Translate this page into:
Chemical characterization and antioxidant of polysaccharide extracted from Dioscorea bulbifera
⁎Corresponding author. sirlei@utfpr.edu.br (Sirlei Dias Teixeira)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The air yam (Dioscorea bulbifera) is an unconventional vegetable that produces a reserve stem, which has polysaccharides in its composition. These biopolymers, in turn, have aroused industrial interest due to many of their properties and applications in several areas, such as agro-food, chemical, pharmaceutical, textile, metallurgical, oil, among others. The objective of this work is to characterize the polysaccharide present in the raw and pre-purified air yams, as well as to verify its potential antioxidant capacity. The FTIR analysis showed in the two samples, a characteristic OH band at approximately 3250 cm−1, which is characteristic of polysaccharides. Through the SEM it was possible to analyze the morphology of the samples and verify that they are composed of films or “sheets” without measurable format. Furthermore, by XRD the samples were found to be an amorphous material, with peaks of crystallinity being observed at approximately 21.54° and 23.62° 2θ. Thermal analysis indicates that the NDP sample presents three regions of mass loss, the first one running up to 100 °C and may be associated with water loss; the second occurs from 150 °C, and occurs in several steps that can be visualized by DTG, and the third from 550 °C. The organic matter present in the sample was about 84%. The DP (Dialysed Polysaccharide) sample, in turn, also presented three regions of mass loss, the first one occurring up to 150 °C, the second from 175 °C and the third, from 425 °C. All these mass losses occur in only one stage. The organic matter present in the sample was approximately 80%. The samples presented potential antioxidant activity when analyzed by DPPH, ABTS, FRAP, OH radical removal, H2O2 removal and reducing power. The obtained results are very important, as they reveal information about the chemical characteristics and potential bioactivity of the dialysed and non-dialysed polysaccharide of D. bulbifera.
Keywords
Antioxidants
XRD
FTIR
SEM
Thermal Analysis
1 Introduction
From the earliest days of mankind, various cultures and peoples have already made use of plants or plant-derived products for the treatment of different diseases in both humans and animals. In recent years, numerous known plant species have been used for the development of drugs and products that are beneficial to human health (Abbasi et al., 2013; Bieski et al., 2015). Brazil, as a country with a continental dimension, has an enormous biotechnological potential, due to the great biodiversity of fauna and flora. Hence the importance of the study and characterization of plants and products derived from them, in order to identify new bioactive compounds and to elucidate their mechanisms of action.
Dioscorea bulbifera, also known as air yam, is an unconventional vegetable, originating from the Asian and African continents. It was introduced in Brazil by the Portuguese colonizers. It produces a reserve stem that can be used in cooking as a substitute for potato, but the plant is still somewhat unknown and therefore has low commercial value (Brasil, 2010). In the literature there are few reports on this tuber, but some indicate the presence of saponins, flavonoids, terpenoids, phenolic compounds, tannins, cardiac glycosides, alkaloids, oxalates, organic acids and polysaccharides (Adeosun et al., 2016, Eleazu et al., 2013, Shajeela et al., 2013; Yang et al., 2016).
The polysaccharides are the result of condensation of a large number of aldoses and ketoses, possess high molecular weight, and great structural complexity; and inorganic compounds are also present (Simões et al., 2010, Le Costaouëc et al., 2017).
Polysaccharides can be extracted from different raw materials, including fungi, bacteria and plants. Polysaccharides from plants (plant polysaccharides) may be present in algae, plant exudates, seeds, fruits, cereals and tubers (Cunha et al., 2009).
Natural polysaccharides have aroused a biotechnological interest, since some have immunomodulatory, antioxidant, anticoagulant, anti-inflammatory, antitumor, antimicrobial, hypoglycemic and antiviral capacity (Meng et al., 2016; Xie et al., 2016; Wang et al., 2016; Zhang et al., 2016).
The objective of this work is to obtain and purify the polysaccharide present in D. bulbifera, characterizing it by FTIR, SEM, DR-X and Thermal Analysis, in addition to evaluating the antioxidant capacity of the polysaccharide, with the intention of contributing to the development of products and add commercial value to this unconventional vegetable.
2 Materials and methods
2.1 Plant material
The collection of the botanical material was carried out in the interior of the municipality of São Lourenço do Oeste, Santa Catarina, in March 2017. The identification was carried out and the exsiccate-specimen deposited in the Herbarium of the Federal Technological University of Paraná, Campus Pato Branco, under the number HPB 1103.
