Translate this page into:
Characterization of pectinase from Geotrichum candidum AA15 and its potential application in orange juice clarification
⁎Corresponding author. msohail@uok.edu.pk (Muhammad Sohail)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
Purpose
Pectinase is used in fruit juice industry to increase the juice yield and to enhance clarification process by reducing turbidity and viscosity. The current study was focused on characterizing pectinase preparation from Geotrichum candidum AA15 and to evaluate its application in clarification of orange juice.
Methods
The pectinase preparation from AA15 was characterized to determine optimum temperature and pH for its activity and thermal stability under acidic environment. Considering the desirable attributes exhibited by the enzyme, it was applied for orange juice clarification and factors affecting the clarification process were optimized using response surface methodology (RSM) approach.
Results
This enzyme exhibited the highest activity when incubated at 35 °C for 25 min in a buffer of pH 5. The enzyme did not lose any activity for up to 1 h at 30 °C. Whereas, activity was declined to 82% and 67% when incubated for 1 h at 35 °C and 40 °C, respectively. Moreover, stability of the enzyme when placed in the buffer of pH 2–3 was remarkable. The characteristics of extracted pectinase to work at mesophilic temperature under acidic environment presented it as suitable to be applied in fruit juice processing industries. Consequently, the factors affecting orange juice clarification, including temperature, treatment duration and enzyme loadings were optimized using RSM approach and clarification yield of 61% was obtained under optimized conditions.
Conclusion
The pectinase preparation was found suitable to clarify orange juice and the data depicted the possible candidature of the strain for future biotechnological applications.
Keywords
Box Behnken design
Pectinase
Thermal stability
Yeast
1 Introduction
Pectinases exhibit vital importance in the present biotechnological field. They are widely utilized in bioscouring of cotton, retting and degumming of plant fibers, coffee and tea fermentation, extraction of vegetable oil, paper bleaching and treatment of pectic wastewater. In food processing industries, it is employed for the extraction and clarification of fruit juices (Baumann, 1981). Microbial pectinases share approximately 25% sales of food enzymes worldwide. The major proportion of which is obtained from filamentous fungi, particularly from A. niger (Jayani et al., 2005, dos Santos et al., 2016). Commercial preparations of pectinases from fungal source contain a mixture of pectinolytic enzymes with other proteins. Although, a single type of pectinase activity is needed for some of the industrial processes such as for the clarification of orange juice. Thus the alternate sources of pectinase need to be evaluated. Yeasts produce different enzymes in considerable amount and could be an efficient source for the production of pectinase. Yeasts offer several advantages over filamentous fungi such as many species are considered as GRAS and generally produce single type of pectinase (Lessa et al., 2018). Furthermore, shorter generation time of yeasts could be exploited to decrease the cost of the enzyme by getting more titers in relatively shorter fermentation period (Padma et al., 2011). Literature revealed the pectinolytic potential of several yeast genera including Saccharomyces, Kluyveromyces, Rhodotorula, Cryptococcus, Geotrichum and Candida (Vaughn et al., 1969; Wimborne and Rickard, 1978; Federici, 1985; Barnby et al., 1990).
The catalytic attributes and the stability of an enzyme under various physio-chemical environments are significant for their commercialization. The biochemical characteristics of enzyme assist in retaining its intended activity level for a more extended period and improving stability (Celestino et al., 2006). The catalytic properties and molecular weight of pectinase vary in different microorganisms (Kaur et al., 2004). However, yeasts and molds are known to produce pectinases which can work in acidic conditions such as in fruit juices (Kumar and Sharma, 2012).
Presence of various components of fruits, such as pectin, makes its juice unclear (Vaillant et al., 1999) and the elevated level of pectin results in colloid formation which is a commonly faced problem during clear fruit juice production. Although, filtration can be done to separate these suspended pulp particles but the existence of pectin offers some resistance because of its fibre-like molecular structure (Sulaiman et al., 1998). Alternatively, pectinolytic enzymes have been utilized for depectinization of various juices including apple, orange, mosambi, banana, lemon, mango, pineapple, grape, guava, papaya and date syrup (Patidar et al., 2018). Pectinases flocculate pectin by making its complex with proteins, thereby, the resulting juice exhibits lesser viscosity which ultimately enhances the filtration, flavor and color. Pretreatment of fruit juices and other pectin containing liquid foods with pectinases has been evaluated to impregnate flow during microfiltration, ultrafiltration and reverse osmosis since the presence of pectin results in fouling of membrane and consequently in flux reduction (Rai et al., 2004). Indeed the flux of depectinized apple juice was found to increase by more than two fold compare to the untreated juice (Rao et al., 1987).
