Translate this page into:
Characterization of the volatile profile from six different varieties of Chinese chives by HS-SPME/GC–MS coupled with E. NOSE
⁎Corresponding authors at: College of Horticulture, Gansu Agricultural University, Lanzhou 730070, China. xiaoxm@gsau.edu.cn (Xuemei Xiao), yujihuagg@163.com (Jihua Yu)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objectives
The Chive chives cultivars Han Yu Zi Gen, Fu Jiu Bao F1, Shouguang Du Gen Hong, Xue Jiu, Jiu Xing 22, and Ma Lian Da Ye Bai Gen are recently evolved cultivars, and this would be the first study to characterize for agronomy, quality, volatile profile and E-Nose for aromatic and textural quality.
Methods
These cultivars were selected based on their suitability for aromatic and textural qualities that are desirable for food processing. The cultivars Han were analyzed for agronomy and quality indices by methods in general use in modern laboratories, while their volatile profile was studied by HS-SPME/GC–MS and E-nose analyses.
Results
The cultivars varied for agronomy and quality indices (P < 0.05). The volatile profile showed that Esters, Heterocyclic polymers, Acids, Aldehydes, Hydrocarbons, Sulfides, Ketones, Ethers, Furfuryl derivatives, and Phenols were dominating compound groups. High total volatile contents (17.75 and 17.54 µg/g) were determined in Xue Jiu and Han Yu Zi Gen, respectively, more significant than Fu Jiu Bao F1, Ma Lian Da Ye Bai Gen, Jiu Xing 22, and Shouguang Du Gen Hong (12.22, 11.70, 10.19 and 9.53 µg/g), respectively. PCA showed that W5S and W2W were the farthest points of the first and second principal components, respectively, while W5S, W1S, W3C, W1C and W2W were main sensors classifying cultivars; where sensor W5S had larger contribution rate.
Conclusions
In this study, HS-SPME/GC–MS and E-nose methods accurately detected flavor differences among cultivars. These analyses further suggested that Xue Jiu and Han Yu Zi Gen possessed strong overall flavor characteristics; while Shouguang Du Gen Hong was the lightest in flavor characteristics. Hence it is for the general recommendation that Chinese chive cultivars Xue Jiu and Han Yu Zi Gen may be a choice of the food industry for appropriate utilization.
Keywords
Chinese chive
Agronomy
Quality property
Volatiles
HS-SPME/GC–MS
Electronic nose
1 Introduction
Allium tuberosum Rottler. ex Spreng., is a plant species inherited to Shanxi province of China, belongs to the family Amaryllidaceae, and is cultivated around the world (Friesen et al., 2006, Li et al., 2010, Saini et al., 2013). Once A. tuberosum is planted, the fresh leaves can be harvested 3–4 times (Asavasanti et al., 2017). The plant has a persistent short spathe that opens with 1–3 valves; 14–35 long and sub-equal pedicels; flowers are white-colored, star-like, widely spread, and slight fragrant; also produces irregular depressed spherical black colored seed, 3–4 mm in length. Globin Med (2015) has proved its cultivation in this part in the 10th century and provided evidence that A. tuberosum was cultivated in China even as early as 200 BCE. Its cultivation is reported worldwide now, but its use as a vegetable is more specific in East Asia (China, Korea, Japan, and Mongolia) and South-East Asia (Vietnam, Thailand, Indonesia, Malaysia, Philippines). The plant provides multiple cuttings, and flat leaves are often used to make dumplings with a combination of egg, shrimp, and pork (Gao et al., 2019).
Because of its proven nutritional and therapeutic characteristics, A. tuberosum is valuable in functional food and nutraceutical products. It has been used as a traditional medicine to treat diabetes, hypertension, cancer, inflammation, gastrointestinal issues, and microbial infestations since antiquity. The extracts may be utilized to prevent and treat a variety of disorders (Bede and Zaixiang, 2020). The fresh leaves are cooked and utilized as a traditional medicine for impotence, stomach ulcers, Dyspepsia, nocturnal emission, stomach discomfort, and kidney protection (Chandrashekar et al., 2011). Chandrashekara and Venkatesh (2016) have described chive’s pharmacological and biological activities, such as anticancer, antidiabetic, antioxidant, antibacterial, aphrodisiac, cytoprotective, and hepatoprotective characteristics. The Chinese chives have a potential future as antibacterial and antioxidant reagents, and their crude extract might be a stepping stone toward novel products in the culinary, cosmetic, and medical industries (Lawthienchai et al., 2016).
A. tuberosum crude extracts are believed to have antimicrobial and antioxidant activities, which are primarily associated with its sulfur compounds (Asavasanti et al., 2017). The existence of organosulfur compounds in Allium species has been extensively reported, which mainly included alliin, allicin, ajoene, captopril, etc. (Bede and Zaixiang, 2020). The primary chemical components found in Chinese chives crude hexane extract are 3-Methyl-cyclopentanol and Phenol (Lawthienchai et al., 2016). Sulfur-containing compounds are the strongest odorants, depending on their concentration, chemical purity, and compounds such as 8-mercapto-p-menthan-3-one, p-menthene-8-thiol, 3-mercapto-hexanol, and Oxane® have gained flawless supremacy in imparting natural fruitiness and freshness (Goeke, 2002). Volatile components isolated from Chinese chive by GC and GC–MS account for 88% of the total volatiles. In addition to commonly reported compounds, 27 novels Sulfur-containing components were found in Chinese chive which mainly included sulfide, disulfides, trisulfides, and tetrasulfides with ethyl, butyl, etc. (Pino et al., 2001).
The volatile sulfur-containing compounds in Allium genus have been reported in many past studies (Morris and Thompson 1956, Schwimmer and Mazelis 1963, Mackenzie and Ferns 1977, Block 1992, Block et al., 1992, Pino et al., 2001, Abu-Lafi et al., 2004, Rose et al., 2005, Ledezma and Apitz-Castro 2006, Corzo-Martínez et al., 2007, Kalayarasan et al., 2009). However, Yabuki et al. (2010) characterized the composition of Sulfur-containing constituents in 12 A. tuberosum varieties using GC–MS and HPLC analyses and detected 5 disulfides (dimethyl, allyl methyl, methyl 1-(E)-propenyl, diallyl, and allyl 1-(E)-propenyl), 2 trisulphides (dimethyl and allyl methyl), and 2 vinyldithiins (3-vinyl-[4H]-1,2- and 2-vinyl-[4H]-1,3-) by mass spectral and GC-retention data. Sulfides with a methyl group predominated over those with an allyl group among the identified Sulfur compounds. Sulfides and thiosulfinates found in Allium plants family are also known to have antimicrobial properties against various bacteria and fungi (Ledezma and Apitz-Castro 2006, Kim et al., 2008, Asavasanti et al., 2017), such as H. pylori (Sivam et al., 1997). Sivam et al. (1997) and E. coli O157:H7 (Seo et al., 2001). Yabuki et al. (2010) used HPLC to analyze methiin and alliin contents in Chinese chive cultivars, and chromatogram acquired from extract (cv. Super Green Belt Nira) confirmed clear separation of alliin and methiin. Fresh leaves of all cultivars had higher methiin content (5–12 folds) than alliin. Gîtin et al. (2014) identified Allicin as a key Sulfur molecule compound by GC- MS analysis of the leaf extract detecting disulfides as major sulfur compounds and suggested the potential use of fresh Allium leaves condiment and preservative in the food industry. Although some sulfur compounds in Chinese chives have been identified, unknown substances still need to be explored through a new method. The headspace solid micro-extraction gas-chromatography mass spectrometry (HP-SPME/GC–MS) is an innovative, more rapid, sensitive, and solvent-free method, and it was first introduced in the 1990s and adopted extensively in air, water, soil, and food analysis (Roberts et al., 2000). From gaseous or liquid samples, the analytes are extracted using this method, by absorption on HS-SPME with a skinny polymer coating used to fix the solid fiber surface in injection needle (Pragst, 2007). GC/MS enables the identification of compounds and compares achieved mass spectra of analytes. The method analyzed the volatile components individually and identified compounds in extracted samples, revealed the number of volatile components extracted, and evaluated extraction efficiency in addition to sample amount and extraction duration.
Choice of cultivar is the main way to ensure a high-quality product. In chives, Thiosulphate composition varies significantly in different cultivars due to changes in enzymes associated with cellular factors, including water availability, temperature pH, etc. (Brueckner and Perner, 2006). The varieties and intake typology of Allium species may also vary significantly in chemical properties, and even smaller variations may have importance in relative description and diversity. In Allium cepa varieties, a significant difference in flavonol was determined, which were in the range of 7 to 700 mg kg−1 in red and gold varieties, respectively (Bonaccorsi et al., 2008). Yabuki et al. (2010) found dimethyl disulfide and dimethyl trisulphide in all 12 Chinese chive varieties, and their leaves have markedly higher contents of methiin than alliin. Among six varieties screened, in variety Super-Green-Belt Nira a clear separation of methiin and alliin was confirmed. The factors that affect flavor intensity and quality include genetic, ecological, and cultivation procedures. The chives cultivars showed great variation in their chemical composition and flavor intensities regardless of ecology and cultivation technique (Chen 2006).
The utilization of Chinese chives is most common in far-east (Poojary et al., 2017), particularly in China due to its protective human health effects (Putnika et al., 2019); in humans, its utilization reduces the risk of breast cancer (Pourzand et al., 2016) and cardiovascular diseases (Saljoughian et al., 2018); it is antiviral, antimicrobial, (Bisen and Emerald, 2016), antioxidant, antimutagenic, antiasthmatic, antidiabetic (Nile et al., 2017); antiprotozoal protozoal, antispasmodic, anticarcinogenic, anti-amnesic, anti-inflammatory (Prakash et al., 2007; Santas et al., 2008); neuroprotective, hepatoprotective, hypotensive, hypoglycemic, immuno-modulatory, urease/xanthine oxidase inhibitory, and prebiotic properties (Wang et al., 2013). The mentioned properties are primarily associated with the significant contents of organosulfur compounds (Putnik and Bursać Kovačevć, 2017; Fidelis et al., 2018; Zhu et al., 2018); and many of them are tainted during processing (Giacometti et al., 2018). The extract recovery particularly for food and pharmaceutical use are broadly encouraged (Poojary et al., 2017). The extracts rich in organosulfur compounds and polyphenols possess antimicrobial properties (Barba et al., 2017). However, knowledge about concentrations of organosulfur compounds in newly evolved cultivars for bioavailability is yet to be developed.
