Translate this page into:
Chemical components and functions of Taxus chinensis extract
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Taxus chinensis is a National level protected plant and has been for a long time, since it grows slowly and has low regenerative capacity. Consequently, no large base of raw material forest of taxus was formed. This study used Taxus chinensis as the study object, for analyzing the chemical composition of T. chinensis extract via fourier transform infrared spectroscopy (FT-IR) and gas chromatography/mass spectrometry (GC/MS). 37 types of chemical components were detected. These were mainly ethers (asarone, dibutyl phthalate, diisobutyl phthalate), alcohols (nerolidol, Myo-inositol, 4-C-methyl-, trans-Sinapyl alcohol), acids (3,4-dimethoxycinnamic acid, palmitic acid, oleic Acid), flavonoids (macckiain, formononetin), ketone (4-hydroxy-.beta.-ionone, pseudobaptigenin), phenols (methyleugenol), esters (triacetin), aldehydes (sinapinaldehyde), and pyridines (alpha-methylstyrene). This chemical component could be directly or indirectly used for bioengineering, pharmaceutical engineering, the cosmetic industry, and other chemical industries.
Keywords
Taxus chinensis
Extract
Function
FT-IR
GC/MS
1 Introduction
Taxus chinensis is endemic to China and its main distribution is in the provinces south of the Yangtze River Basin. T. chinensis is a member of the family Taxaceae and the genus Taxus. All species of taxus contain highly toxic ingredients but provide a potentially rich source of biological active diterepenoids (Li et al., 2008; Qiu et al., 2009) They grow slowly and are protected at National level. Taxol (paclitaxel) has been one of the best natural anticancer drugs in the past few decades (Mu and Feng, 2003). It has been widely used for the treatment of breast cancer, lung cancer, ovarian cancer, and part of head and neck cancer. The supply of taxol is extracted from taxus bark (Shen et al., 2017). Relying on other chemical component from taxus due to the scarcity of taxol, slow growth and low paclitaxel. Therefore, study of the extract of chemical components and functions of T. chinensis has become very meaningful.
2 Material and methods
The material of this study was obtained from the T. chinensis trunk. First, we ground three parts of the material (bark, sapwood, and heartwood of T. chinensis) in a micro plant grinding machine to obtain wood powder, and the powder was put into a drying oven, set at a temperature of 100 °C and dry for 6 h to evaporate all free water from the sample.
Then, we used four different experiment levels: “0” represented the untreated wood powder; “1”, “2”, and “3” represented the material for the extraction experiment using ethanol, ethanol & methanol, and ethanol & benzene as solvent (Table 1).
Solvent
Bark
Sapwood
Heartwood
Untreated
–
B0
S0
H0
Extractive
Ethanol
B1
S1
H1
Ethanol & Methanol
B2
S2
H2
Ethanol & Benzene
B3
S3
H3
2.1 FT-IR analysis
The dried T. chinensis powder was filtered through a 200-mesh sieve. This experiment used pure KBr as a solid dispersion medium (Mi et al., 2019). The finely ground T. chinensis powder was dispersed in KBr at 1:100. The range of the spectrum was set to 400–4000 cm−1 (Castaldi et al., 2010; Li et al., 2017a, 2017b; Maréchal and Chanzy, 2000).
2.2 GC/MS analysis
The chromatographic column was HP-5MS (30 m × 250 μm × 0.25 μm), capillary column was elastic quartz, carrier gas was high purity He, flow rate was 1 mL/min, and the shunting mode was used with a split ratio of 20:1. The temperature of GC program started at 50 °C, increased to 250 °C at a rate of 8℃/min, increased to 300℃ at a rate of 5℃/min. MS program scanning quality range was 30–600 amu, ionization voltage was 70 eV, and ionization current (EI) was 150 μA. The ion source temperature was 230℃, quadrupole temperature was 150℃ (Daferera et al., 2000; Ma et al., 2008a, 2008b; Wang et al., 2005).
3 Results and discussion
3.1 Analysis of FT-IR
This analysis used materials extracted via different slovents. Due to the ubiquitous solvent effect, interactions between sample molecules and solvent molecules would change the frequency and intensity of vibration of the sample molecule (Peng et al., 2017a, 2017b). For the same raw material, this may thus obtain different results due to the different solvents.