2.2 Sample preparations
The tubers were washed, peeled and cut to standard size (1 cm3). The vegetable material was bleached with boiling water for 4 min, so that the enzymes responsible for the darkening of the tuber were inactivated (Correia et al., 2008). After application of the short-time thermal process, the material was treated with ethanol (95%) for 2 h on ultrasound, to remove the yellow coloration. Then, the plant material was immersed in water for rehydration.
2.3 Extraction of polysaccharide
The extraction of the polysaccharide present in the air yam was carried out according to the methodology suggested by Maity et al. (2014), with modifications. A 1:1 ratio of sample and water mixture was placed in a water bath at 80 °C for 1 h, then stored at 4 °C for 14 h. Subsequently, the sample was centrifuged at 4 °C, 8000 rpm for 50 min. The supernatant containing the polysaccharide was collected, precipitated with ethanol (95%), lyophilized and stored for further analysis.
A portion of this polysaccharide underwent partial purification by dialysis to obtain the pre-purified (dialysed) polysaccharide (DP), which was subjected to the same analysis as that of the crude (non-dialysed) polysaccharide (NDP).
2.4 Chemical screening
2.4.1 Fourier Transform Infrared Spectroscopy (FTIR)
Fourier Transform Infrared Spectroscopy (FTIR) was performed in the region of 4000–400 cm−1 using the attenuated total reflectance (ATR) method with a Perkin Elmer Spectrometer Frontier.
2.4.2 Scanning electron microscopy (SEM)
Scanning electron microscopy (SEM) was performed using a Hitachi 3000 Scanning Electron Microscope. Images were obtained with a magnification of 200 times.
2.4.3 X-Ray diffraction (XRD)
The X-ray diffractograms were obtained with a Rigaku Mini Flex 600 equipment and were performed with using copper lamp radiation (Cu-Kα) source operating at 15 mA and 40 kV, with a speed of 3° min−1 and a step of 0.02°.
2.4.4 Thermal Analysis
The samples were submitted to Thermogravimetric Analysis (TGA), Differential Thermal Analysis (DTA) and Derived Thermogravimetry (DTG). The mass loss was monitored at a heating rate of 10 °C min−1, between 26 °C and 800 °C with synthetic air flow of 50 mL min−1 using a TA instruments Q 600.
2.5 Antioxidant assay
2.5.1 DPPH free radical scavenging capacity
The determination of the antioxidant activity by the free radical sequestration method DPPH (2,2-diphenyl-1-picrylhydrazyl) was performed according to the methodology described by Brand-Williams et al. (1995), with some modifications: 0.5 mL of the standards or previously diluted sample were added in test tubes, with the subsequent addition of 3.0 mL of ethanol (50% v/v) and 0.3 mL of the DPPH alcohol solution (0.5 mmol L−1). After 45 min of reaction, the reading was performed using a spectrophotometer at the wavelength of 517 nm. For the construction of the standard curve, the synthetic antioxidant Trolox was used in different concentrations (15, 25, 50, 75 and 100 μmol L−1).
2.5.2 Antioxidant activity assay using the ABTS radical cation decolorization method
This method is used to measure the antioxidant activity by capturing the 2,2-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) or ABTS radical, and was performed according to the methodology proposed by Re et al. (1999), with modifications. First, the ABTS radical was generated from the reaction of the ABTS reagent (7 mmol L−1) with potassium persulfate (140 mmol L−1). Such a mixture was maintained at room temperature and no light exposure for a period of 16 h. A solution of ethanol (40% v/v) was diluted with the ABTS solution until it reached absorbance of 0.700 at the 734 nm wavelength. Then, using test tubes, 30 μL of the already diluted samples and 3 mL of the ABTS solution were added. The standard curve was obtained using the synthetic antioxidant Trolox at various concentrations (100, 500, 1000, 1500 and 2000 μmol L−1).
2.5.3 Antioxidant activity assay using the ferric reducing antioxidant power (FRAP) method
The assay was carried out using the methodology suggested by Benzie and Strain (1996) was used; with modifications. The FRAP reagent was prepared by mixing 25 mL of acetate buffer solution (300 mmol L−1, pH 3.6), 2.5 mL of TPTZ solution (10 mmol L−1 in 40 mmol L−1 HCl) and 2.5 mL of FeCl3 (20 mmol L−1) in aqueous solution.
Test tubes containing 100 μL of the properly diluted sample and 3 mL of the FRAP reagent were prepared. The tubes were then stored at 37 °C in a water bath and in the absence of light for 30 min. The calibration curve was obtained using ferrous sulfate standard at different concentrations (200, 500, 1000, 1500, 2000 μmol L−1). The absorbance of the solutions was measured at a wavelength of 595 nm.