There have been fewer detailed studies that identify volatile organic Sulfur compounds in Chinese chives, and no study has compared the Sulfur-containing constituents among various genotypes of Chinese chives developed and grown in the same locality. The cultivars Han Yu Zi Gen, Fu Jiu Bao F1, Shouguang Du Gen Hong, Xue Jiu, Jiu Xing 22 and Ma Lian Da Ye Bai Gen are recent developments in China never these have been in-depth studied before for agronomy, quality, volatile profile and E-Nose examinations. These cultivars were included in this study to compare their suitability for aromatic and textural qualities that are desirable for food processing. Therefore, this investigation was aimed at characterizing the composition of volatile Sulfur-containing constituents, quality characteristics, and agronomy of Chinese chive genotypes.
2 Materials and methods
2.1 Materials
Six Chinese chive genotypes (Han Yu Zi Gen [G1], Fu Jiu Bao F1 [G2], Shouguang Du Gen Hong [G3], Xue Jiu [G4], Jiu Xing 22 [G5], and Ma Lian Da Ye Bai Gen [G6]) were used as a genetic resource. The genotypes were obtained from three markets, G1, G3, G4, and G6 from Alibaba Platform, G2 from Henan Fusheng Seed Industry Co. Ltd, and G5 from Henan Jiuxing Biotechnology Research Institute Co. Ltd. Initially, every 10 seeds were sowed in a nutrition pot. After one month of sowing, in the middle of March 2020 when seedlings became 10 to 15 cm height, they were transplanted in pots using five pots for each genotype in recommended potting media following three replicated CRD. In all, 90 pots were used (Genotypes = 6× plants = 5× Replications = 3), where in each pot 2–3 seedlings were transplanted and after root development, the normally healthy looking five plants were left, and the remaining were removed. The insect pests and disease-free Chinese chive plants were selected from the experimental pots of similar maturity; leaves were picked and crushed for each variety to prepare samples to detect volatile Sulfur-containing compounds. The leaves were picked in such a way to avoid external impurities (Yabuki et al., 2010).
2.2 Methods
2.2.1 Agronomic indices
Shoot, morphological indexes included plant height, number of leaves plant−1, leaf sheath diameter, leaf sheath height, and leaf width. Measurement of Chinese chives plant standing height, length of leaves, and sheath height of six cultivars were evaluated in the laboratory using a measuring tape (0.01 cm); while the leaf width was measured by electronic Vernier caliper (0.01 mm); and the number of plant leaves was counted by visual observation. Six plants of each block were taken for one replication three replications for each variety. The chives’ standing height was measured from the basal part of the leaf sheath i.e. from top of the bulb to tip of the longest bulb. Leaf-sheath length was recorded from the basal part of the leaf sheath to the beginning of the first leaf. The largest diameter of the leaf sheath (around 1 cm above the top of the bulb) was recorded as leaf sheath diameter. Shoot, morphological indexes included (Plant height, number of leaves plant−1, leaf sheath diameter, leaf sheath height, and width of leaf). Measurement of Chinese chives plant standing height, length of leaves and sheath height of six cultivars were evaluated in the laboratory using a measuring tape (0.01 cm); while electronic Vernier caliper measured the leaf width (0.01 mm), and the number of plant leaves was counted by visual observation. Six plants of each block were taken for one replication; three replication for one variety chives standing height measured from the basal part of the leaf sheath (i.e., the top of the bulb) to the tip of the longest bulb. Leaf-sheath length has been recorded from the basal part of the leaf sheath to the beginning of the first leaf. The largest diameter of the leaf sheath (near 1 cm above the top of the bulb) will be recorded as leaf sheath diameter.
2.2.2 Quality indices
The coomassie brilliant blue method was used to estimate the soluble protein content (Sedmak and Grossberg 1977, Wang et al., 2021), while the anthrone–sulfuric acid assay method was used to estimate the soluble sugar content (Wang et al., 2021). The Ascorbic acid (Vitamin-C) was determined by the 2, 6-chlorophenol indophenol staining method (Wang et al., 2021), while the Nitrate content in Chinese chive leaf samples was determined by Salicylic Acid Method using an ultraviolet spectrophotometer (Li et al., 2021). However, Chlorophyll content was determined by the acetone extraction method, as suggested by (Wang et al., 2021).
2.2.3 Characterization of volatile sulfur-containing compounds
2.2.3.1 HS-SPME/GC–MS (headspace solid-phase micro-extraction combined with gas chromatography-mass spectrometry)
Three randomly harvested plants each of six Chinese chive genotypes were soaked in water; and after smearing off the leaves, they were separately shredded gently, followed by crushing in a mortar with 10 ml of deionized water and a small quantity of sea sand to achieve 10 g sample for each plant.
2.2.3.2 HS-SPME analysis
Univariate analyses were employed for this purpose. The Chinese chive samples were taken out of liquid N and ground swiftly to homogenize, and the 1.5 g of homogenate was put in screw-head headspace vial (15 ml) with magnetic stirring rotor and ultrapure water (2 ml) to stir fully. Later on, a 0.75 g of firm Na2SO4 quantity was taken and added 4 μL of difurfuryl sulfide internal standard; and sample vial was tightly fitted using PTFE silicon stopper. Later, the headspace bottle was kept in equilibrium at 70 °C for 15 min on a metal heating agitation platform (500 rpm). After that, the removal and adsorption was performed by inserting the pretreated 85 μm CAR/PDMS SPME fiber into a headspace bottle for 50 min with uninterrupted heating and agitation. When extraction completes, fiber was desorbed into GC injection port for 5 min and subject to GC–MS analysis.
2.2.3.3 GC–MS analysis of volatiles leaf extract
Characterization of volatile sulfur-containing compounds was done by GC–MS analysis of volatiles leaf extract (Yabuki et al., 2010). An Agilent 7890B Gas Chromatograph coupled with an Agilent 7000D Quadrupole Mass Spectrometric Detector (Agilent, USA); and standard mass Spectrometry Library (NIST, 2014) workstation were used for the separation and identification of the Volatile organic compounds (VOCs). ADB-WAX elastic quartz capillary column (30 m × 0.25 mm, 0.25 μm) was used as stationary phase (Agilent, USA).
The VOCs of Chinese chive were analyzed using an Agilent 7890B/7000D GC–MS under the following conditions: capillary column, DB-WAX (30 m × 0.25 mm, 0.25 μm) with He (≥99.999% purity) as the carrier gas at a flow rate of 1 ml/min and splitless mode; initial temperature 40℃ held for 1 min, raised to the 80℃ at 8℃/min, then raised to 130℃ at 2℃/min, and finally raised to 220℃ at 6℃/min held for 3 min; total analysis time, 49 min; MS ionization, EI, 70 eV; MS source, 230 ℃; scan area, 30–660 amu. After the program started, the VOCs were separated and identified by the GC–MS with automatic Deconvolution System (AMDIS) and Mass Spectrometry Library (NIST, 2014). Compared with the Mass Spectrometry Library, only those with a matching score of >70 were identified. The concentration of VOCs was analyzed by the standard internal method, using the formula:.
Where A1 and A2 are the peak areas of determinant and the internal standard, respectively; M1 and M2 are the internal standard and sample, respectively.
2.2.4 Electric-Nose analysis
The determination and analysis were performed with a commercial PEN3.5 E-nose (Airsense Analytics, GmBH, Schwerin, Germany). The system contained ten metal oxide sensors. Before detection, each sample (1.5 g of Chinese chives) was placed in a 15 ml headspace glass vial and closely capped with a PTFE-silicon stopper. The samples were then kept at 70℃ for approximately 65 min for equilibrium. The detection time of sample was 120 s, the cleaning time of sensor was 60 s, and the adjustment time of automatic zero was 10 s. All samples were run with three repetitions. Material type and performance description represented by sensors are shown in the Table 1.
Sensor number
Sensor name
Sensor sensitivity and general description
Detection limits (mg kg−1)
1
W1C
Aromatic organic compounds
Toluene, 10
2
W5S
Very sensitive, broad range sensitivity, reacts to nitrogen oxides
NO2, 1
3
W3C
Ammonia, also used as sensor for aromatic compounds
Benzene, 10
4
W6S
Detection on mainly hydrogen gas
H2, 0.1
5
W5C
Alkanes, aromatic compounds
Propane, 1
6
W1S
Sensitive to methane
CH4, 100
7
W1W
Detection on inorganic sulfur compounds
H2S, 1
8
W2S
Detection on alcohol
CO, 100
9
W2W
Aromatic compounds, inorganic sulfur and organic compounds
H2S, 1
10
W3S
Sensitive to methane and aliphatic organic compounds
CH3, 10
2.2.5 Statistical analysis
The data are mentioned as mean ± standard error (SE) for different experiments, and the genotypes were assessed for their variation in morphological and quality traits by using ANOVA and Tukey’s comparison test (P < 0.05).