Fig. 1 and Table 2 show the results of FT-IR spectra of T. chinensis bark. We could see that in different experimental levels, the same part of the FT-IR spectrum of wood powder differed. The spectrograms show that transmittance of B0 was 76.24% at 3341 cm−1, transmittance of B1, B2, and B3, respective were 61.07%, 58.31%, and 43.87% at 3441 cm−1 (O–H stretching vibration). Furthermore, according to other studies we know that the characterized absorption peaks of cellulose were 2900 cm−1, 1425 cm−1, 1370 cm−1, and 895 cm−1. Transmittance of B0 was 85.21% at 2934 cm−1, transmittance of B1, B2, and B3, respective were 81.58%, 79.75%, and 68.29% at 3441 cm−1 (C–H stretching vibration). At 1370 cm−1 (C–H flexural vibration), transmittance of B0, B1, B2, and B3 were 89.58%, 78.30%, 79.73%, and 68.95%, respectively. This showed that cellulose was hydrolyzed to differently extent and it was hydrolyzed more in mixed solvent of ethanol and benzene.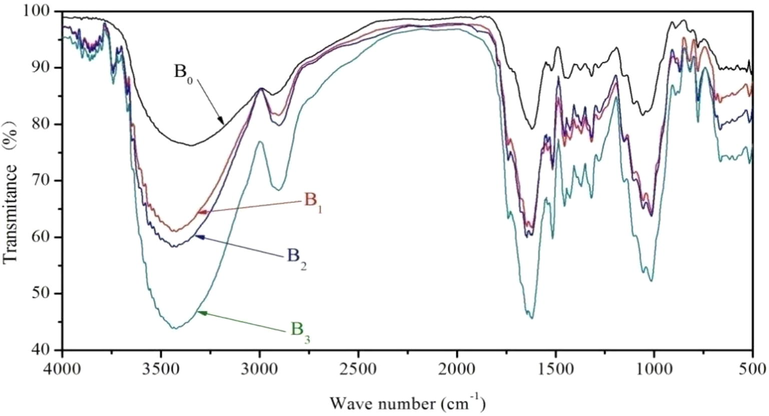
FT-IR spectrum of T. chinensis bark under four treatment methods.
Absorption peak attribution
Absorption peak (cm−1)
Chemical component
B0
B1
B2
B3
O–H Stretching vibration
3341
3441
3441
3441
Cellulose, Hemicellulose, carboxylic acid, alcohol
C–H Stretching vibration
2934
2903
2903
2903
Cellulose
Benzene ring stretching vibration
1618
1618
1618
1618
Lignin
1522
1516
1516
1516
Lignin
1452
1454
1454
1454
Lignin
C–H Flexural vibration
1373
1369
1369
1369
Cellulose, Hemicellulose
S-ring, 5-substituted G-ring
1317
1317
1317
1317
Lignin
C–O Stretching vibration
1059
1056
1056
1056
Cellulose, Hemicellulose
At 1618 cm−1 (benzene ring stretching vibration), transmittance of B0, B1, B2, and B3 were 79.15%, 61.62%, 60.32%, and 45.55%, respectively. At about 1518 cm−1 (benzene ring stretching vibration), transmittance of B0, B1, B2, and B3 were 89.43%, 71.92%, 72.44%, and 59.73%, respectively (Peng et al., 2017a, 2017b). At about 1454 cm−1 (benzene ring stretching vibration), transmittance of B0, B1, B2, and B3 were 88.22%, 76.08%, 77.88%, and 65.47%, respectively. At 1317 cm−1 (S-ring, 5-substituted G-ring), transmittance of B0, B1, B2, and B3 were 88.48%, 76.85%, 77.60%, and 66.90%, respectively. This shows that the transmittance of B1 was close to B2 and their transmittances were weaker than that of B0. The transmittance of B3 was the weakest. This shows that lignin was partially hydrolyzed in the mix solvent of ethanol and benzene, and a small amount of hydrolyed in the ethanol solvent and the mixed solvent of ethanol and methanol.
At about 1056 cm−1 (C–O Stretching vibration), transmittance of B0, B1, B2, and B3 were 81.61%, 66.46%, 65.02%, and 53.70%, respectively. In combination with the above data, this shows that hemicelluloses was partially hydrolyzed by organic solvents (Ma et al., 2008a, 2008b; Río et al., 2007; Schwanninger et al., 2004).
