigger, so the durability is poor. At 25.21 min, through Table 1 we can know that the substance is 7, 9-di-tert-butyl-1-oxaspiro(4,5)deca-6,9-diene-2, 8-dione, and molecular structure. The data from TD-GC/MC can be inferred that these three unsaturated compounds should be the main components of the extracts from Choerospondias axillaris. However, the study on the physiological activity and pharmacological toxicity of these three compounds is not clear. Note: “--” this indicates that nothing is detected.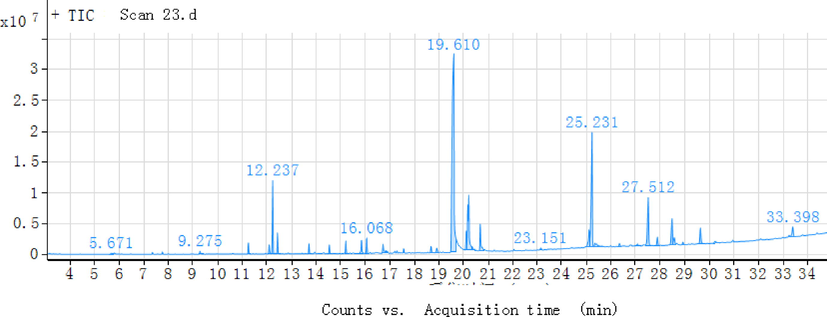
The ion chromatograms of VOC from Choerospondias axillaris by TD-GC/MS.
No.
Retention time (min)
Relative content (%)
Compound Name
1
9.275
0.64
1-(2-Butoxyethoxy)ethanol
2
11.254
1.9
Formamide, N,N-dibutyl-
3
12.086
2
2,2,4-Trimethyl-1,3-pentanediol diisobutyrate
4
12.237
12.2
Ethanol, 2-(2-butoxyethoxy)-,
5
12.426
3.5
Propanoic acid, 2-methyl-, 3-
6
13.712
1.66
Dimethyl phthalate
7
14.544
1.35
2,4-Di-tert-butylphenol
8
15.211
2.08
Benzene, 1,2,3-trimethoxy-5-(2-
9
15.854
2.99
2,2,4-Trimethyl-1,3-pentanediol
10
16.068
2.79
Cedrol
11
17.556
0.76
Methyl tetradecanoate
12
19.61
100
1,2-Benzenedicarboxylic acid,
13
20.114
3.74
Phthalic acid, butyl hex-3-yl ester
14
20.215
20.88
7,9-Di-tert-butyl-1-
15
20.681
6.4
Dibutyl phthalate
16
28.483
7.5
10,11-Dihydro-10-hydroxy-2,3,6-
From the results of Table 1, 16 kinds of organic compounds were detected from the extract of the experiment. Most volatile organic compounds are aldehydes and esters. For example, the substance detected at 12.42 min is 2-methylpropionate-3-heptylenyl ester. Its chemical formula is C11H20O2, a colorless liquid, soluble in ethanol and oil, and generally used for food and spices. The relative content of the substance in the extractant is 3.5%. The substance detected at 15.85 min was 2,2,4- trimethyl-1,3-pentanediol. Its chemical formula is C8H18O2, which is a white crystal, soluble in alcohols, ketones and aromatic solvents, and is difficult to dissolve in water and fatty hydrocarbon. It can produce monoester or diester with a variety of acids. Is a kind of irritant substance, mainly used in polyester manufacturing. The relative content of the substance in the extractant is 2.99%. The substance was detected in 19.6 min, 1,2-benzenedicarboxylic acid. Its chemical formula is C12H14O4, it is colorless transparent oily liquid. It is mixed with ethyl alcohol ether, soluble in acetone, benzene and other organic solvents, insoluble in water. Used for cellulose acetate, it is used to improve the water resistance and elasticity of the products and give appropriate strength. Used for nitrocellulose, products can obtain excellent light resistance and toughness, and no stink. The adhesion of polyvinyl acetate can be improved (Jiang et al., 2017). Since this product is non-toxic, it can be used as the plasticizer of non-toxic adhesive for food packaging. It can also be used as the plasticizer of alkyd resin, butyl rubber and chloroprene rubber, as well as the solvent used as perfume. The relative content of the substance in the extractant is 100%. At 20.2 min, 7, 9-di-tert-butyl-1 was detected, with a relative amount of 20.88%, and its use is unclear.