3 Results
3.1 Agronomic characters in different varieties of Chinese chive
The agronomic record of the Chinese chive cultivars (Table 2) showed that the plants of Jiu Xing 22 produced maximum leaves, followed by Ma Lian Da Ye Bai Gen, Han Yu Zi Gen, Xue Jiu, Fu Jiu Bao F1, and least in cultivar Shouguang Du Gen Hong surpassing by 5.21, 6.95, 13.11, 14.06 and 21.01%, respectively. Maximally taller plants were produced by cultivar Ma Lian Da Ye Bai Gen, followed by Fu Jiu Bao F1, Jiu Xing 22, Han Yu Zi Gen, Xue Jiu, and shortest plants were found in Shouguang Du Gen Hong exceeding by 2.38, 4.60, 5.99, 7.14 and 14.56%, respectively. Similarly, cultivar Ma Lian Da Ye Bai Gen had the longest leaves, followed by Fu Jiu Bao F1, Jiu Xing 22, Han Yu Zi Gen, Xue Jiu, while shortest leaves were noted for Shouguang Du Gen Hong; and Ma Lian Da Ye Bai Gen showed superiority for this trait by 2.57, 5.03, 5.61, 6.74 and 15.20%, respectively. Maximum leaf sheath height was noted in Fu Jiu Bao F1, while Jiu Xing 22, Han Yu Zi Gen, Ma Lian Da Ye Bai Gen, and Shouguang Du Gen Hong ranked 2nd, 3rd, 4th, and 5th, respectively; whereas Xue Jiu remained least. Fu Jiu Bao F1 surpassed 2nd, 3rd, 4th, 5th, and least by 4.07, 4.88, 5.28, 10.98, and 15.85%, respectively. Cultivar Xue Jiu had maximum leaf width, followed by Han Yu Zi Gen, Ma Lian Da Ye Bai Gen, Jiu Xing 22, Fu Jiu Bao F1, and Shouguang Du Gen Hong remained least in this trait; and Xue Jiu showed superiority by 2.78, 5.56, 8.33, 11.11 and 16.67%, respectively. The greatest leaf sheath diameter was recorded in cultivar Shouguang Du Gen Hong, surpassing Xue Jiu (11.57%), Han Yu Zi Gen (12.96%), and Fu Jiu Bao F1 (14.35%), Ma Lian Da Ye Bai Gen (28.24%) and Jiu Xing 22 (33.80%). G1 = Han Yu Zi Gen, G2 = Fu Jiu Bao F1, G3 = Shouguang Du Gen Hong, G4 = Xue Jiu, G5 = Jiu Xing 22, G6 = Ma Lian Da Ye Bai Gen.
Variety
Plant leaves (No.)
Plant height (cm)
Leaf length (cm)
Leaf sheath height (cm)
leaf width (cm)
Leaf sheath diameter (cm)
G1
5.89 ± 0.056ab
29.64 ± 0.282b
27.58 ± 0.488b
2.34 ± 0.049ab
0.35 ± 0.010ab
1.88 ± 0.054abc
G2
5.44 ± 0.147bc
30.78 ± 0.487ab
28.47 ± 0.389ab
2.46 ± 0.080a
0.32 ± 0.006bc
1.85 ± 0.066abc
G3
5.00 ± 0.167c
26.94 ± 0.683c
24.78 ± 0.701c
2.19 ± 0.094ab
0.30 ± 0.000c
2.16 ± 0.021a
G4
5.50 ± 0.347bc
29.28 ± 0.570b
27.25 ± 0.500b
2.07 ± 0.065b
0.36 ± 0.015a
1.91 ± 0.037ab
G5
6.33 ± 0.167a
30.08 ± 0.699ab
27.75 ± 0.603b
2.36 ± 0.099a
0.33 ± 0.006abc
1.43 ± 0.076c
G6
6.00 ± 0.096ab
31.53 ± 0.282a
29.22 ± 0.338a
2.33 ± 0.096ab
0.34 ± 0.011ab
1.55 ± 0.062bc
3.2 Quality property in different varieties of Chinese chive
The quality profile of Chinese chive was determined, and data (Table 3) revealed that soluble protein among six cultivars (0.37 and 1.97 mg/g) was higher in Shouguang Du Gen Hong than Han Yu Zi Gen (10.15%), Xue Jiu (19.29%), Ma Lian Da Ye Bai Gen (39.03%), Fu Jiu Bao F1 (73.60%) and Jiu Xing 22 (81.22%). The deviation in soluble sugar (1.26 to 3.24%) among cultivars revealed greater values Ma Lian Da Ye Bai Gen than Xue Jiu (35.80%), Fu Jiu Bao F1 (49.38%), Han Yu Zi Gen (55.86%), Shouguang Du Gen Hong (58.33%) and Jiu Xing 22 (61.11%). The vit-C among cultivars (7.34–7.55 mg/g) was higher in Jiu Xing 22 than Han Yu Zi Gen (0.66%), Fu Jiu Bao F1 (1.32%), Shouguang, Du Gen Hong (1.85%), Xue Jiu (2.52%) and Ma Lian Da Ye Bai Gen (2.78%). Likewise, nitrate among cultivars (7135.42–8974.23 µg/g) was higher in Xue Jiu than Ma Lian Da Ye Bai Gen (9.22%), Jiu Xing 22 (19.79%), Fu Jiu Bao F1 (23.89%); Shouguang Du Gen Hong (47.53%) and Han Yu Zi Gen (53.92%). However, total leaf chlorophyll of six Chinese chive cultivars (1.51–2.04 mg/g) was higher in Jiu Xing 22 over Shouguang Du Gen Hong (4.90%), Xue Jiu (6.37%), Han Yu Zi Gen (14.22%), Fu Jiu Bao F1 (21.08%) and Ma Lian Da Ye Bai Gen (25.98%).
Variety
Soluble protein (mg/g)
Soluble sugar (%)
Vitamin-C (mg/g)
Nitrate content (µg/g)
Total Chlorophyll content (mg/g)
G1 = Han Yu Zi Gen
1.77 ± 0.104a
1.43 ± 0.091c
7.50 ± 0.018ab
4135.42 ± 52.4c
1.75 ± 0.026b
G2 = Fu Jiu Bao F1
0.52 ± 0165b
1.64 ± 166bc
7.45 ± 0.012bc
6830.59 ± 390.0b
1.61 ± 0.023c
G3 = Shouguang Du Gen Hong
1.97 ± 0.142a
1.35 ± 0.121c
7.41 ± 0.007bcd
4708.88 ± 248.8c
1.94 ± 0.065a
G4 = Xue Jiu
1.59 ± 0.178a
2.08 ± 0.171b
7.36 ± 0.038 cd
8974.23 ± 75.8a
1.91 ± 0.041a
G5 = Jiu Xing 22
0.37 ± 0.165b
1.26 ± 0.105c
7.55 ± 0.035a
7197.92 ± 317.5b
2.04 ± 0.016a
G6 = Ma Lian Da Ye Bai Gen
1.20 ± 0.180ab
3.24 ± 0.162a
7.34 ± 0.074d
8146.39 ± 219.4a
1.51 ± 0.048c
3.3 Characterization of volatile profile in different varieties of Chinese chives
3.3.1 Volatile components measured by HS-SPME/GC–MS
The volatile profile (Table 4) shows the characterization of volatile compounds in six A. tuberosum genotypes using HS-SPME/GC–MS analysis detected enormously higher count of constituents (97) in 15 groups classified as: Aldehydes (10), Hydrocarbons (7), Esters (11), Acids (10), Sulfides (7), Ketones (7), Ethers (6), Alkaloids (2), Heterocyclic polymers (11), Cyclomethicone (5), Polyolefins (2), Furfuryl and furan derivatives (5), Phenols (5), Diterpenes (2), Terpenoids (1) and others (5). The detected compounds varied among genotypes and more compounds (56) were detected in Xue Jiu, followed by Jiu Xing 22 (51) and Ma Lian Da Ye Bai Gen (51), Fu Jiu Bao F1 (44), Shouguang Du Gen Hong (42) and least (40) number of compounds was detected in genotype Han Yu Zi Gen. Total volatile contents were almost equal in cultivars Xue Jiu (17.75 µg/g) and Han Yu Zi Gen (17.54 µg/g) and greater than cultivars Fu Jiu Bao F1 (12.22 µg/g), Ma Lian Da Ye Bai Gen (11.70 µg/g), Jiu Xing 22 (10.19 µg/g) and Shouguang Du Gen Hong (9.53 µg/g). Total volatile contents of cultivar Xue Jiu was greater than cultivars Han Yu Zi Gen (1.20%), Fu Jiu Bao F1 (45.25%), Shouguang Du Gen Hong (86.25%), Jiu Xing 22 (74.19%), and Ma Lian Da Ye Bai Gen (51.71%); whereas, total volatiles content of cultivar Han Yu Zi Gen was greater than cultivars Fu Jiu Bao F1 (43.54%), Shouguang Du Gen Hong (84.05%), Jiu Xing 22 (72.13%), Ma Lian Da Ye Bai Gen (49.91%). i. The isolated volatile constituents are cohesively organized with the GC–MS automatic deconvolution system and their comparison was made with standard mass spectrum in the NIST 14 library (MS match index ≥ 70%). ii. The values of the volatile constituents associated with MS match index <70% were not included. iii. RT or Retention time (min) of detected volatile compounds on capillary column DB-WAX. iv. 06 Chinese chives cultivars are G1 = Han Yu Zi Gen, G2 = Fu Jiu Bao F1, G3 = Shouguang Du Gen Hong, G4 = Xue Jiu, G5 = Jiu Xing 22, G6 = Ma Lian Da Ye Bai Gen. v. The values for each volatile constituent are average of three replicates for each genotype. vi. Total volatile contents in cultivars were almost equal in G4 (17.75%) and G1 (17.54%) and greater than rest of the cultivars. vii. Total volatile contents of G4 was greater than G1 (1.20%), G2 (45.25%), G3 (86.25%), G5 (74.19%), G6 (51.71%). viii. Total volatiles content of G1 was greater than G2 (43.54%), G3 (84.05%), G5 (72.13%), G6 (49.91%).
Group (No.)