Tropical fruit juices are popular throughout the world owing to their taste and are utilized in place of traditional caffeine-containing drinks such as coffee and tea, and carbonated soft drinks (Jagtiani et al., 1988). Among tropical fruits, citrus fruits and juices serve as primary source of our daily requirement of vitamin C as well as also provide some additional supplementary nutrients. Orange is one of the important tropical citrus fruit originated from Asia (Kareem and Adebowale, 2007). Pakistan is included in one of those countries where a variety of fruits are produced that are grown under different climatic conditions such as apple, pear and cherries (grow under cold climate), apricots, grapes, pomegranates and melons (grow under warm climate) and banana, mango, guava, date and citrus fruits (i.e. tropical or sub-tropical fruits). Pakistan exports significant quantity of these fruit juices to different countries and currently, 38 fruit processing units are operating in Pakistan. The industrial processing of orange juices requires enzymatic clarification due to its hazy appearance. Several studies have been reported the optimization of enzymatic clarification process of tropical fruits juices including orange juice (Lee et al., 2006; Kareem and Adebowale, 2007). The enzymatic degradation of pectin is affected by different process variables including enzyme concentration, temperature and treatment duration (Rai et al., 2004). These factors need to be optimized for maximum clarification of juices. A conventional approach for the determination of optimal conditions is varying one factor at a time while keeping other factors at a constant level. Although, this strategy is simple and straight, however, it is time and labor intensive and does not explain the interactive effect among the factors. Considering these limitations, statistical tools have been developed to investigate interaction among process variables and to determine optimal level of significant variables to get the maximum yield. Response surface methodology (RSM) is one of the efficient statistical tool that analyzes multiple parameters and their interactions in relatively fewer number of experiments thus requires less time. Previously, the tool has been employed for the optimization of production of ligninolytic enzymes from Rhizopus (dos Santos et al., 2017) and clarification of various tropical fruit juices using mold pectinases (Lee et al., 2006). Yeast pectinases have not been studied using statistical tools to evaluate their suitability in juice clarification. Previously, we reported about statistical optimization of immobilization of indigenous strain, AA15 of Geotrichum candidum, on corncob and for subsequent production of pectinase (Ejaz et al., 2018). In this study, pectinase preparation from AA15 was characterized to determine its activity and stability profiles at varying temperature and pH. Furthermore, the crude enzyme was applied for clarification of orange juice and three of the variables were optimized using RSM.
2 Materials and methods
2.1 Production of pectinase
G. candidum AA15 was retrieved from the Departmental culture collection and cultivated to produce pectinase under optimized conditions as reported previously (Ahmed et al., 2019). The medium was centrifuged at 3000×g for 15 min and cell-free culture supernatant (CFCS) was obtained for further studies.
2.2 Pectinase assay
Pectinase activity in CFCS was evaluated by determining the amount of galacturonic acid by DNS method (Miller, 1959) using buffered citrus pectin (0.5% w/v) as a substrate and galacturonic acid as a standard. One unit of pectinase activity was defined as the amount of enzyme required to produce 1 µmole of galacturonic acid under standard assay conditions.
2.3 Effect of temperature on pectinase activity and stability
The effect of temperature on the pectinase activity by AA15 was investigated by performing the enzyme assay as mentioned in the previous section except for varying temperature from 25 to 65 °C. Pectinase activity (IU mL−1) was plotted against the temperature and optimum temperature was determined. Thermal stability of pectinase was assessed by placing CFCS at various temperatures (25–65 °C) for 1 h, chilled on ice for 15 min, followed by performing pectinase assay to determine the residual enzyme activity.
2.4 Influence of pH on the activity and stability of pectinase
The effect of pH on pectinase activity by the strain AA15 was investigated by subjecting CFCS to pectinase assay in presence of different buffers (pH 2.5–8) at optimum temperature (35 °C). To determine the effect of pH on the stability of pectinase by AA15, CFCS was equilibrated in buffers of various pH and placed in a water bath at 35 °C for 3 h. Aliquots were collected with an interval of 30 min and residual activity was determined under standard assay conditions. The buffers tested include Glycine-HCl (50 mM, pH 2.5–3.5), sodium acetate (50 mM, pH 4.0–5.5), citrate phosphate (50 mM, pH 6.0–7.0) and Tris-HCl (50 mM, 7.5–9.0).