2.5.4 Sequestration activity of the hydroxyl radical
The hydroxyl radical sequestration activity was determined following the procedure of Liu et al. (2010), with modifications. A test tube was prepared containing 5 mL FeSO4 (1.5 mmol L−1), 0.35 mL H2O2 (6 mmol L−1), 0.15 mL sodium salicylate (20 mmol L−1) and 1 mL of the already diluted polysaccharide. As standard for comparison, ascorbic acid and glucose were used at the same sample concentration. For control 1, all reagents were placed in another tube, replacing the sample with water. As control 2, a mixture containing all reagents and the sample, except sodium salicylate, was prepared. After the incubation time of 1 h at 37 °C, the absorbance of the salicylate-hydroxylated complex was measured with a spectrophotometer at 562 nm. The percentage of removal was calculated using the equation below:
In which A1 represents the absorbance of the sample, ascorbic acid or glucose. A0 is the absorbance of control 1 and A2 is the absorbance of control 2.
2.5.5 H2O2 removal activity
The ability to remove H2O2 was determined according to Liu et al. (2010). A solution was prepared with 1 mL of freshly prepared H2O2 (0.1 mmol L−1), 1 mL of previously diluted polysaccharide, 0.1 mL of ammonium molybdate (3% w/v), 10 mL of H2SO4 (2 mol L−1), and 7 mL potassium iodide (KI) (1.8 mol L−1). The mixture was titrated with Na2S2O3 (5 mmol L−1) until the yellow color disappeared. For comparison, the removal capacity of vitamin C and glucose at the same sample concentration was determined. The removal activity was calculated according to the following equation:
In which V0 represents the volume of the Na2S2O3 solution used to titrate the control mixture and V1 the titration volume of the sample-containing mixtures.
2.5.6 Reducing power
The reducing power was evaluated according to Liu et al. (2010) by mixing 2.5 mL of the diluted polysaccharide with 2.5 mL of potassium ferricyanide (1% w/v) and incubating the mixture at 50 °C for 20 min. The reaction was terminated by the addition of 2.5 mL of TCA solution (10% w v trichloroacetic acid) followed by addition of 5 mL of deionized water and 1 mL of ferric chloride solution (0.1% w/v) and the absorbance of the solution was measured at 700 nm. To compare the reducing power, vitamin C and glucose were used in the same sample concentration. A higher absorbance of the mixture will indicate greater reducing power of the sample.
3 Results and discussion
3.1 Chemical screening
The FTIR spectra of the NDP samples (Fig. 1A) and PD (Fig. 1B) are similar to each other, but with some different absorption bands. In both spectra there is a band attributed to the presence of OH at 3260 cm−1 and 3250 cm−1, respectively, these bands are considered to be characteristic of polysaccharides (Jahanbin et al., 2017; Liu et al., 2017; Meng et al., 2017; Rashid et al., 2018; Ren et al., 2017; Souza et al., 2015). The existing band at 2938 cm−1 corresponds to the vibration of C–H stretching, especially methyl (Liu et al., 2017; Meng et al., 2017; Rashid et al., 2018; Ren et al., 2017; Silva-Leite et al., 2017). In the DP spectrum we can observe a band in the region of 2360 cm−1, characteristic of CO2 absorption, which often appears due to CO2 saturation present in the analysis environment (Silverstein et al., 2006). The presence of pyranose was observed in 1020 cm−1 (Fig. A) and 1030 cm−1 (Fig. B), a characteristic band of carbohydrates that may indicate the its presence (Liu et al., 2017; Meng et al. 2017; Ren et al., 2017; Silva-Leite et al., 2017). It is important to emphasize that this band is better defined in the DP sample, which can be associated to the removal of impurities and the pre-purification to which the sample was submitted. Another important difference is found in the region of 1730 cm−1 (Fig. A) and 1720 cm−1 (Fig. B) characteristic of compounds that present carbonyl, almost nonexistent in the NDP sample and well defined in the DP sample. However, the absorptions between 1200 and 800 cm−1, observed in both spectra, are also characteristic of carbohydrates (Souza et al., 2015).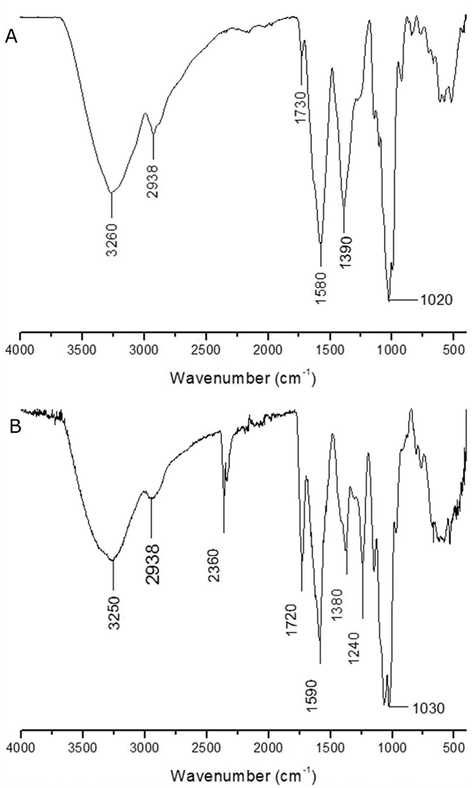
FTIR spectrum of (A) NDP and (B) DP of the D. bulbifera.