Fig. 2 and Table 3 show the results of FT-IR spectra of T. chinensis sapwood. The characterized absorption peaks of cellulose, at about 2898 cm−1 (C–H Stretching vibration), transmittance of B0, B1, B2, and B3 were 79.08%, 86.75%, 78.24%, and 81.12%, respectively. At about 1426 cm−1 (CH2 Flexural vibration, CH2 Scissor vibration), transmitance of B0, B1, B2, and B3 are 80.59%, 86.04%, 81.34%, and 75.80%, respectively. At about 1373 cm−1 (C–H Stretching vibration), transmittance of B0, B1, B2, and B3 are 81.78%, 89.72%, 84.33%, and 77.23%, respectively. The results show that cellulose was hydrolyzed in small amount. The characterized absorption peaks of hemicellulose, at 1742 cm−1 (C=O Stretching vibration), transmittance of B0, B1, B2, and B3 were 85.05%, 79.01%, 75.14%, and 79.02%, respectively. The results show that a small amount of hemicellulose was hydrolyzed. The characterized absorption peaks of lignin, at about 1653 cm−1 (C=O Stretching vibration), transmittance of B0, B1, B2, and B3 were 81.14%, 76.87%, 74.97%, and 75.48%, respectively. At 1512 cm−1 (benzene ring stretching vibration), transmittance of B0, B1, B2, and B3 were 78.23%, 70.36%, 66.33%, and 69.21%, respectively. At about 1460 cm−1 (C–H Flexural vibration, CH2, CH3 asymmetric flexural), transmittance of B0, B1, B2, and B3 were 80.44%, 80.52%, 76.73%, and 74.56%, respectively. The results show that a small amount of lignin was hydrolyzed.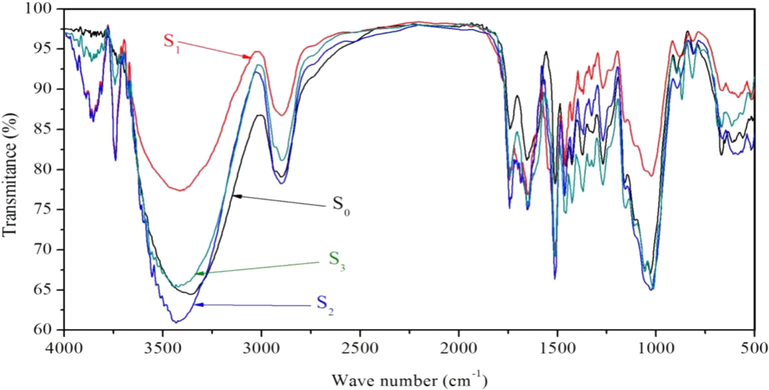
FT-IR spectrum of T. chinensis sapwood under four treatment methods.
Absorption peak attribution
Absorption peak (cm−1)
Chemical component
S0
S1
S2
S3
O–H Stretching vibration
3360
3412
3412
3412
Cellulose, Hemicellulose, carboxylic acid, alcohol
C–H Stretching vibration
2899
2897
2899
2897
Cellulose
C=O Stretching vibration
1742
1742
1744
1740
Hemicellulose
1655
1653
1653
1653
Lignin
Benzene ring stretching vibration
1512
1512
1514
1512
Lignin
C–H Flexural vibration, CH2, CH3 Asymmetric flexural vibration
1458
1462
1462
1456
Lignin
CH2 Flexural vibration, CH2 Scissor vibration
1427
1425
1425
1427
Cellulose, Lignin
C–H Stretching vibration
1373
1379
1369
1371
Cellulose, Hemicellulose,
G-ring, Acyloxy CO–O stretching vibration
1269
1271
1269
1269
Lignin
C–O Stretching vibration
1030
1024
1026
1014
Cellulose, Hemicellulose, Lignin
Fig. 3 and Table 4 show the results of FT-IR spectra of T. chinensis heartwood. The characterized absorption peaks of cellulose, at about 2902 cm−1 (C–H Stretching vibration), transmittance of B0, B1, B2, and B3 were 75.01%, 81.94%, 82.15%, and 92.46%, respectively. At about 1426 cm−1 (CH2 Flexural vibration, CH2 Scissor vibration), transmittance of B0, B1, B2, and B3 were 76.57%, 84.74%, 78.73%, and 91.43%, respectively. The results show that a small amount of cellulose was hydrolyzed. The characterized absorption peaks of hemicellulose, at 1740 cm−1 (C=O Stretching vibration), transmittance of B0, B1, B2, and B3 were 85.26%, 87.79%, 75.88%, and 85.02%, respectively. The results show that a small amount of hemicellulose was hydrolyzed. The characterized absorption peaks of lignin, at about 1650 cm−1 (C=O Stretching vibration), transmittance of B0, B1, B2, and B3 were 77.63%, 83.51%, 67.08%, and 84.60%, respectively. At 1513 cm−1 (Benzene ring stretching vibration), transmittance of B0, B1, B2, and B3 were 70.47%, 81.23%, 65.93%, and 77.87%, respectively. At about 1458 cm−1 (C–H Flexural vibration, CH2, CH3 Asymmetric flexural), transmittance of B0, B1, B2, and B3 were 74.74%, 84.23%, 76.30%, and 87.96%, respectively. The results show that lignin was partially hydrolyzed in the mix solvent of ethanol and methanol, and a small amount of hydrolyed in the others solvent.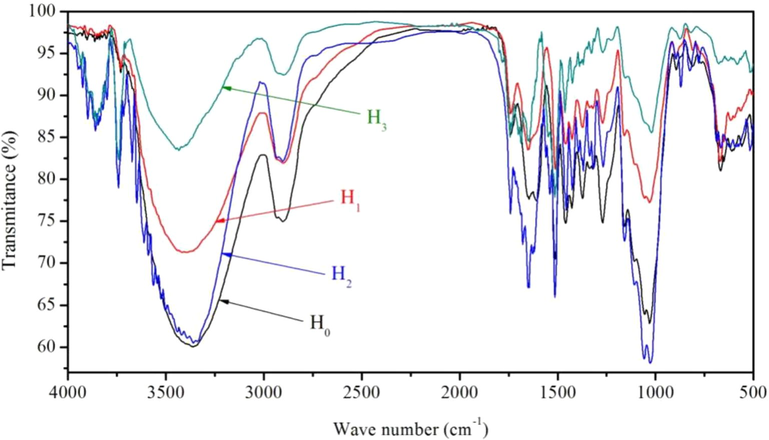
FT-IR spectrum of T. chinensis heartwood under four treatment methods.