2.5 Juice extraction process
Oranges were procured locally, properly washed with tap water and cut into two halves with a clean knife. A screw type juice extractor was used to obtain juice manually.
2.6 Enzymatic treatment
The potential of pectinase preparation of AA15 was evaluated for the clarification of orange juice by performing some preliminary experiments that provided an idea to adjust the levels of each variable in the subsequent experimental design. Box Behnken design (BBD) in response surface methodology (RSM) was adopted in this study. The selected variables were incubation temperature, X1 (30–40 °C), duration of treatment, X2 (60–180 min), and concentration of enzyme, X3 (4–8 v/v %). Water bath was used to maintain the temperature of the enzymatic treatment to the desired level. The pH of the juice was hold constant at natural value (3.3–4.2) as the variation in pH is not a common practice for industrial clarification process of juice (Rai et al. 2004). For each experiment, 25 ml of juice was treated as described in Table 1.
Experiment no.
Temperature (°C) X1
Time (min) X2
Enzyme concentration (%v/v), X3
Clarity (%) (Experimental value)
Clarity (%) (Predicted value)
1
30
60
6
11
12
2
40
60
6
0
0
3
30
180
6
36
39
4
40
180
6
14
13
5
30
120
4
36
29
6
40
120
4
10
8
7
30
120
8
25
27
8
40
120
8
0
6
9
35
60
4
17
22
10
35
180
4
50
53
11
35
60
8
32
29
12
35
180
8
48
42
13
35
120
6
0
0
14
35
120
6
0
0
15
35
120
6
0
0
A design of 15 combinations was generated using Minitab 17. The response function measured was clarity of juice. The response variable (Y) was related to coded variables by a second order polynomial equation as given below.
The coefficients of the polynomial were shown by b0 (constant term), b1, b2 and b3 (linear coefficient), b12, b13 and b23 (interactive coefficient) andb11, b22 and b33 (quadratic coefficient). Minitab 17 was used to generate analysis of variance (ANOVA) table that also aided to determine the effect and regression coefficients of individual linear, quadratic and interactive terms. F-value at a probability of 0.05 i.e. 95% confidence limit was used to assess the significances of all terms in the polynomial. Contour plots were created from the regression coefficients to visualize the interactive effect of variables.
2.7 Clarity analysis
After each experimental run (as suggested by BBD) the pectinase was denatured by heating the suspension at 90 °C for 5 min. Pulp and particles from the treated juice were separated by centrifugation at 3000×g for 10 min. Supernatant was used to analyze clarity of the juice by taking absorbance at 660 nm (OD660) in a spectrophotometer against distilled water as a reference blank.
3 Results and discussions
Pectinase is an industrially important enzyme that has many commercial applications, such as, in food juice industry. In this study, pectinase activity in crude preparation from G. candidum AA15 was characterized and studied for its possible application in clarification of orange juice.
3.1 Effect of temperature on activity and stability of pectinase
Temperature is considered as one of the important aspect for the activity and stability of an enzyme. The studies on the activity of pectinase by G. candidum AA15 revealed that the enzyme shows its maximum activity at 35 °C (Fig. 1a) and any further increase in temperature reduces the activity drastically. The reduction of enzyme activity at elevated temperatures is perhaps by the thermal denaturation of the enzyme (Amin et al., 2013). The optimal temperature for pectinase activity from AA15 was comparable to the optimum temperature (30 °C) for pectinase by another strain of G. candidum (Hang and Woodams, 1992). Although, thermophilic enzymes are stable for long period of time at high temperatures during the pretreatment of various substances in industries and they also serve as an ecofriendly alternate as compare to harsh chemicals (Dhiman et al., 2013). However, the enzymes work at mesophilic or sub-mesophilic temperatures are applied efficiently in food processing industries to preserve the nutritional and sensory properties (Nakagawa et al., 2005).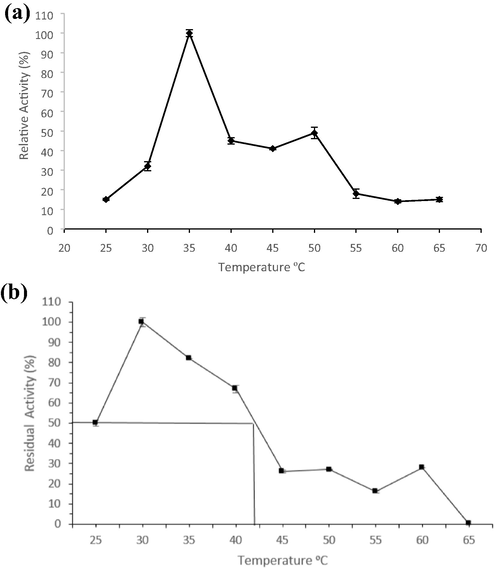
Effect of temperature on (a) activity and (b) stability of pectinase by G. candidum.