As shown by the images obtained through SEM (Fig. 2), it is possible to observe that both the NDP (Fig. 2A) and the DP (Fig. 2B) have very similar morphology, both of which have the characteristic of forming films or “sheets” with no defined and measurable format. The difference is that NDP presents in a more compacted form, with the “sheets” more closely packed and denser when compared to NP. Studies with other polysaccharides detected morphology very similar to those found in this work. Pan et al., (2017) conducted studies with a heteropolysaccharide of corn silk, the micrograph of which was very similar to DP (Fig. 2B), they associated the pores present in the sample to the lyophilization process; Yu et al. (2017), studied the polysaccharide present in American ginseng and the same presented structure similar to the dry leaves and laminated structures as those observed for DP and NDP in the present study. Zhao et al. (2017), analyzed the existing polysaccharide in Dioscorea hemsleyi, which also appeared in sheet form very similar to the morphologies of NDP and DP.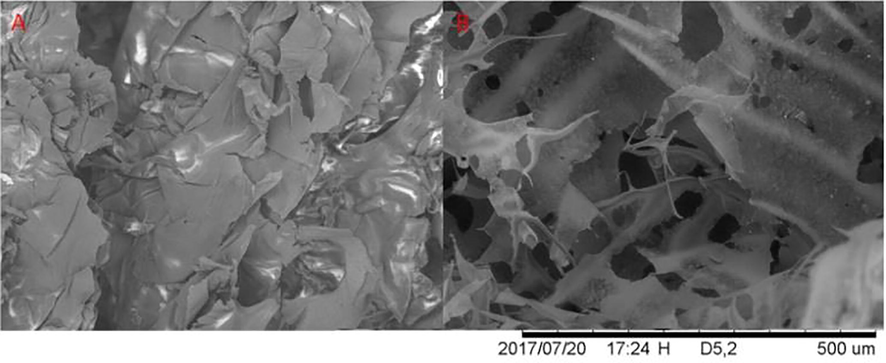
Scanning electron microscopy of (A) NDP and (B) DP, magnification of 200 times.
To evaluate the crystallinity of the DP and NDP polysaccharide samples, XRD analysis was used, the results are shown in Fig. 3. The diffractograms of the NDP and DP samples show crystallinity peaks at 21.54° and 23.62° 2θ (Degrees) in both samples. However, most of the material is amorphous, which is very common in samples of this nature. This profile was also observed in several studies with different types of polysaccharides (Ballesteros et al., 2016; Zhang et al., 2017). It is also observed that the DP sample (Fig. 3) presented more intense and defined peaks, which indicates that the partial purification of the sample improved the crystallinity. In the literature, there are some studies on starch, which is also a polysaccharide, obtained from different species of Dioscorea, including D. bulbifera itself. However, the diffractograms of these starches are completely different when compared to the diffractograms of DP and NDP, which makes it possible to state that DP and NDP are not samples composed of starch (Hornung et al., 2017).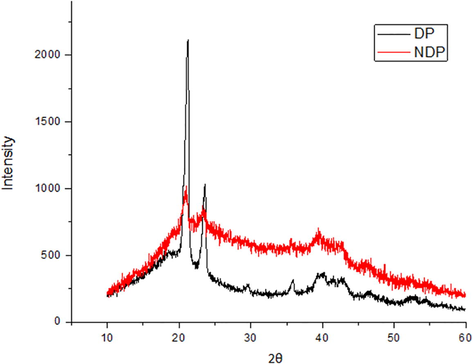
X-ray Diffractogram of NDP and DP.