3.2 Chemical group change characteristics of choerospondias axillaris and residues
FTIR is a rapid detection technology, with a high sensitivity, and it is commonly used in the identification of chemical bonds and functional groups in compounds (Suksuwan et al., 2015; Jović et al., 2014; Yi et al., 2018). In this experiment, the changes of the Choerospondias axillaris and extracted residues’ chemical groups were shown in Fig. 2, in the range from 4000 cm−1 to 400 cm−1.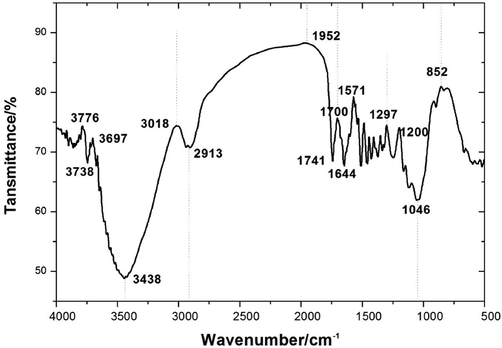
Infrared spectra of VOC of benzene/ ethanol extractives from Choerospondias axillaris.
As shown in infrared spectroscopy of Fig. 2, it showed a total of 22 absorption peaks examined in Choerospondias axillaris sample. First, there was absorption peak at 3438 cm−1, indicating that there was the O–H stretching vibration present, which having alcohols compound: 2-ethyl-1-Butanol. In 1741 cm−1, there was a strong absorption peak, 2913 cm-1 and 1046 cm-1also has two absorption peaks. The first strong absorption peaks are caused by C-O stretching, the last two are aldehyde group C–H stretching vibration, prove the existence of aldehydes compounds. In 1300 cm−1-1000 cm−1, there are two peaks, respectively, C-O-C symmetric stretching vibration (weak peak near 1140 cm−1-1030 cm−1) and asymmetric stretching vibration (strong peak near 1300 cm−1-1150 cm−1) (Adams et al., 2007; Roscini et al., 2010). In the vicinity of 3440 cm−1 also can often observe the weaker C = O stretching vibration frequency doubling of absorption. It can be determined that there are esters. And at 1118 cm−1, it is probably ethers. There are several absorption peaks of 900 cm−1 to 750 cm−1, which can determine the cis trans configuration of the compound. 1732 cm−1-1729 cm−1 and 732 cm−1-1503 cm-1carbonyl C=O double bond stretching vibration and C–H in-plane bending vibration; 1370 cm-1C-O and C–C frame vibration, 674 cm−1-664 cm-1 C–C scale, 1510 cm−1 –1510 cm−1 near the benzene ring of vibration, etc., corresponding to a variety of oxygen-containing organic compounds, such as phenols, aldehydes, acids, ketones and other substances. In summary, Choerospondias axillaris mainly contains fat hydrocarbon and aromatic structure and various kinds of oxygen-containing functional groups. In addition, it can be concluded that organic solvent extraction does not made compound groups of Choerospondias axillaris changed.
3.3 Volatility characteristic of Choerospondias axillaris by thermostability analysis
Changes in Choerospondias axillaris from 30 °C to 250 °C are shown in Fig. 3. There are three distinct phases of heat loss willow cheilophila: the first phase of the initial temperature is 30 to 50 °C, the change of weight from 3.455906 mg to 3.455906 mg, this part of the quality loss is one of the most rapidly. At this point, the DTG curve also declines from the maximum value, indicating that the TG content decreases at a slower rate (Elbaz et al., 2015). In the second phase, from 50 °C to 200 °C, this one phase curve flattens the quality, there are fewer. This part of mass loss should be caused by evaporation or volatilization of small amount of water. At this point DTG is also leveling off, basically staying the same. The third part is from 200 °C to the end of 247 °C, quality change from 3.178024 mg to 3.109858 mg. The DTG curves of this part of the rise, this is because the decreased from 200 °C to TG quality rate begin to ascend (Cuetos et al., 2009; Jia et al., 2012; Paama et al., 2000). According to the DTG curve, the order of mass loss rate was observed as follows: the first, the third and the second stages. There are two critical temperature turning points in the whole heating process. At these temperatures, the mass of Choerospondias axillaris changes dramatically, which may be caused by chemical changes, such as the breakdown of macromolecules into smaller molecules that are more volatile (Sasca et al., 2010).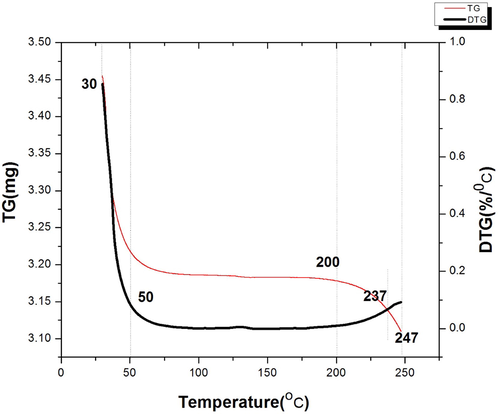
Thermogravimetric curve of Choerospondias axillaris.