Absorption peak attribution
Absorption peak (cm−1)
Chemical component
H0
H1
H2
H3
O–H Stretching vibration
3360
3393
3360
3433
Cellulose, Hemicellulose, carboxylic acid, alcohol
C–H Stretching vibration
2907
2900
2900
2897
Cellulose
C=O Stretching vibration
1740
1740
1740
1744
Hemicellulose
C=O Stretching vibration
1647
1651
1651
1653
Lignin
Benzene ring stretching vibration
1514
1512
1514
1514
Lignin
C–H Flexural vibration, CH2, CH3 Asymmetric flexural vibration
1458
1456
1456
1462
Lignin
CH2 Flexural vibration, CH2 Scissor vibration
1427
1427
1425
1423
Cellulose, Lignin
G-ring, Acyloxy CO-O stretching vibration
1271
1271
1267
1271
Lignin
C-O Stretching vibration
1030
1030
1030
1020
Cellulose, Hemicellulose, Lignin
According to FT-IR analysis, the absorption peaks did not indicate significant migration. This indicates that in the extraction process, the chemical components of the samples did not change too much.
3.2 Analysis of GC/MS
Table 5 shows that: via analysis of GC–MS, in the bark of T. chinensis, the experiment of extracting with ethanol solution, nine types of chemical components which occupied 53.93% of total peaks areas were identified. There were benzene,1,2,3-trimethoxy-5-(2-propenyl)- (47.50%), 4,6-diamino-3-[4-methoxybenzyl]-1H-pyrazolo[3,4-d]pyrimidine (3.21%), 1,2-Benzenedicarboxylic acid, butyl 8-methylnonyl ester (0.63%), nerolidol (0.55%) and estra-1,3,5(10)-trien-17.beta.-ol (0.55%).
No.
Compound
B1
B2
B3
RT (min)
Area (%)
RT (min)
Area (%)
RT (min)
Area (%)
1
.alpha.-Methylstyrene
–
–
–
–
5.51
0.59
2
Triacetin
–
–
–
–
11.83
0.29
3
Methyleugenol
12.76
0.32
12.76
0.23
12.75
0.27
4
Benzene,1,4-dimethoxy-2,3,5,6-tetramethyl-
14.51
0.33
14.51
0.36
14.51
0.39
5
Benzene,1,2,3-trimethoxy-5-(2-propenyl)-
15.12
47.50
15.12
29.53
15.14
46.23
15.19
0.36
6
4-Hydroxy-.beta.-ionone
15.19
0.46
–
–
15.19
0.43
7
Asarone
–
–
15.22
0.53
–
–
8
Nerolidol
15.22
0.55
–
–
15.23
0.58
9
4-Allyl-2,6-Dimethoxyphenol
–
–
15.82
0.15
–
–
10
Benzene,1,2,3-trimethoxy-5-(2-propenyl)-
–
–
16.48
0.31
16.48
0.25
11
1,2-Benzenedicarboxylic acid, bis(2-methylpropyl)
–
–
–
–
19.39
3.31
12
3,4-Dimethoxycinnamic acid
–
–
–
–
19.89
0.59
13
2,2-Dimethyl-6-methylene-1-[3,5-dihydroxy-1-
–
–
–
–
20.45
0.26
14
Estra-1,3,5(10)-trien-17.beta.-ol
20.46
0.55
20.46
0.35
–
–
15
1,2-Benzenedicarboxylic acid, butyl 8-methylnonyl ester
20.55
0.63
–
–
–
–
16
Dibutyl phthalate
–
–
–
–
20.55
2.32
17
.gamma.-Sitosterol
–
–
–
–
25.59
0.34
18
1H-2,8a-Methanocyclopenta[a]cyclopropa[e]cyclodecen-11-one, 1a,2,5,5a,6,9,10,10a-octahydro-5,5a,6-trihydroxy-1,4-bis(hydroxymethyl)-1,7,9-trimethyl-, [1S-(1.alpha.,1a.alpha.,2.alpha.,5.beta.,5a.beta.,6.beta.,8a.alpha.,9.alpha.,10a.alpha.)]-
25.72
0.38
–
–
25.72
0.36
19
4,6-Diamino-3-[4-methoxybenzyl]-1H-pyrazolo[3,4-d]pyrimidine
27.33
3.21
–
–
27.33
3.11
In the experiment of extracting the mixed solution of ethanol and methanol, seven types of chemical components were identified which occupied 31.82% of total peaks areas. There were benzene,1,2,3-trimethoxy-5-(2-propenyl)- (29.89%) and asarone (0.53%).