Through the TG curve of the NDP sample (Fig. 4) it is possible to verify a mass loss before 100 °C, confirmed by the DTG peak at 57.98 °C, which can be associated with sample dehydration, that can be confirmed by the slight decrease at the beginning of the DTA and which characterizes an endothermic reaction. A second mass loss is verified from 150 to 500 °C, but when the DTG is analyzed, it can be seen that this step actually unfolds in several other steps. In DTG there are two peaks that overlap in the region of 150–300 °C. In addition, in the region of 310–550 °C there are several decomposition peaks, indicating a large loss of mass, which occurs in several stages. From 550 to 700 °C it is verified by the TG that the sample suffers another mass loss and the well-defined peak observed at approximately 677.9 °C in the DTG proves that this event occurs in only one step. Through the DTA we can verify a well-defined exothermic peak at 688.72 °C, but throughout the process it is possible to observe other exothermic peaks that are not so defined. Therefore, it can be said that the NDP sample is not very thermally stable and has several stages of decomposition, stabilizing at a temperature close to 700 °C. It can also be stated that the amount of organic matter of the NDP sample reaches 84% and the residue of the thermal analysis consists of inorganic compounds present in the sample.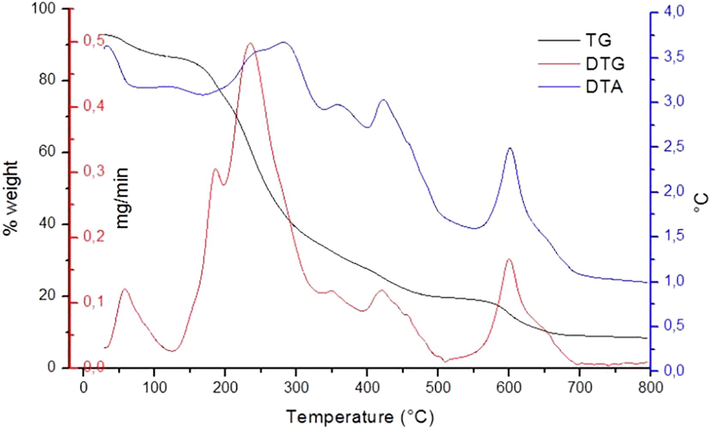
TGA, DTA and DTG curves of the NDP sample.
In Fig. 5, it can be seen from the TG curve that the DP sample has three stages of mass loss, which occur in a single step, and can be seen in both TG and DTG. The first decomposition reaction observed at TG occurs before the temperature at 150 °C and is confirmed by the DTG peak at 63.23 °C, which may be linked to sample dehydration. The second mass loss observed by the TG is between 175 and 400 °C and is confirmed by the well-defined peak of the DTG at 286.34 °C, this being the most significant step in the process. Finally, the last observed mass loss occurs in the range of 425–600 °C in TG, and the DTG peak in 454.73 °C corresponds to this decomposition. Through the DTA, two exothermic peaks are verified during the analysis, they occur very close to, or at the same temperature as the DTG peaks. In this way, it can be affirmed that the decomposition of the DP sample occurs in three well defined stages, and can be associated to the dialysis of the sample, since many impurities were eliminated in this step. The decomposition stabilizes near the temperature of 600 °C, which is lower than the stabilization of the NDP sample. This may be due to the lower amount of organic matter present in the sample, due to having been subjected to the dialysis process, besides the ease in the degradation of the organic matter present in the sample in comparison with the previous one. It was possible to determine by TG analysis that the amount of organic matter present in the sample reaches 80%, not too much lower than the one found for the NDP sample.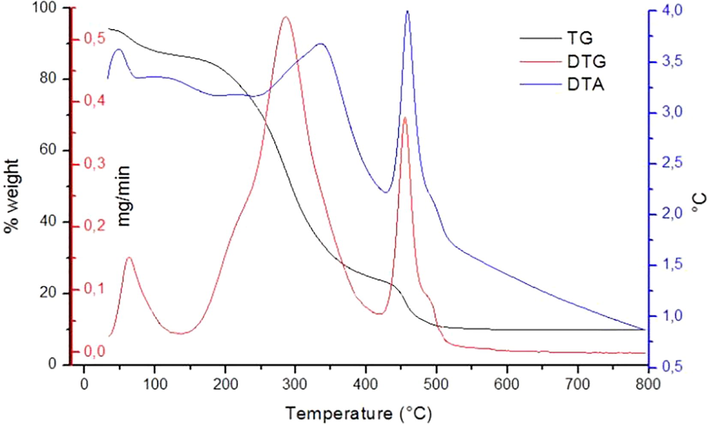
TGA, DTA and DTG curves of the DP sample.