Name of compound
RT (min)
Compound content in genotypes (µg/g)
MS match index (%)
Molecular formula
CAS
G1
G2
G3
G4
G5
G6
Aldehydes
1
Benzene, 1,3-dimethyl-
5.83
0.056
0.122
0.082
–
0.011
–
92.98
C8H10
108–38-3
2
Benzene, propyl-
7.04
0.119
–
–
–
–
–
76.18
C9H12
103–65-1
3
2-Hexenal, (E)-
7.47
0.626
0.347
0.217
0.147
0.186
0.158
86.94
C6H10O
6728–26-3
4
(E)-1-Methyl-2-(prop-1-en-1-yl)
8.37
0.640
0.720
0.483
1.108
0.445
1.264
79.79
C4H8S2
23838–19-9
5
Benzenamine, 3,5-dimethyl-
14.25
0.074
–
–
–
–
–
75.90
C8H11N
108–69-0
6
Undecanal
15.44
–
–
–
0.012
–
–
70.11
C11H22O
112–44-7
7
(E)-1-Allyl-2-(prop-1-en-1-yl)
15.57
0.301
0.136
0.272
0.701
0.215
0.129
82.03
C6H10S2
122156–02-9
8
2,4-Heptadienal, (E,E)-
15.71
–
0.016
0.009
0.012
0.016
0.009
71.47
C7H10O
4313–03-5
9
Benzaldehyde, 3-ethyl-
25.37
0.309
0.238
0.137
0.219
0.351
0.148
89.01
C9H10O
34246–54-3
10
Benzene, 1-(1,1-dimethylethyl)-3-ethyl-
31.42
–
0.022
–
0.008
0.01
–
77.94
C12H18
14411–56-4
Hydrocarbons
11
psi.,.psi.-Carotene
0.20
–
–
0.011
0.06
0.006
0.008
80.44
C42H64O2
13833–01-7
12
Dodecane
6.40
–
0.044
0.023
–
–
–
84.33
C12H26
112–40-3
13
p-Xylene
6.70
0.542
–
–
–
–
–
84.92
C8H10
106–42-3
14
Styrene
8.23
0.338
0.791
0.549
0.01
0.008
0.007
86.44
C8H8
100–42-5
15
Cyclopentanecarboxaldehyde
14.80
0.059
0.152
0.092
0.37
0.403
0.407
79.86
C8H12O
1000154–24-0
16
1H-3a,7-Methanoazulene
18.76
0.044
0.008
–
–
0.005
–
80.29
C15H24
469–61-4
17
Cyclohexene,1-methyl-3-(1-methylethyl)-
21.25
–
0.022
–
0.028
0.012
0.053
76.20
C10H18
13828–31-4
Esters
18
Monomethyl carbonotrithioate
1.81
–
0.017
0.002
–
–
–
84.08
C2H4S3
1113–26-4
19
S-Methyl methanethiosulfinate
23.31
–
–
0.022
–
–
–
75.14
C2H6OS2
13882–12-7
20
3,7,11,15-Tetramethylhexadec
36.02
–
0.081
–
–
–
–
77.83
C22H42O2
76337–16-1
21
3,7,11,15-Tetramethylhexadec-2
36.04
–
–
0.132
0.261
0.309
–
87.48
C22H42O2
76337–16-1
22
Cyclodecasiloxane, eicosamethyl-
37.49
–
–
–
0.041
0.035
0.046
79.66
C20H60O10Si10
18772–36-6
23
Ethyl 13-methyl-tetradecanoate
40.36
–
–
–
–
–
0.051
80.07
C17H34O2
1000336–61-5
24
Ethyl 9-hexadecenoate
42.50
–
–
–
–
–
0.121
92.96
C18H34O2
54546–22-4
25
Z-(13,14-Epoxy)tetradec acetate
47.24
–
–
–
0.036
0.021
0.05
78.28
C16H28O3
1000131–33-2
26
Ethyl 9,12,15-octadecatrienoate
47.38
–
–
–
–
–
0.153
92.46
C20H34O2
1000336–77-4
27
2,2-Dimethyl-6-methylene
47.68
0.213
–
–
–
–
–
70.75
C14H24O4
1000212–02-6
28
Dasycarpidan-1-methanol, acetate
47.81
–
–
0.066
–
0.013
0.002
73.73
C20H26N2O2
55724–48-6
Acids
29
3-Pyridinecarboxylic acid
0.17
–
0.005
–
–
–
–
73.71
C32H39NO10
51906–00-4
30
Alanine
1.65
–
0.023
–
0.019
0.026
–
70.89
C3H7NO2
56–41-7
31
Tetradecanoic acid
38.29
–
0.265
–
0.037
–
0.146
83.89
C16H32O2
124–06-1
32
Hexadecanoic acid
42.16
0.466
0.224
0.803
0.399
0.582
0.569
86.00
C18H36O2
628–97-7
33
3-Hydroxypropanoic acid
42.48
–
0.018
–
0.02
–
–
73.01
C15H26O3
1000196–34-0
34
Oleic Acid
44.20
–
0.008
0.01
0.004
0.003
–
73.58
C18H34O2
112–80-1
35
Z-8-Methyl-9-tetradecenoic acid
44.20
–
–
–
0.037
–
0.029
78.37
C15H28O2
1000130–84-5
36
Linoleic acid
46.32
–
–
0.068
0.044
0.09
0.131
86.80
C20H36O2
544–35-4
37
Phthalic acid
46.52
–
–
–
–
0.049
–
70.28
C20H28O4
1000309–06-9
38
9,12,15-Octadecatrienoic acid
47.37
–
0.03
0.109
0.102
0.099
–
81.82
C20H34O2
1191–41-9
Sulfides
39
Sulfide, allyl methyl
3.33
1.04
–
–
–
–
–
77.89
C4H8S
10152–76-8
40
Ethylbenzene
5.62
0.049
0.061
0.04
0.014
–
–
90.12
C8H10
100–41-4
41
Diallyl disulphide
15.34
1.179
0.219
0.207
0.786
0.191
0.276
90.63
C6H10S2
2179–57-9
42
Trisulfide, methyl 2-propenyl
19.94
0.625
0.892
0.583
1.167
0.709
0.797
84.99
C4H8S3
34135–85-8
43
Tetrasulfide, dimethyl
26.84
0.102
0.026
0.054
–
–
–
77.43
C2H6S4
5756–24-1
44
Trisulfide, di-2-propenyl
28.53
0.74
0.085
0.167
0.833
0.232
0.047
75.26
C6H10S3
2050–87-5
45
(Z)-1-Allyl-3-(prop-1-en-1-yl)
29.17
–
–
–
0.051
–
–
79.18
C6H10S3
382161–75-3
Ketones
46
Acetoin
3.31
–
0.434
0.175
–
0.048
–
73.54
C4H8O2
513–86-0
47
Cyclohexanone, 2,2-dimethyl-
12.13
0.189
–
–
–
–
–
71.56
C8H14O
1193–47-1
48
Cyclohexene,1-propyl-
18.48
–
–
–
0.014
–
–
80.41
C9H16
2539–75-5
49
6,6-Dimethylhepta-2,4-diene
19.06
0.103
–
–
–
0.012
0.011
72.28
C9H16
1000195–03-3
50
N,2,4,6-Tetramethylbenzenamine
22.11
0.14
0.06
0.029
0.062
0.057
0.051
85.50
C10H15N
13021–14-2
51
2-Pentadecanone, 6,10,14-trimethyl-
39.82
–
–
0.042
0.069
0.063
0.027
76.11
C18H36O
502–69-2
52
Ethanone,1-(2-hydroxy-5-methylphenyl)
41.10
0.533
0.224
0.166
0.201
0.08
0.153
81.32
C9H10O2
1450–72-2
Ethers
53
(R)-(-)-2-Amino-1-propanol
1.35
0.042
–
–
–
0.006
0.021
78.23
C3H9NO
35320–23-1
54
Dimethyl ether
3.16
1.55
0.398
0.181
0.244
0.138
0.146
80.26
C2H6O
115–10-6
55
Methyl propyl ether
3.17
0.019
–
–
–
–
–
77.14
C4H10O
557–17-5
56
Disulfide, dimethyl
4.91
1.279
1.406
1.106
1.685
0.906
0.993
88.52
C2H6S2
624–92-0
57
Disulfide, methyl 2-propenyl
8.81
0.659
0.38
0.432
0.705
0.441
0.438
81.86
C4H8S2
2179–58-0
58
Dimethyl trisulfide
11.77
2.557
2.843
2.045
5.175
1.968
3.295
92.26
C2H6S3
3658–80-8
Alkaloids
59
Cyclooctasiloxane, hexadecamethyl-
26.10
–
–
–
0.107
–
0.124
80.32
C16H48O8Si8
556–68-3
60
Cyclononasiloxane, octadecamethyl-
33.68
–
–
–
0.066
0.077
0.072
87.36
C18H54O9Si9
556–71-8
Heterocyclic polymers
61
2-Propanamine
1.43
–
–
0.008
–
–
–
75.60
C3H9N
75–31-0
62
2-Pyrrolidinone
5.22
0.746
–
–
–
–
0.03
79.03
C11H19NO
14293–08-4
63
Thiophene, 3,4-dimethyl-
7.66
0.104
0.206
0.07
0.059
0.015
0.099
92.19
C6H8S
632–15-5
64
3H-1,2-Dithiole
16.53
–
0.003
–
–
–
–
71.24
C3H4S2
288–26-6
65
2,4-Octadienal, (E,E)-
19.52
–
–
–
–
0.158
–
72.76
C8H12O
30361–28-5
66
2-Cyclohexen-1-one, 4-ethyl-3
19.93
–
0.012
–
–
–
–
71.04
C10H16O
17622–46-7
67
Oxime-, methoxy-phenyl-
29.80
0.895
0.918
0.326
0.511
0.384
0.233
74.93
C8H9NO2
1000222–86-6
68
2-Vinyl-4H-1,3-dithiine
31.43
–
–
0.006
–
–
–
64.86
C6H8S2
80028–57-5
69
Butylated Hydroxytoluene
34.35
–
0.034
–
–
–
–
79.87
C15H24O
128–37-0
70
3-Buten-2-one
36.69
0.113
–
–
0.006
–
–
72.16