3.4 Component characteristics of pyrolyzates from extractive
The total ion chromatograms of the samples studied by Py-GC–MS are shown in Fig. 4. And the relative content of each component has been counted by area normalization. The MS data is analyzed by using the NIST standard MS map and publicly published books and papers, and then identify each component (Ribechini et al., 2009; Bo et al., 2015; Khabbaz et al., 2015). And the analytical results of the six samples were listed in Tables 2.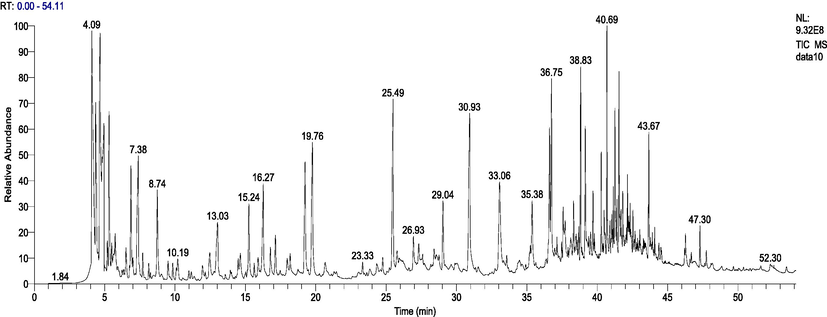
The total ion flow diagram of Choerospondias axillaris by Py-GC/MS.
NO.
Retention time (min)
Relative content (%)
Compound Name
1
4.09
5.65
Carbamic acid, monoammonium salt
2
4.36
4.33
Acetic anhydride
3
4.67
7.46
hydroxy-Acetic acid
4
4.81
1.56
Acetic acid
5
4.95
4.02
Ammonium acetate
6
5.19
0.54
2-Butenal
7
5.31
2.94
1-hydroxy-2-Propanone
8
5.5
0.53
1,4,2,5 Cyclohexanetetrol
9
5.7
0.70
Furan, 2,5-dimethyl-
10
5.74
0.67
Tolycaine
11
6.86
2.02
1,2-Ethanediol, monoacetate
12
7.38
3.14
Propanoic acid, 2-oxo- methyl ester
13
8.74
2.01
Furfural
14
10.19
0.50
4-Hexen-2-one
15
12.46
0.47
2(5H)-Furanone
16
12.47
6.26
2(5H)-Furanone
17
13.03
2.03
2-Cyclopenten-1-one, 2-hydroxy-
18
14.64
0.64
2-Propenamide, N-(aminocarbonyl)-
19
15.24
1.65
Phenol
20
16.27
2.53
N-Butyl-tert-butylamine
21
16.78
0.65
1,3,5-Cycloheptatriene, 1-methoxy-
22
17.14
0.84
1,2-Cyclopentanedione, 3-methyl-
23
18.18
0.50
Phenol, 2-methyl-
24
19.24
3.08
p-Cresol
25
19.76
3.61
Phenol, 2-methoxy-
26
20.67
0.66
6-Oxa-bicyclo[3.1.0]hexan-3-ol
27
25.49
3.99
Creosol
28
29.04
1.29
Phenol, 4-ethyl-2-methoxy-
29
30.93
5.14
2-Methoxy-4-vinylphenol
30
33.06
3.90
Phenol, 2,6-dimethoxy-
31
33.34
0.46
Phenol, 2,6-dimethoxy-
32
35.38
1.59
Phenol, 2-methoxy-4-(1-propenyl)-, (Z)-
33
36.62
2.57
1,2,4-Trimethoxybenzene
34
36.75
3.52
trans-Isoeugenol
35
37.58
0.81
Bicyclo[5.3.0]decane, 2-methylene-5-(1-methylvinyl)-8-methyl-
36
37.72
0.76
à-Guaiene
37
38.32
0.77
5-tert-Butylpyrogallol
38
38.83
2.40
Benzene, 1,2,3-trimethoxy-5-(2-propenyl)-
39
39.16
1.93
3′,5′-Dimethoxyacetophenone
40
40.28
0.92
.gama.-eudesmol
41
40.69
2.63
2-Naphthalenemethanol, decahydro-à,à,4a-trimethyl-8-methylene-, [2R-(2à,4aà,8aá)]-
42
41.14
0.49
Phenol, 2,6-dimethoxy-4-(2-propenyl)-
43
41.26
1.74
Phenol, 2,6-dimethoxy-4-(2-propenyl)-
44
41.55
2.04
6-Isopropenyl-4,8a-dimethyl-1,2,3,5,6,7,8,8a-octahydro-naphthalen-2-ol
45
41.69
0.64
Ethanone, 1-(4-hydroxy-3,5-dimethoxyphenyl)-
46
41.82
0.62
Diepicedrene-1-oxide
47
42.15
0.56
6-Isopropenyl-4,8a-dimethyl-1,2,3,5,6,7,8,8a-octahydro-naphthalen-2-ol
48
43.67
1.11
Tricyclo[4.4.0.0(2,7)]dec-8-ene-3-methanol, à,à,6,8-tetramethyl-, stereoisomer
49
46.28
0.56
cis-Vaccenic acid
50
47.3
0.61
Benzene, 1,1′-(1-methylethylidene)bis[4-methoxy-