Determining the antioxidant activity through the DPPH, ABTS and FRAP methods, it was possible to verify that the NDP sample presented higher antioxidant activity when analyzed using the DPPH and FRAP methods, while the DP sample was highlighted when analyzed by the ABTS method, as can be seen in Table 1.
Method
NDP
DP
DPPH (mg eq. Trolox g−1)
0.94 ± 0.02
0.28 ± 0.01
ABTS (mg eq. Trolox g−1)
165.00 ± 2.36
623.33 ± 4.71
FRAP (mmol Fe2+ g−1)
1.056 ± 0.001
0.175 ± 0.001
From these results it can be inferred that, for the DPPH and FRAP methods, the antioxidant activity determined in the samples is closely linked to the impurities present, because when the polysaccharide was pre-purified the antioxidant activity decreased considerably, especially when considering the results obtained by the FRAP method. The DP sample presented higher antioxidant capacity when evaluated by the ABTS method, indicating that, in this case, the impurities probably decreased the quantified antioxidant activity (Table 1).
Oligosaccharides extracted from the peanut shell (Rico et al., 2018), presented antioxidant activity, evaluated by the DPPH and ABTS methods. Values ranged from 9.6 to 11.8 mg eq. Trolox g−1 for DPPH, therefore, superior when compared to the values found for the NDP and DP samples extracted from the air yam tuber, (0.94 and 0.28 mg eq. Trolox g−1 for DPPH, respectively). The ABTS results for the peanut shell were between 34.6 and 53.0 mg eq. Trolox g−1, which in this case are much lower than those determined for the polysaccharide extracted from the air yam tuber, NDP and DP (165.00 and 623.33 mg eq. Trolox g−1, respectively). The analysis of polysaccharide present in different varieties of the tuberous lotus root (Yi et al., 2017) detected antioxidant activity by the FRAP method, and the results ranged from 0.10 to 1.85 mmol Fe2+ g−1 values similar to those found for the D. bulbifera polysaccharide, (0.175 and 1.056 mmol Fe2+ g−1), values that may be justified by the similarity of the polysaccharide sources.
Polysaccharides generally present lower antioxidant activity than that which is found for other plant extracts and/or metabolites, but are still relevant and very interesting results, especially when considering the application of these polysaccharides, since this property will be found in the final product.
In the OH removal experiment, it can be seen that vitamin C can remove almost 100% of the present hydroxyl radical (Fig. 6), since it is considered a positive control, whereas the negative control glucose removes only around 10%, being the negative control for the assays.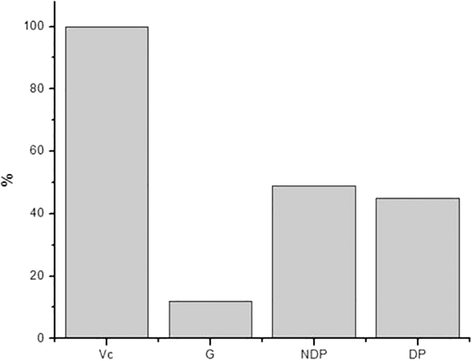
Removal of the hydroxyl radical by the samples tested.
Fig. 6 shows that the NDP and DP samples showed removal of 49% and 45%, respectively, that is, they remove almost half of the hydroxyl radical, which is a satisfactory result considering that the hydroxyl radical, when present in the body, has free access to cell membranes and can cause tissue damage. A study with the polysaccharide extracted from Sophorae tonkinensis Radix showed the ability to remove up to 74% of the hydroxyl radical (Cai et al., 2018).
Although hydrogen peroxide is not very reactive, it induces cell aging and can attack cells due to the high penetrability of cell membranes, therefore the importance of its removal (Giese et al., 2015). Fig. 7 shows the results obtained for the NDP and DP samples in the H2O2 removal, as well as the glucose and vitamin C samples.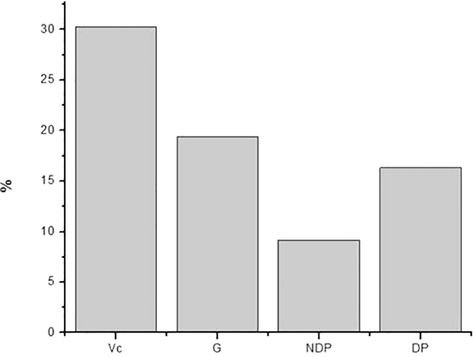
Removal of H2O2 by the samples.