C13H20O2
23267–57-4
71
3-(4,8,12-Trimethyltridecyl)thiophene
45.21
–
0.347
–
–
–
–
71.22
C20H36S
102037–89-8
Cyclomethicones
72
Cyclotrisiloxane, hexamethyl-
2.34
–
–
–
0.509
0.213
0.042
86.84
C6H18O3Si3
541–05-9
73
Cyclotetrasiloxane, octamethyl-
3.68
–
–
–
0.195
0.236
0.001
76.59
C8H24O4Si4
556–67-2
74
Cyclopentasiloxane, decamethyl-
5.93
–
–
–
0.049
–
0.034
83.83
C10H30O5Si5
541–02-6
75
Cyclohexasiloxane, dodecamethyl-
9.67
–
–
–
0.114
0.155
0.133
92.49
C12H36O6Si6
540–97-6
76
Cycloheptasiloxane, tetradecamethyl-
17.20
–
–
–
0.134
0.035
0.168
88.84
C14H42O7Si7
107–50-6
Polyolefins
77
(2-Aziridinylethyl)amine
1.33
–
–
–
–
–
0.009
72.89
C4H10N2
4025–37-0
78
Cyclohexa-1,3-diene, 5,6-diethyl-
22.07
–
–
–
0.015
0.012
–
78.43
C10H16
1000154–64-0
Furfuryl and furan derivatives
79
Furan, 2,3-dihydro-
4.44
0.186
–
–
0.175
0.054
0.086
79.15
C4H6O
1191–99-7
80
Furan, 2-ethyl-
12.56
–
–
0.029
0.066
0.064
0.061
89.35
C6H8O
3208–16-0
81
Furan, 2-hexyl-
19.45
–
–
–
0.138
–
–
70.62
C10H16O
3777–70-6
82
Furfuryl sulfide
41.58
0.143
0.143
0.143
0.143
0.143
0.143
80.85
C10H10O2S
13678–67-6
83
2(4H)-Benzofuranone
43.46
0.144
–
–
–
–
–
73.05
C11H16O2
17092–92-1
Phenols
84
Phenol, 2,6-bis(1,1-dimethylethyl)-
34.72
0.154
–
–
–
–
–
89.13
C17H27NO2
1918–11-2
85
2,4-Di-tert-butylphenol
43.15
0.094
–
–
–
–
–
80.16
C14H22O
96–76-4
86
Phenol, 2,5-bis(1,1-dimethylethyl)-
43.16
–
0.004
–
–
–
0.002
75.77
C14H22O
5875–45-6
87
Phenol, 2-(1-methylethyl)-
45.96
–
0.116
0.046
0.07
–
0.059
74.99
C11H15NO2
2631–40-5
88
Pheno1,4-diol, 2,3-dimethyl-5
46.52
–
0.081
0.044
0.041
–
–
70.84
C9H9F3O2
1000127–49-8
Diterpenes
89
Phytol, acetate
35.24
–
–
0.354
0.374
0.478
0.252
83.28
C22H42O2
1000375–01-4
90
Phytol
47.69
–
–
0.123
0.221
0.366
0.269
82.98
C20H40O
150–86-7
Terpenoids
91
trans-.beta.-Ionone
35.23
–
0.036
–
–
–
–
78.60
C13H20O
79–77-6
Others
92
1,5-Cyclooctadiene, 3-t-butyl-
14.14
0.159
–
–
–
–
–
78.03
C12H20
1000152–17-9
93
(2E,4S,7E)-4-Isopropyl-1
18.84
0.033
–
–
–
–
–
73.03
C15H26O
198991–79-6
94
3H-1,2-Dithiol-3-one
35.88
0.176
–
–
0.03
0.008
0.012
72.33
C4H4OS2
3620–10-8
95
1,1′-Biphenyl, 2,2′,5,5′-tetramethyl-
41.75
–
–
–
0.043
0.048
0.139
80.38
C16H18
3075–84-1
96
3-n-Hexadecylthiophene
45.22
–
–
0.068
–
–
–
76.85
C20H36S
1000360–37-5
Total content of volatile compounds (µg/g)
–
17.54
12.22
9.53
17.75
10.19
11.70
–
–
–
The cultivar Han Yu Zi Gen contained 5 individual volatile compounds with >1 concentration which included Diallyl disulfide (1.179 µg/g), Sulfide, allyl methyl (1.75 µg/g), Dimethyl ether (1.55 µg/g), Dimethyl trisulfide (2.557 µg/g), and Disulfide, dimethyl (1.279 µg/g). In cultivar Fu Jiu Bao F1, the concentration of individual volatiles was >1 only in two constituents such as Dimethyl trisulfide (2.843 µg/g) and Disulfide, dimethyl (1.406 µg/g); while in cultivar Shouguang Du Gen Hong, also two individual volatiles were determined with >1 volatile concentration including Dimethyl trisulfide (2.045 µg/g) and Disulfide, dimethyl (1.106 µg/g). In cultivar Xue Jiu the concentration of four individual volatile compounds was >1 which included Dimethyl trisulfide (5.175 µg/g), (E)-1-Methyl-2-(prop-1-en-1-yl) (1.108 µg/g), Trisulfide, methyl 2-propenyl (1.167 µg/g) and Disulfide, dimethyl (1.68 µg/g);. In contrast, in cultivar Jiu Xing 22, only one volatile compound had concentration >1 (Dimethyl trisulfide 1.968 µg/g). The volatiles had their concentration >1 in cultivar Ma Lian Da Ye Bai Gen included (E)-1- Methyl-2-(prop-1-en-1-yl) (1.264 µg/g), Dimethyl trisulfide (3.295 µg/g) and Disulfide, dimethyl (1.00 µg/g). Cultivars Han Yu Zi Gen and Xue Jiu were determined to have superiority in flavor substance due to higher contents of sulfur and ether as compared to the rest of the tested cultivars.
The study also revealed that all the tested genotypes commonly contain 17 volatiles such as (E)-1-Allyl-2-(prop-1-en-1-yl), (E)-1-Methyl-2-(prop-1-en-1-yl), 2-Hexenal, (E), Benzaldehyde, 3-ethyl, Cyclopentanecarboxaldehyde, Hexadecanoic acid, Diallyl disulfide, Trisulfide, methyl 2-propenyl, Ethanone,1-(2-hydroxy-5-methyl phenyl), Dimethyl trisulfide, Disulfide, dimethyl, Disulfide, methyl 2-propenyl, Styrene, Oxime-, methoxy-phenyl, Thiophene, 3,4-dimethyl, Furfuryl sulfide and N,2,4,6-Tetramethyl-benzenamine. Since, the genotypes had significant variation in identified compounds as well as in their contents;. However, in Furfuryl and furan derivatives group, Furfuryl sulfide [Bis(furan-2-ylmethyl)sulfane] (Molecular formula: C10H10 O2S, avg. mass 194.250 da) was detected in all the genotypes in equal content (0.143 µg/g).
The volatile profile (HS-SPME/GC–MS analysis) of the Chinese chives’ cultivars detected Aldehydes, Hydrocarbons, Esters, Acids, Sulfides, Ketones, Ethers, Alkaloids, Heterocyclic polymers, Cyclomethicone, Polyolefins, Furfuryl and furan derivatives, Phenols, Diterpenes, and Terpenoids. The study showed that cultivars Xue Jiu and Jiu Xing 22 had equally greater volatiles as compared to Ma Lian Da Ye Bai Gen, Fu Jiu Bao F1, Shouguang Du Gen Hong, and Han Yu Zi Gen; and 17 kinds of volatile were common to all genotypes. The genotypes showed marked variation in identified constituents and their contents. There was only constituent [Furfuryl sulfide [Bis (furan-2-ylmethyl) sulfane] found to have equal content in all six genotypes (0.143 µg/g). This compound is based on glucose, and it was present in an equal concentration in all tested genotypes (Okaru and Lachenmeier, 2017). The furfural is highly useful as a flavoring agent in many foods and alcoholic and non-alcoholic drinks. The furfural in its numerous derivatives occurs broadly as a natural constituent of the food supply. So, its’ consistent concentration in test crops regardless of genotypes is symbolically the specialty of this plant to use or to add as a natural constituent of certain foods (Eric, 2018).