In the experiment for extracting the mixed solution of ethanol and benzene, 15 types of chemical components were identified, which occupied 59.32% of total peaks areas. These were: benzene,1,2,3-trimethoxy-5-(2-propenyl)- (46.23%), 1,2-benzenedicarboxylic acid, bis(2-methylpropyl) (3.11%), 4,6-diamino-3-[4-methoxybenzyl]-1H-pyrazolo[3,4-d]pyrimidine (3.11%), dibutyl phthalate (2.32%), alpha-methylstyrene (0.59%), 3,4-dimethoxycinnamic acid (0.59%), nerolidol (0.58%) and 4-hydroxy-.beta.-ionone (0.43%).
Table 6 shows that via analysis of GC–MS, in the sapwood of T. chinensis, the experiment of extracting with ethanol solution, eight types of chemical components were identified, which occupied 35.09% of total peaks areas. These were benzene,1,2,3-trimethoxy-5-(2-propenyl)- (30.61%), gamma-sitosterol (2.29%) and 1 h-2,8a-Methanocyclopenta[a]cyclopropa[e]cyclodecen-11-one,1a,2,5,5a,6,9,10,10a-octahydro-5,5a,6-trihydroxy-1,4-bis(hydroxymethyl)-1,7,9-trimethyl-,[1S-(1.alpha.,1a.alpha.,2.alpha.,5.beta.,5a.beta.,6.beta.,8a.alpha.,9.alpha.,10a.alpha.)]- (1.20%).
No.
Compound
S1
S2
S3
RT (min)
Area (%)
RT (min)
Area (%)
RT (min)
Area (%)
1
.alpha.-Methylstyrene
–
–
–
–
5.51
0.61
2
Triacetin
–
–
–
–
11.83
0.19
3
Methyleugenol
12.76
0.19
–
–
12.75
0.25
4
Benzene,1,4-dimethoxy-2,3,5,6-tetramethyl-
14.51
0.23
14.51
0.30
14.51
0.23
5
Benzene,1,2,3-trimethoxy-5-(2-propenyl)-
15.11
30.61
15.12
18.10
15.12
29.69
15.18
0.14
6
4-Hydroxy-.beta.-ionone
15.18
0.18
–
–
15.19
0.19
7
6-epi-Shyobunol
15.22
0.16
–
–
15.22
0.22
8
Myo-Inositol, 4-C-methyl-
–
–
–
–
17.61
5.16
9
Diisobutyl phthalate
–
–
–
–
19.39
2.13
10
Dibutyl phthalate
–
–
–
–
20.55
1.49
11
Benzene,1,2,3-trimethoxy-5-(2-propenyl)-
–
–
20.85
0.23
–
–
12
2-[4-methyl-6-(2,6,6-trimethylcyclohex-1-enyl)hexa-1,3,5-trienyl]cyclohex-1-en-1-carboxaldehyde
23.62
0.23
–
–
23.29
0.21
23.62
0.25
13
1H-2,8a-Methanocyclopenta[a]cyclopropa[e]cyclodecen-11-one, 1a,2,5,5a,6,9,10,10a-octahydro-5,5a,6-trihydroxy-1,4-bis(hydroxymethyl)-1,7,9-trimethyl-, [1S-(1.alpha.,1a.alpha.,2.alpha.,5.beta.,5a.beta.,6.beta.,8a.alpha.,9.alpha.,10a.alpha.)]-
25.50
1.2
23.62
0.1
24.18
0.21
24.38
0.27
14
.gamma.-Sitosterol
25.68
2.29
–
–
25.50
0.52
25.68
2.00
15
4,6-Diamino-3-[4-methoxybenzyl]-1H-pyrazolo[3,4-d]pyrimidine
–
–
27.33
2.57
–
–
16
S-Indacene-1,7-dione,2,3,5,6-tetrahydro-3,3,4,5,5,8-hexamethyl-
–
–
27.67
14.46
–
–
17
Macckiain
–
–
28.67
1.62
–
–
29.11
3.50
In the experiment for extracting the mixed solution of ethanol and methanol, seven types of chemical components were identified, which occupied 41.02% of total peaks areas. These were benzene,1,2,3-trimethoxy-5-(2-propenyl)- (18.24%), s-indacene-1,7-dione,2,3,5,6-tetrahydro-3,3,4,5,5,8-hexamethyl- (14.46%), macckiain (5.12%) and 4,6-diamino-3-[4-methoxybenzyl]-1H-pyrazolo[3,4-d]pyrimidine (2.57%).
In the experiment for extracting the mixed solution of ethanol and benzene, 13 types of chemical components were identified, which occupied 43.62% of total peaks areas. These were benzene,1,2,3-trimethoxy-5-(2-propenyl)- (29.69%), Myo-inositol, 4-c-methyl- (5.16%), gamma-sitosterol (2.52%), diisobutyl phthalate (2.13%) and dibutyl phthalate (1.49%).