It can be observed that the NDP and DP samples had a capacity to remove 9% and 16% of H2O2, respectively. The values obtained for the NDP and DP samples are very interesting when compared to the positive standard, vitamin C, which showed only 30% removal of H2O2, even though it is a widely used standard antioxidant. In addition, it is important to note that H2O2 removal is higher in PD, which shows that the impurities present in the NDP sample do not present this type of antioxidant activity. Studies with laminarin showed a 19% H2O2 removal power, a value very close to that found in the DP sample (Fig. 7) (Giese et al., 2015).
The reducing power of the samples is verified according to the final absorbance, the higher the absorbance the greater the reducing power of the samples. In Fig. 8 it is observed that the reducing power of the NDP sample was 0.30, whereas of the DP sample it was 0.089. It can be stated that the reducing power of the samples is related to the impurities, because the dialysed polysaccharide presented lower reducing power to the one in the NDP sample.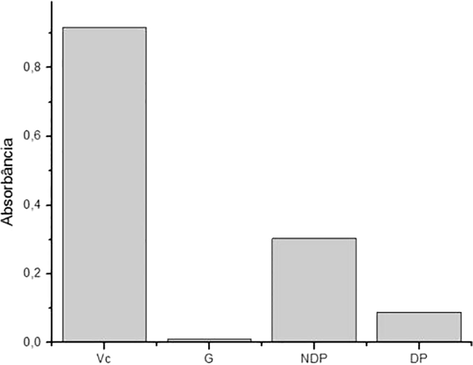
Reducing power of the samples tested.
A study with the polysaccharide present in the barley evaluated its antioxidant activity by the reducing power methodology and obtained absorbance values ranging from 0.09 to 0.28, (Ahmad et al., 2016) values that are close to those found in the present work.
4 Conclusions
By means of the performed analyzes, it can be observed that both the NDP and DP samples are composed of a polysaccharide, which is an amorphous material with crystalline peaks and with a film-like aspect. It was also possible to define its stages of thermal decomposition. The samples presented significant antioxidant activity when compared with other reports of polysaccharides found in the literature. These results may guide future works with this material, enabling the development of applications for the polysaccharide, especially in the area of food safety as biofilm matrix.
Acknowledgements
The authors gratefully acknowledge the Central Analysis from Federal Technological University of Parana (UTFPR) for the assisting in the development of experimental assays and the Araucaria Research Foundation for financial support.
References
- Ethnobotanical survey of medicinally important wild edible fruits species used by tribal communities of Lesser Himalayas-Pakistan. J. Ethnopharmacol.. 2013;148:528-536.
- [Google Scholar]
- Antibacterial activities and phytochemical properties of extracts of Dioscorea bulbifera Linn (Air Potatoe) tubers and peels against some pathogenic bacteria. J. Phytopharm.. 2016;5:20-26.
- [Google Scholar]
- Germination and microwave processing of barley (Hordeum vulgare L) changes the structural and physicochemical properties of β-D-glucan and enhances its antioxidant potential. Carbohydr. Polym.. 2016;153:696-702.
- [Google Scholar]
- Extraction of polysaccharides by autohydrolysis of spent coffee grounds and evaluation of their antioxidant activity. Carbohydr. Polym.. 2016;157:258-266.
- [Google Scholar]
- The ferric reducing ability of plasma (FRAP) as a measure of “Antioxidant Power”: The FRAP assay. Anal. Biochem.. 1996;239:70-76.
- [Google Scholar]
- Ethnobotanical study of medicinal plants by population of Valley of Juruena Region, Legal Amazon, Mato Grosso. Brazil. J. Ethnopharmacol.. 2015;173:383-423.
- [Google Scholar]
- Use of a free radical method to evaluate antioxidant activity. LWT - Food Sci. Technol.. 1995;28:25-30.
- [Google Scholar]
- Brasil, 2010. Manual de Hortaliças Não-Convencionais.
- Structural characterization, antioxidant and hepatoprotective activities of polysaccharides from Sophorae tonkinensis Radix. Carbohydr. Polym.. 2018;184:354-365.
- [Google Scholar]
- Phytochemical composition and antifungal actions of aqueous and ethanolic extracts of the peels of two yam varieties. Med. Aromat. Plants.. 2013;2:2-5.
- [Google Scholar]
- Efeitos do processamento industrial de alimentos na estabilidade de vitaminas. Alim. Nutr.. 2008;19:83-95.
- [Google Scholar]
- Polissacarídeos da biodiversidade brasileira: Uma oportunidade de transformar conhecimento em valor econômico. Quim. Nova.. 2009;32:649-660.
- [Google Scholar]
- Free-radical scavenging properties and antioxidant activities of botryosphaeran and some other β-D-glucans. Int. J. Biol. Macromol.. 2015;72:125-130.