3.3.2 Response curve and flavor radar chart
The curve (Fig. 1) shows the response of various sensor types (W5S, W2W, and W1C), which are the main sensor types for six cultivars. The response curve revealed that with the increase in sampling time, the response signal of each sensor was gradually stabilized. The sensors and their main applications used in E-nose analysis distributed as W1C: Sensitive to aromatic compounds, W5S: Very sensitive to oxynitride, W3C: Sensitive to ammonia and aromatic compounds such as benzene; W6S: Sensitive to hydrogen; W5C: Sensitive to alkanes such as propane and aromatic compounds; W1S: Sensitive to methane; W1W: Sensitive to sulfur compounds such as hydrogen sulfide; W2S: Sensitive to alcohols and aldehydes and ketones; W2W: Sensitive to aromatic compounds and organic sulfur compounds; W3S: Sensitive to alkanes such as methane Cultivars Xue Jiu and Han Yu Zi Gen were relatively superior in volatile contents than other tested cultivars and characterized for aromatic, broad-methane, alkanes and sulfur-organic compounds. Fu Jiu Bao F1 designated in broad-alcohol and sulph-chlor groups; Shouguang Du Gen Hong was characterized as aromatic and methane-aliph; Jiu Xing 22 fell under broad range and aroma-aliph categories, while Ma Lian Da Ye Bai Gen was placed in hydrogen group (Fig. 2). The distinctive flavor constituents befalling generally in Chinese chive or in individual cultivars are controlled by specific biologically active sulfur-organic components. Some of the cultivars contained aromatic compounds and organic sulfur compounds. Cultivars Xue Jiu and Han Yu Zi Gen not only have aromatic and organic sulfur compounds, but their contents were higher than the other four companion cultivars. The fingerprint profiles of the six Chinese chives cultivars showing similarity, and W5S is the largest sensor, followed by W2W. Fig. 2 concludes that Xue Jiu and Han Yu Zi Gen were stronger in overall flavor characteristics than the rest of the evaluated cultivars.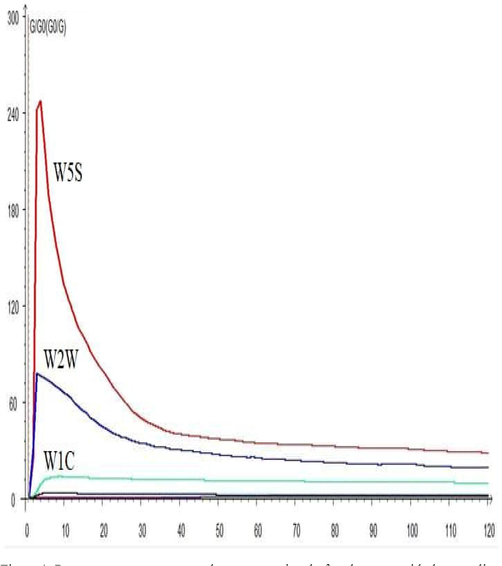
Response curve represents the response signal of each sensor with the sampling time of six Chinese chives cultivars.
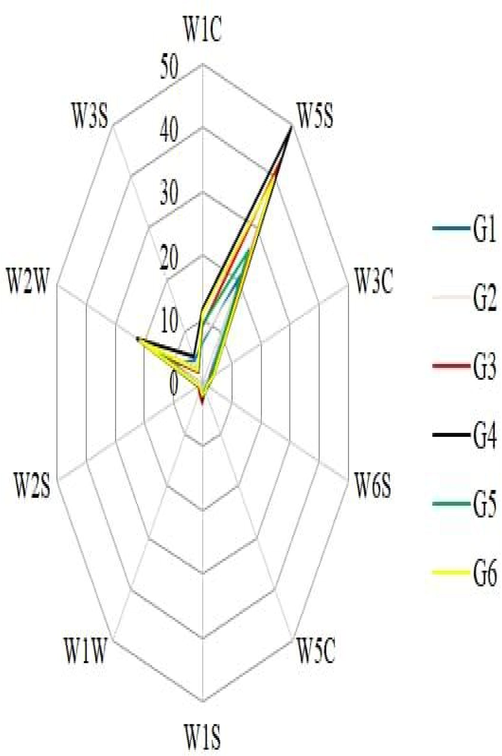
RFC (Radar fingerprint chart) of the volatile constituents in six Chinese chive cultivars.
3.3.3 Principal component analysis (PCA)
PCA is a clustering method of analysis of the dataset; it could be derived from a two-dimensional PCA illustrated as Figs. 3A and 3B, where six Chinese chives cultivars and ten sensors develop the related groups of volatiles. The sum of the first two main components reached 98.655%, out of which the main axis-1 characterized 93.06% of the total variance, while main exis-2 characterized 5.59% of the whole variance. It suggests that the order difference of various Chinese chives cultivars can be highly distinguished. The sum of these two components is >90%, so we distinguished them according to six cultivars. The first principal component can be divided into four categories. G1 and G2 are in the first group; G5 is in the second group; G3 and G6 in the third group; and G4 is in the fourth group. Distinguished cultivars G1 and G2, G3 and G6 by the second principal component, that is to say, first, it is distinguished by first principal component and indistinguishable is distinguished by the second principal component.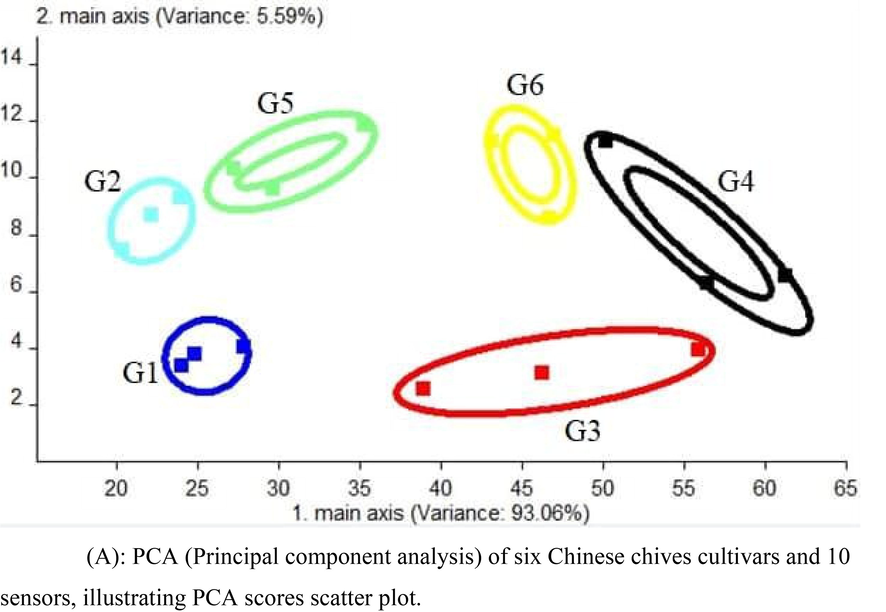
PCA (Principal component analysis) of six Chinese chives cultivars and 10 sensors, illustrating PCA scores scatter plot.
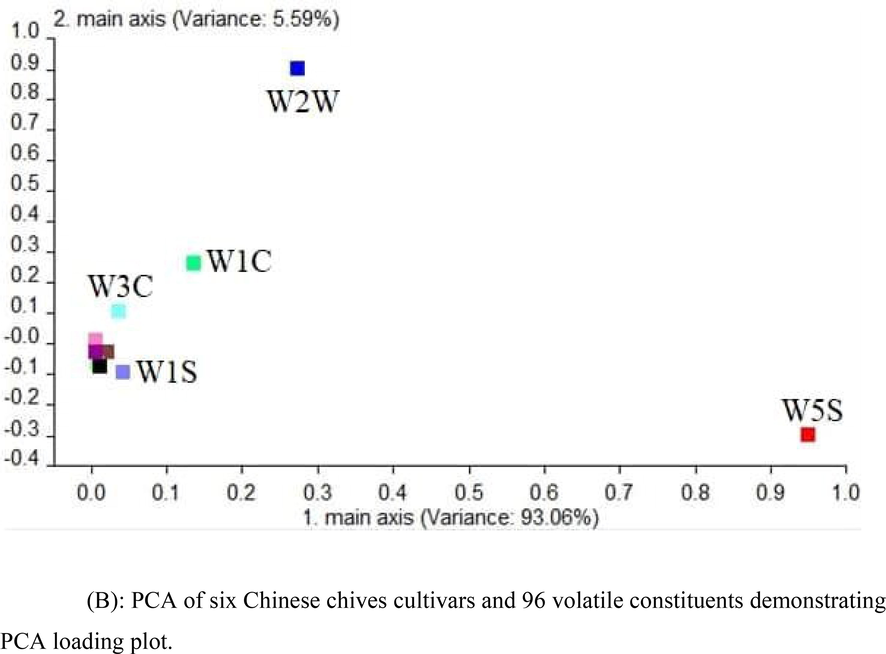
PCA of six Chinese chives cultivars and 96 volatile constituents demonstrating PCA loading plot.
Fig. 3B is the PCA of odor data of electronic nose showing a diagram of the PCA analysis of genotypes with sensors W5S, W1S, W3C, W1C, and W2W as primary sensors. It indicates that the farther each sensor was from the origin of the coordinate, the greater was the rate of contribution. Regarding the classification of six tested Chinese chive cultivars, the sensor W5S had a larger contribution rate. To increase recognition of the sensor, more sensors were added to distinguish the mixed types. Using PCA analysis for performance verification of sensor array, it is clearly seen that W5S is the farthest point of the first principal component and the intensity of the response is most significant. However, W2W is the farthest point of the second principal component, and the intensity of the response is the second. Therefore, sensors W5S and W2W could be selected as sensor arrays for mixed types.
4 Discussion
The crop cultivars may potentially vary in agronomic and quality traits, but under similar soil and climatic environment, such variation might have an association with the genetic makeup of the parental materials. In leaves number, cultivar Jiu Xing 22 surpassed the rest of cultivars, Ma Lian Da Ye Bai Gen produced tallest plants with longest leaves, maximum leaf sheath height was found in Fu Jiu Bao F1, Xue Jiu had maximum leaf width, and Shouguang Du Gen Hong surpassed rest of tested cultivars in leaf sheath diameter. This suggested that Chinese chive cultivars tested in this study showed significant (P < 0.05) variation in foliage and height of the plants. (Gao et al., 2018) have observed significant differences (P < 0.05) between Chinese chive varieties for their physical and chemical profiles. The experimental results reported by (Saini et al., 2013) revealed that Chinese chive varieties varied significantly for their agronomic profile.