Table 7 shows that vie analysis of GC–MS, in the heartwood of T. chinensis, the experiment of extracting with ethanol solution, 12 types of chemical components were identified, which occupied 43.28% of total peaks areas. These were formononetin (17.71%), Myo-inositol, 4-C-methyl- (8.19%), pseudobaptigenin (5.40%), pseudobaptigenin (4.79%), macckiain (2.32%), nerolidol (1.51%), dibenz[a,c]cyclohexane,2,4,7-trimethoxy- (1.07%) and oleic acid (0.82%).
No.
Compound
H1
H2
H3
RT (min)
Area (%)
RT (min)
Area (%)
RT (min)
Area (%)
1
.alpha.-Methylstyrene
–
–
–
–
5.51
0.91
2
Triacetin
–
–
–
–
11.83
0.37
3
Benzene,1,4-dimethoxy-2,3,5,6-tetramethyl-
–
–
14.45
0.28
–
–
4
Benzene,1,2,3-trimethoxy-5-(2-propenyl)-
14.12
0.42
15.03
4.69
15.09
1.81
15.09
0.28
5
Nerolidol
15.21
1.51
15.15
1.27
15.21
7.04
6
Phenol,2,6-dimethoxy-4-(2-propenyl)
–
–
15.77
0.19
–
–
7
[1,1′-Bicyclopropyl]-2-octanoic acid, 2′-hexyl-, methyl ester
–
–
16.26
0.11
16.35
0.49
16.32
0.09
8
Benzene,1,2,3-trimethoxy-5-(2-propenyl)-
–
–
16.44
0.13
–
–
9
3-O-Methyl-d-glucose
–
–
–
–
17.23
3.33
10
Myo-Inositol, 4-C-methyl-
18.76
8.19
18.18
3.79
18.39
2.50
18.44
0.81
18.75
7.91
18.80
3.26
11
1,2-Benzenedicarboxylic acid, bis(2-methylpropyl) easter
–
–
–
–
19.39
3.82
12
Palmitic acid
20.46
0.31
–
–
–
–
13
Dibutyl phthalate
–
–
–
–
20.55
2.76
14
Sinapinaldehyde
20.82
0.18
20.78
0.3
–
–
15
trans-Sinapyl alcohol
–
–
20.90
0.46
–
–
16
Oleic Acid
22.56
0.82
–
–
–
–
17
Phenol,4-methyl-2-[5-(2-thienyl)pyrazol-3-yl]-
–
–
23.38
0.83
–
–
18
1H-Cyclopropa[3,4]benz[1,2-e]azulene-5,7b,9,9a-tetrol,1a,1b,4,4a,5,7a,8,9-octahydro-3-(hydroxymethyl)-1,1,6,8-tetramethyl-,5,9,9a-triacetate,[1aR-(1a.alpha.,1b.beta.,4a.beta.,5.beta.,7a.alpha.,7b.alpha.,8.alpha.,9.beta.,9a.alpha.)]-
25.64
0.28
–
–
–
–
19
.gamma.-Sitosterol
–
–
–
–
25.67
4.99
20
Dibenz[a,c]cyclohexane,2,4,7-trimethoxy-
25.87
0.47
–
–
–
–
26.55
0.60
21
S-Indacene-1,7-dione,2,3,5,6-tetrahydro-3,3,4,5,5,8-hexamethyl-
27.67
4.79
–
–
–
–
22
Macckiain
29.12
2.32
–
–
–
–
23
Formononetin
31.57
17.71
–
–
–
–
24
Pseudobaptigenin
32.00
5.40
–
–
–
–
In the experiment for extracting the mixed solution of ethanol and methanol, 10 types of chemical components were identified, which occupied 12.14% of total peaks areas. There were benzene,1,2,3-trimethoxy-5-(2-propenyl)- (4.69%), Myo-inositol, 4-C-methyl- (3.79%) and nerolidol (1.27%).
In the experiment for extracting the mixed solution of ethanol and benzene, 10 types of chemical components were identified, which occupied 40.00% of total peaks areas. These were Myo-inositol, 4-C-methyl- (14.48%), nerolidol (7.04%), gamma-sitosterol (4.99%), 1,2-benzenedicarboxylic acid, bis(2-methylpropyl) easter (3.82%), 3-O-methyl-d-glucose (3.33%), dibutyl phthalate (2.76%), benzene,1,2,3-trimethoxy-5-(2-propenyl)- (1.81%) and alpha-methylstyrene (0.91%).
In conclusion, the extractive chemical components differed depending on the solvent used and the part of the wood. The chemical components of bark were the least, only about 10% of the whole tree; therefore, the extracts of bark were fewest. There was less cellulose and pentose in the bark. Heartwood had more organic solvent extraction and fewer lignin and cellulose than sapwood.
3.3 Functions of chemical components
Triacetin is also called Glyceryl Triacetate. It can be used as a cosmetic biocide, solvent and plasticizer of cosmetic. Experts have concluded that the use of triacetin in cosmetic formulations is safe. It is often used as a carrier for flavors and fragrances (Fiume, 2003). Transesterification of triacetin and methanol can be used with homogeneous alkali catalysts to produce biodiesel (López et al., 2005).