- [Google Scholar]
- Enhancement of the functional properties of Dioscoreaceas native starches: Mixture as a green modification process. Thermochim. Acta.. 2017;649:31-40.
- [Google Scholar]
- Isolation, purification and structural characterization of a new water-soluble polysaccharide from Eremurus stenophyllus (boiss. & buhse) baker roots. Carbohydr. Polym.. 2017;178:386-393.
- [Google Scholar]
- New structural insights into the cell-wall polysaccharide of the diatom Phaeodactylum tricornutum. Algal Research.. 2017;26:172-179.
- [Google Scholar]
- Characterization and antioxidant activity of two low-molecular-weight polysaccharides purified from the fruiting bodies of Ganoderma lucidum. Int. J. Biol. Macromol.. 2010;46:451-457.
- [Google Scholar]
- Purification, characterization, and bioactivities of a polysaccharide from mycelial fermentation of Bjerkandera fumosa. Carbohydr. Polym.. 2017;167:115-122.
- [Google Scholar]
- Structure elucidation and antioxidant properties of a soluble β-d-glucan from mushroom Entoloma lividoalbum. Int. J. Biol. Macromol.. 2014;63:140-149.
- [Google Scholar]
- Isolation, purification, structural analysis and immunostimulatory activity of water-soluble polysaccharides from Grifola Frondosa fruiting body. Carbohydr. Polym.. 2017;157:1134-1143.
- [Google Scholar]
- Antitumor polysaccharides from mushrooms: A review on the structural characteristics, antitumor mechanisms and immunomodulating activities. Carbohydr. Res.. 2016;424:30-41.
- [Google Scholar]
- Physicochemical properties and antidiabetic effects of a polysaccharide from corn silk in high-fat diet and streptozotocin-induced diabetic mice. Carbohydr. Polym.. 2017;164:370-378.
- [Google Scholar]
- Extraction purification and characterization of galactomannan from fenugreek for industrial utilization. Carbohydr. Polym.. 2018;180:88-95.
- [Google Scholar]
- Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med.. 1999;26:1231-1237.
- [Google Scholar]
- Structural characterization and inhibition on α-glucosidase activity of acidic polysaccharide from Annona squamosa. Carbohydr. Polym.. 2017;174:1-12.
- [Google Scholar]
- Valorization of peanut shells: Manufacture of bioactive oligosaccharides. Carbohydr. Polym.. 2018;183:21-28.
- [Google Scholar]
- Polysaccharide rich fractions from barks of Ximenia americana inhibit peripheral inflammatory nociception in mice antinociceptive effect of Ximenia americana polysaccharide rich fractions. Brazilian J. Pharmacogn.. 2017;27:339-345.
- [Google Scholar]
- Identificação Espectrométrica de. Compostos Orgânicos; 2006.
- Farmacognosia: da planta ao medicamento (6th ed). Editora UFSC: Florianópolis; 2010.
- Purified polysaccharides of Geoffroea spinosa barks have anticoagulant and antithrombotic activities devoid of hemorrhagic risks. Carbohydr. Polym.. 2015;124:208-215.
- [Google Scholar]
- Anti-diabetic polysaccharides from natural sources: A review. Carbohydr. Polym.. 2016;148:86-97.
- [Google Scholar]
- Recent advances in bioactive polysaccharides from Lycium barbarum L., Zizyphus jujuba Mill, Plantago spp., and Morus spp.: Structures and functionalities. Food Hydrocoll.. 2016;60:148-160.
- [Google Scholar]
- Exploration in the cascade working mechanisms of liver injury induced by total saponins extracted from rhizoma Dioscorea bulbifera. Biomed. Pharmacother.. 2016;83:1048-1056.
- [Google Scholar]
- Activity diversity structure-activity relationship of polysaccharides from lotus root varieties. Carbohydr. Polym. 2017
- [CrossRef] [Google Scholar]
- Isolation, purification, characterization and immunostimulatory activity of polysaccharides derived from American ginseng. Carbohydr. Polym.. 2017;156:9-18.
- [Google Scholar]
- A mini-review of chemical and biological properties of polysaccharides from Momordica charantia. Int. J. Biol. Macromol.. 2016;92:246-253.
- [Google Scholar]
- Properties of soluble dietary fiber-polysaccharide from papaya peel obtained through alkaline or ultrasound-assisted alkaline extraction. Carbohydr. Polym.. 2017;172:102-112.
- [Google Scholar]
- The effect of different extraction techniques on property and bioactivity of polysaccharides from Dioscorea hemsleyi. Int. J. Biol. Macromol.. 2017;102:847-856.
- [Google Scholar]