According to the results of Py-GC–MS analysis, 50 peaks were detected in Table 2. The results show that the content of more substances are as follows: hydroxy-Acetic acid (7. 46%), Carbamic acid-monoammonium salt (5.65%), Ammonium acetate (4.02%), 2(5H)-Furanone (6.26%), 2-Methoxy-4-vinylphenol (5.14%), trans-Isoeugenol (3.52%), Ammonium acetate (4. 02%), Propanoic acid, 2-oxo- methyl ester (3.14%),1-hydroxy-2-Propanone (2.94%), N-Butyl-tert-butylamine (2.53%), p-Cresol (3.08%), 1,2,4-Trimethoxybenzene (2.57%). The main components of these detected compounds are esters, acids, phenols, anthraquinones and ketones. By analyzing the main functions and functions of different compounds, Choerospondias axillaris can be more effectively and fully utilized and exerted.
4 Conclusion and discussion
Many extracts were extracted in benzene/ethanol extract, mainly used as plasticizer, but from the extraction of saliva extract-butanol, carbamic acid phenol and sheen – three oxygen radicals, its function is not clear. In addition, a variety of useful substances, such as benzene, methyl ketone, 2 (5 h) – Furanone, hexanal, ammonium acetate, 2-Propanone, propionic acid, their function is different, in the actual processing and utilization, can according to different functions, the production of various kinds of useful products and making fully effective use of resources. FTIR results further confirmed the organization structure of Choerospondias axillaris and extracting of residue is consistent. It suggests that the sample of Choerospondias axillaris contains esters, aldehydes, alcohols, ether, etc., and display, organic solvent extraction no organized with Choerospondias axillaris change significantly.
There are three obvious stages of thermal loss. During the whole process of thermal gravity, the DTG curve is in the first decline, then the latter tends to be gentle, and finally the trend is improved. There are two key temperature tipping points of 50 °Celsius and 200 °Celsius. At these temperatures, the quality of the salivary bacteria changes significantly, such as the decomposition of large molecules into small molecules that are more volatile. The main pyrolysis products include ester, acid, alcohol, aldehyde, ether, enyl acid, ammonium, anhydride, phenol, ketone, furan and heterocyclic compounds. There is a big difference between the properties and functions of matter. Therefore, the application prospect is also very different.
Through the pyrolysis law and pyrolysis products of Choerospondias axillaris, the new pathway of Choerospondias axillaris resource utilization and the application of Choerospondias axillaris in other fields are revealed, which provides a scientific basis for the maximum resource utilization of Choerospondias axillaris.
Acknowledgement
This project is supported by the science and technology project of Hunan Province, China (2016NK2154), and the National Natural Science Foundation of China (31172257).