Apart from ability of an enzyme to work optimally at higher temperature, thermostability in terms of capability of an enzyme to resist thermal denaturation is also a limiting factor that dictates utilization of an enzyme at commercial scale (Bhatti et al., 2006). The thermal stability profile of pectinase from G. candidum AA15 was investigated in absence of the substrate and the data showed that there was less than 50% loss in activity at temperatures ranged between 25 and 40 °C even after 1 h while it retained 100% of initial activity when incubated at 30 °C for 1 h (Fig. 1b). It showed that pectinase from AA15 is reportedly more stable than polygalacturonase by G. candidum where 50% loss in activity was observed within 1 h at 40 °C and complete loss was observed within 15 min at 50 °C (Hang and Woodams, 1992). Mid-point of thermal inactivation (Tm) for pectinase by AA15 was calculated as 42 °C. The stability of pectinases at different temperatures is a significant attribute and pectinase from G. candidum AA15 could be potentially applied in fruit juice processing industries as it is stable up to 40 °C for 1 h.
3.2 Influence of pH on activity and stability of pectinase
Enzyme activity is greatly influenced by pH as the binding of substrate and catalysis are affected by the charge distribution on substrate and enzyme molecules. (Kulkarni et al., 2007). Blanco et al. (1994), reported that most of the pectinases obtained from yeasts have an optimal pH range of 3.5–5.5. The activity of pectinase from AA15 was determined in presence of buffers of various pH and it was found that the enzyme was more active towards acidic pH (from 3 to 5.5) with an optimal activity at pH 5. The pectinase activity was drastically decreased when enzymatic reaction was carried out at a pH higher than 5 and towards alkaline side, although, a slight increment in the activity was observed at pH 7.5 indicating the presence of isoform of the enzyme (Fig. 2a). Owing to its optimal activity under acidic environment, the pectinase from G. candidum AA15 has a prospect to be applied in fruit juice industries and winemaking.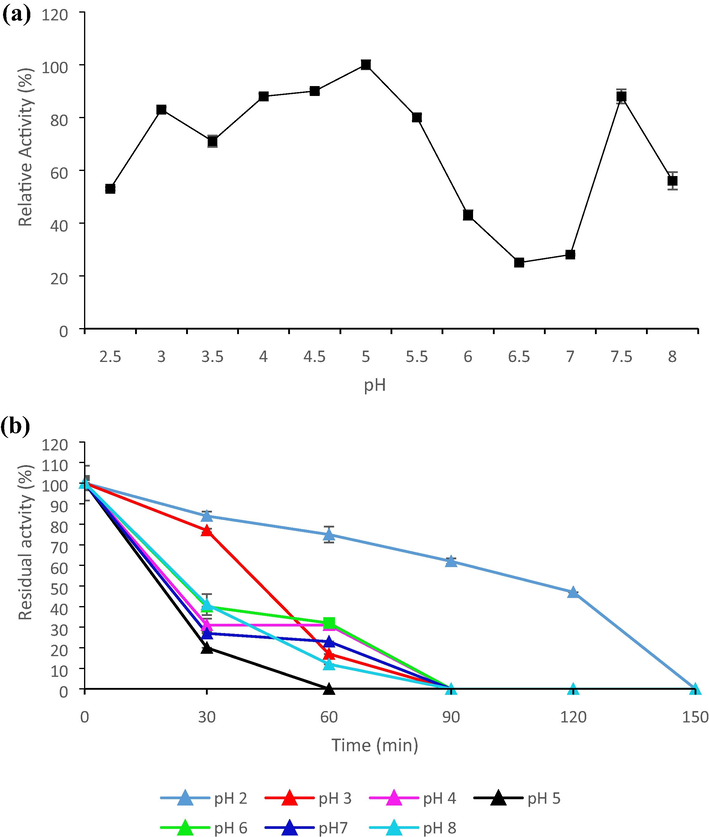
Effect of pH on pectinase (a) activity and (b) stability by G. candidum AA15.