Methyleugenol has many functions, such as anti-fungal, anti-bacterial, anti-nematode, toxic effects on pathogens, and causing antifeedant and anti-pollination in insect herbivores (Huang et al., 2002; Tan and Nishida, 2012). As an added flavoring substance, methyleugenol is also a component present in the traditional diet (Smith et al., 2002).
Isolated asarone can function against excitotoxic neuronal death in primary cultured rat cortical cells. It has neuroprotective action (Cho et al., 2002). Asarones from the rhizomes of Acorus tatarinowii is considered as a new drugs for treating depression (Han et al., 2013), Asarone is the active components in Acorus tatarinowii Schott, which is the traditional Chinese medicine and has been used to treat epilepsy for several thousands of years (Deng et al., 2010).
Nerolidol is widely used in different industries, used in food flavoring, detergents and cleansers (Chan et al., 2016) Nerolidol has antifungal activity (Lee et al., 2007) and antiulcerogenic activity (Klopell et al., 2014a, 2014b). It can be used for the skin to improve skin lesions infected by M. gypseum. Nerolidol may be an effective supplement to topical antifungal drugs for clinical relief of dermatophytosis. Significantly improved oxidative stability using 4-allyl-2,6-dimethoxyphenol as an additive (Klopell et al., 2014a, 2014b). 3,4-Dimethoxycinnamic acid is a prospective dietary compound for prophylaxis of neurodegenerative diseases (Zanyatkin et al., 2017).
Dibutyl phthalate is used as a plasticizer in elastomers, resin solvent, textile lubricating agent and adhesives. Dibutyl phthalate can also be used as a perfume solvent in the production of cosmetics and as a lubricant for aerosol valves, a skin emollient, a suspension agent for solids in aerosols, and an antifoamer. Dibutyl phthalate is known to be a developmental and repreoductive toxicant. It may have an adverse effect on the uterus of rodents, at least in part of which is responsible for the loss of early embryos (Ema et al., 2000; Higuchi et al., 2003).
For FT-IR analysis, the absorption peaks did not migrate significantly. This shows that the chemical components of the samples did not change severely in the process of extraction. The absorption peak of FT-IR had some change due to the difference of extract solvents, and the chemical components were hydrolyzed to some extent.
In the analysis of GC/MS, 37 types of chemical components were detected. The extractive chemical components were different depending on the solvent used and the part of the wood. The chemical components of bark were fewest with only about 10% of the whole tree; therefore, the extracts of bark were the fewest. There was less cellulose and pentose in the bark. Heartwood had more organic solvent extraction and fewer lignin and cellulose than sapwood.
The following is part of the chemical components and functions:
Triacetin can be used as a cosmetic biocide, solvent in cosmetic formulations, plasticizer, and is commonly used as carrier for flavors and fragrances. Methyleugenol has anti-fungal, anti-bacterial, anti-nematode, and toxic effects on pathogens and is a traditional diet and as added flavoring substance. Asarone has neuroprotective action and it is a new therapeutic agent for curing depression. Nerolidol is widespread across shampoos, perfumes, detergents, cleansers, food flavoring, and antifungal drugs. 3,4-Dimethoxycinnamic acid can prevent neurodegenerative diseases. Dibutyl phthalate is used as a plasticizer in elastomers, textile lubricating agent, resin solvent, and in safety glass, printing inks, paper coatings, adhesives, skin emollient, and lubricants for aerosol valves.
Conflict of interest
All the authors hereby agreed and confirm that there is no conflict of interest for this research work and publication of this paper.
Acknowledgments
This work was financially supported by the National Natural Science Fundation of China (31872697). The Hunan Science Fund for Distinguished Young Scholars (16JJ1028).
References
- Study of sorption processes and FT-IR analysis of arsenate sorbed onto red muds (a bauxite ore processing waste) J. Hazard. Mater.. 2010;175:172-178.
- [Google Scholar]
- Nerolidol: a sesquiterpene alcohol with multi-faceted pharmacological and biological activities. Molecules. 2016;21:529.
- [Google Scholar]
- Protection of cultured rat cortical neurons from excitotoxicity by asarone, a major essential oil component in the rhizomes of Acorus gramineus. Life Sci.. 2002;71:591-599.
- [Google Scholar]
- GC-MS analysis of essential oils from some Greek aromatic plants and their fungitoxicity on Penicillium digitatum. J. Agric. Food Chem.. 2000;48:2576-2581.
- [Google Scholar]
- Development of gas chromatography/mass spectrometry following headspace solid-phase microextraction for fast determination of asarones in plasma. Rapid Commun. Mass Spectr. RCM. 2010;20:2120-2126.
- [Google Scholar]
- Effects of dibutyl phthalate on reproductive function in pregnant and pseudopregnant rats. Reprod. Toxicol.. 2000;14:13.
- [Google Scholar]
- Antidepressant-like effects of essential oil and asarone, a major essential oil component from the rhizome of Acorus tatarinowii. Pharm. Biol.. 2013;51:589-594.