The quality profile indicated that cultivar Shouguang Du Gen Hong showed superiority in soluble protein content, soluble sugar was highest in Ma Lian Da Ye Bai Gen, Jiu Xing 22 contained highest vitamin-C and superior in total leaf chlorophyll; while Xue Jiu was richest in nitrate among the tested Chinese chive cultivars. It is notable that not a single cultivar showed linear dominance for agronomic and quality traits. Alan et al. (2018); Alan et al. (2019) reported that Chinese chive cultivars vary significantly for various agronomic and quality traits such as foliage, nitrates and chlorophyll content. (Guohua et al., 2006) concluded that Chinese chive seeds and leaves had high levels of nutritionally important components such as minerals (Guohua et al., 2006); while (Alan et al., 2019) reported that Chinese chive seed contained high amounts of crude protein (12.3%); while in our study, the highest crude protein in leaves remained 1.97%. This indicates that the crude protein widely differs in seed and leaves of Chinese chives. The variation in crude protein may also be linked with the genetic makeup of cultivars used by different researchers under different ecologies. Volatile profile showed that esters, heterocyclic polymers, acids, aldehydes, hydrocarbons, sulfides, ketones, ethers, furfuryl derivatives and phenols were dominating compound groups. High total volatile contents (17.75 and 17.54 µg/g) were determined in Xue Jiu and Han Yu Zi Gen, respectively, greater than rest of the cultivars. The Furfuryl sulfide was detected in all the six tested cultivars in equal content (0.143 µg/g). The fruits and vegetables are dried before preservation to reduce water activity, but traditional drying adversely affects thermally labile bioactive compounds (organosulfur compounds) which may affect functional qualities (Kathori et al., 2020); these compounds are responsible for antimicrobial and antioxidant activities (Niu et al., 2020); where flavonoids possess strong antibacterial and antioxidant activity (Górniak et al. 2019). The utilization of Chinese chives affects human health positively (Putnika et al., 2019). Yabuki et al., (2010) characterized the composition of Sulfur-containing constituents in Chinese chive using 12 varieties and detected five disulfides, two trisulphides, and two vinyldithiins. They also tentatively identified two novel compounds. The results of (Goeke, 2002) and (Pino et al., 2001) also showed similarity to our research findings.
E-nose analysis reveals that distinguishing volatiles were scattered as aromatic, broad range, sulfur organic, hydrogen, arom-aliph, broad methane, broad alcohol, and methane aliph. Among genotypes, Xue Jiu and Han Yu Zi Gen were found to have greater contents of the volatiles in addition to the quality of aromatic and sulfur organic constituents. The fingerprint profiles suggested similarity in Xue Jiu and Han Yu Zi Gen, and W5S is the largest sensor, followed by W2W. This indicates that Xue Jiu and Han Yu Zi Gen were genotypes with strong overall flavor characteristics as compared to the rest of the tested genotypes. Similar study results have been reported by (Welke et al., 2014). Generally, the practical contribution of the volatile is considered more promising when its concentration is >1 (Tchabo et al., 2017). However, in our study, seven volatiles had contents greater than >1 µg/g. Wei et al. (2021) detected 24 volatiles with >1 µg/g content to describe the odor of the cabbage samples, and volatiles were categorically designated as aromatic, pungent, fruity, fatty, and floral by the radar fingerprint chart. The pungent odor could be considered as the strongest scent, and ethers have the primary role in forming this odor (Rajkumar et al., 2017). The odor descriptions of dimethyl disulfide and dimethyl trisulfide are generally described as Allium, and sulfurous aroma, and these were determined in entire samples having a vital role in the development of fragrance in Allium species. The aroma is mainly consisted of (E)-2-hexenal, hexanal, 3-hexenal and (Z)-3-hexen-1-ol. (E)-2-hexenal and their contents confirm green aroma that contributes to the fragrance of specific Chinese chive genotypes (Yalcin and Kavuncuoglu, 2014). The distinctive flavor constituents befalling in Allium species or genotypes are dominated by biologically active organosulfur compounds (Gyawali et al., 2006, Yalcin and Kavuncuoglu, 2014). Using a scatter diagram, one can purposely find potential volatile substances of each cultivar, and then the difference information can be developed to help to make a broad level evaluation of volatiles of Chinese chives cultivars (Khalil et al., 2018).
5 Conclusions
This study deals with the characterization of six Chinese chives cultivars for their agronomy, chemical composition, organosulfur compounds as well as textural and aromatic quality characteristics (E-Nose analysis). The cultivars subjected to study included Han Yu Zi Gen, Fu Jiu Bao F1, Shouguang Du Gen Hong, Xue Jiu, Jiu Xing 22 and Ma Lian Da Ye Bai Gen. Apart from the analysis of agronomic and quality indices, these newly developed cultivars were first time studied in depth to compare their suitability for aromatic and textural qualities that are desirable for food processing. The cultivars were analyzed for agronomy and quality indices by methods in general use in modern laboratories; while their volatile profile was studied by HS-SPME/GC–MS and E-nose analyses. The cultivars varied for agronomy and quality indices (P < 0.05). The agronomic measurement of Chinese chive concluded that in leaves number, cultivar Jiu Xing 22 surpassed rest of cultivars, Ma Lian Da Ye Bai Gen produced tallest plants with longest leaves, maximum leaf sheath height was found in Fu Jiu Bao F1, Xue Jiu had maximum leaf width, and Shouguang Du Gen Hong surpassed rest of cultivars in leaf sheath diameter. In quality case, cultivar Shouguang Du Gen Hong showed superiority in soluble protein content; soluble sugar was highest in Ma Lian Da Ye Bai Gen; Jiu Xing 22 contained highest vitamin-C and total leaf chlorophyll; while Xue Jiu was richest in nitrate content. Thus, not a single cultivar showed linear dominance for agronomic and quality traits. Fresh leaf samples of six Chinese chive genotypes were analyzed to characterize volatile profile by HS-SPME/GC–MS and e-nose techniques. The major radicals in fresh leaves of Chinese chive were (E)-1 Allyl, (E)-1-Methyl, Hexadecanoic acid, Diallyl disulfide, Sulfide allyl, Trisulfides, Dimethyl ether, Benzaldehyde, Hexenal, N,2,4,6-Tetramethyl benzenamine, trans-beta.-Ionone, Dimethyl trisulfide, Disulfide, dimethyl, Oxime, and Furfuryl sulfide. The cultivars showed marked variation in identified constituents and their contents. There was only constituent [Furfuryl sulfide [Bis(furan-2-ylmethyl) sulfane] found to have equal content in all six genotypes (0.143 µg/g). The aromatic characteristic might be distinguished based on PCA, electronic-nose and HS-SPME/GC–MS results. The analyses suggested that Xue Jiu and Han Yu Zi Gen were cultivars with strong overall flavor characteristic and these cultivars have aromatic and organic sulfur compounds, but their contents were higher than the other four companion cultivars. The study also proved that HS-SPME/GC–MS and electronic-nose methods might have the accuracy to detect flavor differences in Chinese chives cultivars. It is suggestible that fresh Chinese chive could be viable to utilize in the food industry based on their richness in physicochemical properties, volatiles, and odor qualities. The volatile profile and e-nose analysis suggested that Xue Jiu and Han Yu Zi Gen were cultivars with strong overall flavor characteristic, while Shouguang Du Gen Hong was lightest in flavor characteristics among tested cultivars.
Acknowledgements
This work was supported by the Education science and technology innovation project of Gansu Province (GSSYLXM-02); Research Program Sponsored by State Key Laboratory of Aridland Crop Science, Gansu Agricultural University (No. GSCS-2020-12); Natural Science Foundation of Gansu Province (20JR10RA513); Special project of central government guiding local science and technology development (ZCYD-2020-5). The authors extend their appreciation to the Researchers Supporting Project number (RSP-2021/219), King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- The use of the ‘cryogenic’gc/ms and on-column injection for study of organosulfur compounds of the Allium sativum. Journal of Food Composition and Analysis. 2004;17(2):235-245.
- [Google Scholar]
- Production of Chinese chive (Allium tuberosum) as potted herb. Acta Horticulturae.. 2019;1251:135-138.
- [Google Scholar]
- Production of Chinese chive (Allium tuberosum) as potted herb. XXX International Horticultural Congress IHC2018. Acta Hortic. (1251):135-138.
- [Google Scholar]
- Effect of extraction methods on antibacterial activity and chemical composition of chinese chives (Allium tuberosum rottl. Ex spreng) extract. KMUTNB Int J Appl. Sci Technol. 2017;10(2):97-106.
- [Google Scholar]
- Bioaccessibility of bioactive compounds from fruits and vegetables after thermal and nonthermal processing. Trends in Food Science & Technology. 2017;67:195-206.
- [Google Scholar]
- D. Bede L. Zaixiang Dietary Polysaccharides from Allium Species: A Critical Review in Dietary Polysaccharides from Allium Species: Extraction, Characterization, Bioactivity, And Potential Utilization Act Scie Agri 4 2 01 15.
- Nutritional and therapeutic potential of garlic and onion (Allium sp.) Current Nutrition & Food Science. 2016;12(3):190-199.
- [Google Scholar]
- Allium chemistry: GC-MS analysis of thiosulfinates and related compounds from onion, leek, scallion, shallot, chive, and Chinese chive. Journal of Agricultural and Food Chemistry.. 1992;40(12):2431-2438.
- [Google Scholar]
- The organosulfur chemistry of the genus allium–implications for the organic chemistry of sulfur. Angewandte Chemie International Edition in English. 1992;31(9):1135-1178.
- [Google Scholar]
- Block Eric., 2018. Organosulfur compound. Encyclopedia Britannica, 14 Aug. 2018, https://www.britannica.com/science/organosulfur-compound. Accessed 19 January 2022.
- Flavonol glucosides in allium species: A comparative study by means of HPLC–DAD–ESI-MS–MS. Food Chemistry. 2008;107(4):1668-1673.
- [Google Scholar]
- Distribution of nutritive compounds and sensory quality in the leaves of chives (Allium schoenoprasum L.) Journal of applied botany and food quality. 2006;80(2):155.
- [Google Scholar]
- Isolation, structural elucidation and immunomodulatory activity of fructans from aged garlic extract. Phytochemistry.. 2011;72(2–3):255-264.