Stability of enzyme at different pH is also crucial for its industrial applications. A change in pH causes reversible and irreversible effects on the enzyme activity. The protonation of amino acids of an enzyme is one way to exert reversible effect while the alteration in the structurally important groups of amino acids leads to irreversible change in the native structure of enzyme. Enzyme activity is reduced by irreversible changes whereas reversible effects do not affect the activity of enzyme (McDermid et al., 1988). The stability of pectinase from AA15 was studied at 35 °C in presence of various buffers and residual activity was noted. The data revealed stability of the enzyme at pH 2 and 3 for 30 min; thereafter a decline in the activity was observed. However, it retained up to 50% activity at pH 2 till 2 h (Fig. 2b). The drastic effect was more pronounced when pH was increased from 3. Martos et al. (2013), reported about stability of pectinase from Wickerhanomyces anomalus at a pH range of 3–6 for a longer duration. Nevertheless, moderate stability of pectinase from AA15 under acidic conditions indicated its suitability to be applied for the clarification of various fruit juices.
3.3 Statistical analysis of BBD
The data of characterization of pectinase from AA15 showed that the enzyme can be applied for fruit juice clarification, therefore, studies were conducted to evaluate the potential of the enzyme for the clarification of orange juice. Three of the factors affecting clarification process i.e. temperature, time and enzyme concentration were optimized by employing a statistical tool, Box Behnken design (BBD). In the design of 15 experimental runs, clarity of orange juice was taken as response and the data showed that the response varied greatly from 0 to 50% when conditions of the process varied (Table 1). It indicated that the response depends on the conditions and optimization of the parameters can improve the clarity of the juice. After analysis, regression coefficients for the second order polynomial equation and for linear, quadratic and interactive terms were determined (Table 2) that revealed adequacy of the model with a R2 value of 0.95. The R2 value closer to 1 indicates that the empirical model better fits the actual data. Statistical significance of the model was further affirmed by considering the analysis of variance (ANOVA) that presented F-value of 12.28 and p-value of 0.007 < 0.05 (Table 2). df = Degree of freedom; F-value = Fishers’ function; p-value = level of confidence. R2 = 0.956, R2 (adjusted) = 0.878.
Terms
Main effect
Coefficient
Sum of Squares
df
Mean square
F-value
p-value
Model
−0.00
4364.10
9
484.90
12.28
0.007
X1-Temperature
−21.00
−10.50
882.00
1
882.00
22.33
0.005
X2-Time
22.00
11.00
968.00
1
968.00
24.51
0.004
X3-Enzyme concentration
−2.00
−1.00
8.00
1
8.00
0.20
0.672
X1X1
−3.75
−1.87
75.60
1
12.98
0.33
0.591
X2X2
34.25
17.13
905.69
1
1082.83
27.41
0.003
X3X3
39.25
19.63
1422.06
1
1422.06
36.00
0.002
X1X2
−5.50
−2.75
30.25
1
30.25
0.77
0.422
X1X3
0.50
0.25
0.25
1
0.25
0.01
0.940
X2X3
−8.50
−4.25
72.25
1
72.25
1.83
0.234
Error
197.50
5
39.50
Total
14
3.3.1 Effect of temperature, time and enzyme concentration on clarity
Clarity is a significant index of commercially available juices (Sin et al., 2006). The presence of pectic substances causes the turbidity in fresh juices. Furthermore, pectin molecules exhibit high water holding capacity which causes the formation of cohesive network structure and makes the juice viscous. Pectinolytic enzymes degrade the pectin molecules present in juices and reduce the turbidity and viscosity (Abdullah et al., 2007). The regression analysis of BBD for clarity of orange juice by pectinase preparation of AA15 revealed significant effect of variables including temperature and time on the response of the process, as well as, the significant quadratic effects of time and enzyme concentration. The remaining interactions were not found statistically significant. The quadratic equation in coded form can be given as: where Y is response, X1, X2 and X3 are temperature (°C), time (min) and enzyme concentration (%v/v), respectively. With the highest coefficient value of 11, incubation time was found the most important factor affecting the clarification of orange juice whereas the rest were temperature (10) and enzyme concentration (1), respectively.