- [Google Scholar]
- Effects of dibutyl phthalate in male rabbits following in utero, adolescent, or postpubertal exposure. Toxicol. Sci.. 2003;72:301-313.
- [Google Scholar]
- Insecticidal properties of eugenol, isoeugenol and methyleugenol and their effects on nutrition of Sitophilus zeamais, Motsch. (Coleoptera: Curculionidae) and Tribolium castaneum, (Herbst) (Coleoptera: Tenebrionidae) J. Stored Prod. Res.. 2002;38:403-412.
- [Google Scholar]
- Nerolidol, an antiulcer constituent from the essential oil of Baccharis dracunculifolia DC (Asteraceae) Zeitschrift Fur Naturforschung C A J. Biosci.. 2014;62:537-542.
- [Google Scholar]
- Oxidation stability of biodiesel fuels and blends using the Rancimat and PetroOXY methods. Effect of 4-allyl-2,6-dimethoxyphenol and catechol as biodiesel additives on oxidation stability. Front. Chem.. 2014;2:43.
- [Google Scholar]
- Antifungal effect of eugenol and nerolidol against Microsporum gypseum in a guinea pig model. Biol. Pharm. Bull.. 2007;30:184.
- [Google Scholar]
- Chemistry of Chinese taxus, Taxus chinensis var. mairei. Biochem. Sys. Ecol.. 2008;36:266-282.
- [Google Scholar]
- Chemical structure characteristics of wood/lignin composites during mold pressing. Polym. Compos.. 2017;38:955-965.
- [Google Scholar]
- Chemical structure of hemicellulosic polymers isolated from bamboo bio-composite during mold pressing. Polym. Compos.. 2017;38:2009-2015.
- [Google Scholar]
- Transesterification of triacetin with methanol on solid acid and base catalysts. Appl. Catal. A General. 2005;295:97-105.
- [Google Scholar]
- Ma, Q.Z., Peng, W.X., Zhang, D.Q., 2008a. Effect of nano TiO2 on state of cure and pyrolytic reaction of phenol-formaldehyde resin. In: IEEE International Conference on Nano/micro Engineered and Molecular Systems, IEEE, 574–577.
- Ma, Q.Z., Zhang, D.Q., Peng, W.X., 2008b. Determination of Chemical Components of Benzene/Ethanol Extractives of Chinese-Fir Wood by GC/MS. In: The 2nd International Conference on Bioinformatics and Biomedical Engineering, 3196–3198.
- The hydrogen bond network in I β, cellulose as observed by infrared spectrometry. J. Mol. Struct.. 2000;523:183-196.
- [Google Scholar]
- Research on regional clustering and two-stage SVM method for container truck recognition. Discrete Cont. Dynamical Sys. Ser. S. 2019;12:1117-1133.
- [Google Scholar]
- A novel controlled release formulation for the anticancer drug paclitaxel (Taxol): PLGA nanoparticles containing vitamin E TPGS. J. Contr. Rel. Official J. Contr. Rel. Soc.. 2003;86:33-48.
- [Google Scholar]
- Adsorption characteristics of sulfur powder by bamboo charcoal to restrain sulfur allergies. Saudi J. Biol. Sci.. 2017;24:103-107.
- [Google Scholar]
- Characteristics of antibacterial molecular activities in poplar wood extractives. Saudi J. Biol. Sci.. 2017;24:399-404.
- [Google Scholar]
- High throughput sequencing technology reveals that the taxoid elicitor methyl jasmonate regulates microRNA expression in Chinese taxus (Taxus chinensis) Gene. 2009;436:37-44.
- [Google Scholar]
- Component of non-woody plant lignins and cinnamic acids by Py-GC/MS, Py/TMAH and FT-IR. J. Analytical Appl. Pyrol.. 2007;79:39-46.
- [Google Scholar]
- Effects of short-time vibratory ball milling on the shape of FT-IR spectra of wood and cellulose. Vib. Spectrosc.. 2004;36:23-40.
- [Google Scholar]
- A deep Q-learning network for ship stowage planning problem. Pol. Marit. Res.. 2017;24:102-109.
- [Google Scholar]
- Safety assessment of allylalkoxybenzene derivatives used as flavouring substances — methyl eugenol and estragole. Food Chem. Toxicol. Int. J. Publ. British Indust. Biol. Res. Assoc.. 2002;40:851.
- [Google Scholar]
- Methyl eugenol: its occurrence, distribution, and role in nature, especially in relation to insect behavior and pollination. J. Insect Sci.. 2012;12:56.
- [Google Scholar]
- Chemical component and antifungal activity of essential oil isolated from Chamaecyparis formosensis Matsum. wood. Holzforschung. 2005;48:3008-3299.
- [Google Scholar]
- Inhibition of prion propagation by 3,4-dimethoxycinnamic acid. Phytother. Res. Ptr.. 2017;31:1046-1055.
- [Google Scholar]