- [Google Scholar]
- Immunostimulatory properties of fructans derived from raw garlic (Allium sativum L.) Bioactive carbohydrates and dietary fibre.. 2016;8(2):65-70.
- [Google Scholar]
- Chives: Handbook of herbs and spices, Elsevier. In: Handbook of Herbs and Spices. Elsevier; 2006. p. :337-346.
- [Google Scholar]
- Biological properties of onions and garlic. Trends in food science & technology. 2007;18(12):609-625.
- [Google Scholar]
- In vitro antioxidant and antihypertensive compounds from camu-camu (Myrciaria dubia McVaugh, Myrtaceae) seed coat: A multivariate structure- activity study. Food and Chemical Toxicology. 2018;120:479-490.
- [Google Scholar]
- Relationship between Chinese chive (Allium tuberosum) and its putative progenitor a. Ramosum as assessed by random amplified polymorphic DNA (rapd). University of California Press; 2006. p. :134-142. Documenting domestication
- Isolation and identification of new chemical constituents from Chinese chive (Allium tuberosum) and toxicological evaluation of raw and cooked Chinese chive. Food and Chemical Toxicology. 2018;112:400-411.
- [Google Scholar]
- Q. Gao L.i. Song J. Sun H.-Q. Cao L. Wang H. Lin F. Tang Repellent action and contact toxicity mechanisms of the essential oil extracted from Chinese chive against Plutella xylostella larvae Archives of insect biochemistry and physiology. 100 1 2019 e21509 e21509.
- Extraction of bioactive compounds and essential oils from Mediterranean herbs by conventional and green innovative techniques: A review. Food Research International. 2018;113:245-262.
- [Google Scholar]
- Sulfur compounds identification and quantification from allium spp. Fresh leaves. Journal of food and drug analysis. 2014;22(4):425-430.
- [Google Scholar]
- Sulfur-containing odorants in fragrance chemistry. Sulfur Reports.. 2002;23(3):243-278.
- [CrossRef] [Google Scholar]
- Water resistance, mechanical properties and biodegradability of methylated- cornstarch/poly (vinyl alcohol) blend film. Polymer Degradation and stability. 2006;23(3):243-278.
- [Google Scholar]
- Comprehensive review of antimicrobial activities of plant flavonoids. Phytochem. Rev.. 2019;18(1):241-272.
- [Google Scholar]
- Effect of γ-irradiation on volatile compounds of dried welsh onion (Allium fistulosum L.) Radiation Physics and Chemistry. 2006;75(2):322-328.
- [Google Scholar]
- Diallyl sulfide enhances antioxidants and inhibits inflammation through the activation of nrf2 against gentamicin-induced nephrotoxicity in wistar rats. European Journal of pharmacology. 2009;606(1–3):162-171.
- [Google Scholar]
- Chemical composition and antimicrobial activity of the essential oils of selected apiaceous fruits. Future Journal of Pharmaceutical Sciences. 2018;4(1):88-92.
- [Google Scholar]
- Thiosulfinates from Allium tuberosum L. Induce apoptosis via caspase-dependent and-independent pathways in pc-3 human prostate cancer cells. Bioorganic & medicinal chemistry. 2008;18(1):199-204.
- [Google Scholar]
- Chemical profile and bioactivity of Chinese chives (Allium tuberosum Rottl. Ex Spreng) crude extracts under different solvent extractions. International Journal of Advanced Biotechnology and Research.. 2016;7(4):2209-2221.
- [Google Scholar]
- Ajoene the main active compound of garlic (Allium sativum): A new antifungal agent. Revista Iberoamericana de Micologia. 2006;23(2):75-80.
- [Google Scholar]
- Effect of led spectrum on the quality and nitrogen metabolism of lettuce under recycled hydroponics. Frontiers in Plant Science. 2021;12:1159.
- [Google Scholar]
- Phylogeny and biogeography of Allium (amaryllidaceae: Allieae) based on nuclear ribosomal internal transcribed spacer and chloroplast rps16 sequences, focusing on the inclusion of species endemic to china. Annals of Botany.. 2010;106(5):709-733.
- [Google Scholar]
- The composition of volatiles from different parts of Allium tuberosum plants. Phytochemistry. 1977;16(6):763-764.
- [Google Scholar]
- The identification of (+) s-methyl-l-cysteine sulfoxide in plants. Journal of the American Chemical Society. 1956;78(8):1605-1608.
- [Google Scholar]
- Utilization of quercetin and quercetin glycosides from onion (Allium cepa L.) solid waste as an antioxidant, urease and xanthine oxidase inhibitors. Food Chemistry. 2017;235:119-126.
- [Google Scholar]
- Optimization of Chinese Chive Juice as a Functional Feed Additive. Appl. Sci.. 2020;10:6194.
- [CrossRef] [Google Scholar]
- The Food and Beverage Occurrence of Furfuryl Alcohol and Myrcene-Two Emerging Potential Human Carcinogens? Toxics. 2017;5(1):9.
- [Google Scholar]
- Volatile constituents of chinese chive (Allium tuberosum Rottl. Ex Sprengel) and Rakkyo (Allium chinense g. Don) Journal of agricultural and food chemistry. 2001;49(3):1328-1330.
- [Google Scholar]
- Stability and extraction of bioactive sulfur compounds from Allium genus processed by traditional and innovative technologies. Journal of Food Composition and Analysis. 2017;61:28-39.
- [Google Scholar]
- Thermochemical transformation of sulfur compounds in Japanese domestic Allium. Trends in Food Science & Technology. 2016;52:49-56.
- [CrossRef] [Google Scholar]
- Application of solid-phase microextraction in analytical toxicology. Analytical and Bioanalytical Chemistry. 2007;388(7):1393-1414.
- [Google Scholar]
- Antioxidant and free radical scavenging activities of phenols from onion (Allium cepa) Food Chemistry. 2007;102(4):1389-1393.
- [Google Scholar]
- Fresh-cut apples spoilage and predictive microbial growth under modified atmosphere packaging. In: Rai R., Aswathanarayan J.B., eds. Food safety and protection. Press, Boca Raton, FL: CRC Press; 2017. p. :728.
- [Google Scholar]
- An overview of organosulfur compounds from Allium spp.: From processing and preservation to evaluation of their bioavailability, antimicrobial, and anti-inflammatory properties. Food Chemistry. 2019;276:680-691.
- [Google Scholar]
- Comparative evaluation of physical properties and volatiles profile of cabbages subjected to hot air and freeze drying. LWT. 2017;80:501-509.
- [Google Scholar]
- Solid-phase microextraction method development for headspace analysis of volatile flavor compounds. Journal of Agricultural and Food Chemistry. 2000;48(6):2430-2437.
- [Google Scholar]
- Bioactive s-alk (en)yl cysteine sulfoxide metabolites in the genus allium: The chemistry of potential therapeutic agents. Natural product reports. 2005;22(3):351-368.
- [Google Scholar]
- Comparative study between cultivated garlic (Allium sativum) and wild garlic (Allium tuberosum) Asian Journal of Pharmaceutical Research and Development 2013:108-125.
- [Google Scholar]
- The effects of food essential oils on cardiovascular diseases: A review. Critical Reviews in Food Science and Nutrition.. 2018;58(10):1688-1705.
- [Google Scholar]
- Comparison of the antioxidant activity of two Spanish onion varieties. Food Chemistry. 2008;107(3):1210-1216.
- [Google Scholar]
- Characterization of alliinase of Allium cepa (onion) Archives of Biochemistry and Biophysics. 1963;100(1):66-73.
- [Google Scholar]
- A rapid, sensitive, and versatile assay for protein using coomassie brilliant blue g250. Analytical. 1977;79(1-2):544-552.
- [Google Scholar]
- Antibacterial activity of s-methyl methanethiosulfinate and s-methyl 2-propene-1-thiosulfinate from Chinese chive toward escherichia coli o157: H7. Bioscience, biotechnology, and biochemistry. 2001;65(4):966-968.
- [Google Scholar]
- Helicobacter pylori-In vitro susceptibility to garlic (Allium sativum) extract. Nutr Cancer. 1997;27(2):118-121.
- [CrossRef] [Google Scholar]
- Aroma profile and sensory characteristics of a sulfur dioxide-free mulberry (Morus nigra) wine subjected to non-thermal accelerating aging techniques. Food chemistry. 2017;232:89-97.
- [Google Scholar]
- Anti-inflammatory effects of an aqueous extract of Welsh onion green leaves in mice. Food Chemistry. 2013;138(2–3):751-756.
- [Google Scholar]
- Effect of slow-release fertilizer on soil fertility and growth and quality of wintering chinese chives (Allium tuberm Rottler ex Spreng.) in greenhouses. Scientific Reports. 2021;11(1):1-14.
- [Google Scholar]
- S. Wei X. Xiao L. Wei L. Li G. Li F. Liu J. Xie J. Yu Y. Zhong Development and comprehensive HS-SPME/GC–MS analysis optimization, comparison, and evaluation of different cabbage cultivars (Brassica oleracea L. Var. Capitata L.) volatile components Food Chemistry 340 2021 128166 128166.
- Quantitative analysis of headspace volatile compounds using comprehensive two-dimensional gas chromatography and their contribution to the aroma of chardonnay wine. Food Research International. 2014;59:85-99.
- [Google Scholar]
- Characterisation of volatile sulphur-containing compounds generated in crushed leaves of Chinese chive (Allium tuberosum Rottler) Food Chemistry. 2010;120(2):343-348.
- [Google Scholar]
- Physical, chemical and bioactive properties of onion (Allium cepa l.) seed and seed oil. Journal of Applied Botany and Food. Quality. 2014;87
- [Google Scholar]
- Enzymeassisted extraction of polyphenol from edible lotus (Nelumbo nucifera) rhizome knot: Ultra-filtration performance and HPLC-MS2 profile. Food Research International. 2018;111:291-298.
- [Google Scholar]