Jun-Tao Chen, Ya-Feng Yang, Zhenling Liu and Wan-Xi Peng’s contribution as same as the first author, they were also co-first authors.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Ftir analysis and monitoring of synthetic aviation engine oils. Talanta. 2007;73(4):629-634.
- [Google Scholar]
- Production of aromatic hydrocarbons from catalytic co-pyrolysis of biomass and high density polyethylene: analytical py–gc/ms study. Fuel. 2015;139:622-628.
- [Google Scholar]
- Anaerobic co-digestion of poultry blood with ofmsw: ftir and tg-dtg study of process stabilization. Environ. Technol.. 2009;30(6):571-582.
- [Google Scholar]
- The use of a water extract from the bark of choerospondias axillaris in the treatment of second decree burns. Scand. J. Plast. Reconstr. Surg. Hand Surg.. 1996;30(2):139-144.
- [Google Scholar]
- Tg/dtg, ft-icr mass spectrometry, and nmr spectroscopy study of heavy fuel oil. Energy Fuels. 2015;29(12), acs energyfuels.5b01739
- [Google Scholar]
- Study on variations of seedling traits in choerospondias axillaris geographic provenances. Forest Res.. 2003;16(2):177-182.
- [Google Scholar]
- In vitro and in vivo antioxidant activity of aqueous extract from choerospondias axillaris fruit. Food Chem.. 2008;106(3):888-895.
- [Google Scholar]
- Thermal study on light crude oil for application of high-pressure air injection (hpai) process by tg/dtg and dta tests. Energy Fuels. 2012;26(3):1575-1584.
- [Google Scholar]
- Preparation and properties of novel flame-retardant pbs wood-plastic composites. Arabian J. Chem.. 2017;11(6):844-857.
- [Google Scholar]
- Synthesis and characterizations of nanoporous carbon derived from lapsi (choerospondias axillaris) seed: effect of carbonization conditions. Adv. Powder Technol.. 2015;26(3):894-900.
- [Google Scholar]
- Ftir investigation of solvent effects of n-methyl and n- tert -butyl benzamide. J. Struct. Chem.. 2014;55(8):1616-1622.
- [Google Scholar]
- Py-gc/ms an effective technique to characterizing of degradation mechanism of poly (l-lactide) in the different environment. J. Appl. Polym. Sci.. 2015;78(13):2369-2378.
- [Google Scholar]
- Comparison of bioactivities and phenolic composition of choerospondias axillaris peels and fleshes. J. Sci. Food Agric.. 2016;96(7):2462-2471.
- [Google Scholar]
- Ship identification algorithm based on 3d point cloud for automated ship loaders. J. Coastal Res. 2015:28-34.
- [Google Scholar]
- Research on regional clustering and two-stage svm method for container truck recognition. Discrete and Continuous Dynamical Systems Series S. 2019;12(4–5):1117-1133.
- [Google Scholar]
- Analysis of archaeological samples and local clays using icp-aes, tg-dtg and ftir techniques. Talanta. 2000;51(2):349-357.
- [Google Scholar]
- Impacts of choerospondias axillaris growth on acidity of udic ferrosols in subtropical china. Pedosphere. 2005;15(1):95-102.
- [Google Scholar]
- Py-gc/ms, gc/ms and ftir investigations on late roman-egyptian adhesives from opus sectile: new insights into ancient recipes and technologies. Analytica Chimica Acta. 2009;638(1):79-87.
- [Google Scholar]
- Influence of cell geometry and number of replicas in the reproducibility of whole cell ftir analysis. Analyst. 2010;135(8):2099-2105.
- [Google Scholar]
- The n-butyl amine tpd measurement of brönsted acidity for solid catalysts by simultaneous tg/dtg–dta. Appl. Surf. Sci.. 2010;256(17):5533-5538.
- [Google Scholar]
- Tracking the chemical surface properties of racemic thalidomide and its enantiomers using a biomimetic functional surface on a quartz crystal microbalance. J. Appl. Polym. Sci.. 2015;132(30)
- [Google Scholar]
- Advance in chemical constituents and pharmacological activity of choerospondias axillaris fruit. Food Sci.. 2014;35(13):281-285.
- [Google Scholar]
- The solvent effects on dimethyl phthalate investigated by ftir characterization, solvent parameter correlation and dft computation. Spectrochimica Acta Part A Mol. Biomol. Spectroscopy. 2018;199:412-420.
- [Google Scholar]