BBD, like many other response surface methodologies, offers an option to visualize interactive effects of variables through surface and contour curves. The variables usually interact in a complex manner, such as clarification time has an inverse relation with the enzyme concentration to attain a clear juice at constant temperature (Kilara, 1982). The plots obtained in this study showed a proportional effect of treatment time on the response (Fig. 3a) that could be attributed with the settlement of the fine particles in the juices with time thus results in increase in clarity. Whereas, temperature had a negative effect on the response (Fig. 3b) which is desirable as the processing of foodstuffs at low temperature is usually preferred to maintain their nutritional properties.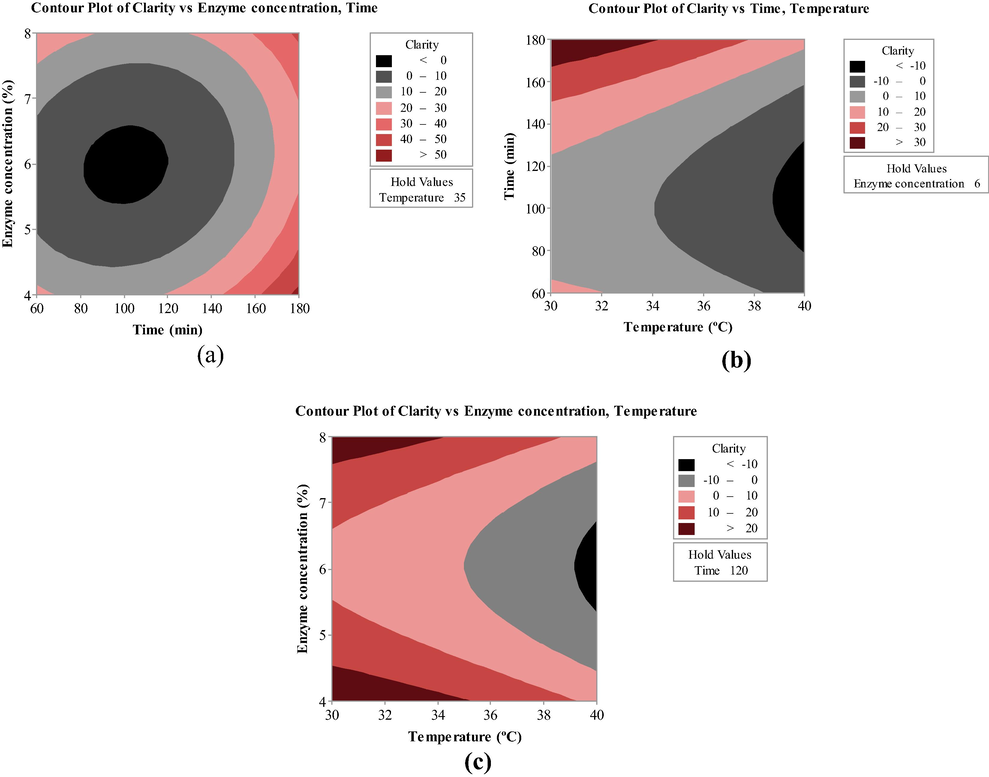
Contour plot showing the interactive effect of (a) enzyme concentration and time (b) time and temperature (c) temperature and enzyme concentration on the clarity of orange juice.
The enzyme concentration is another important factor concerning to clarification of fruit juices as it incurs huge cost. The results indicated an initial negative effect of the amount of pectinase preparation on the clarity of the juice for up to 6%, thereafter, more clarity of the juice was achieved by increasing amount of pectinase preparation (Fig. 3c). Sin et al. (2006) reported previously that the rate of clarification may enhance with the amount of enzyme due to repulsion between positively charged amino acids and other components that results in formation of large sized cloud particles which are eventually settled down.
3.3.2 Optimization
The ultimate goal of BBD was to determine optimum values for each and every variable to get the maximum response. In this study, the optimum clarity of 61% was attained when enzymatic treatment of juice was performed with 4% (v/v) crude pectinase for 180 min at 30 °C. Clarity (61%) obtained by our study was even higher than was reported earlier (50%) for blueberry juice by using 10 units of crude pectinase from A. niger LB23 for 60 min at 40–50 °C (Sandri et al., 2013). The researchers also compared the efficacy of both the crude and purified enzymes and crude extract was found more efficient. It has been explored by Naidu and Panda (2003) that the pectinase activity was more stable in crude preparation than purified form due to the interaction of pectinase with other proteins produced by the same organism. Moreover, the use of crude extract is beneficial to avoid the high cost of enzyme purification processes.
4 Conclusions
After the biochemical characterization of crude pectinase from G. candidum AA15, it appeared that this enzyme could be applied in industries specifically in food-related processes where enzymes active at room temperature are required. The different conditions of treatment revealed that these operating variables greatly influenced the clarity of orange juice. The optimum conditions of these variables were determined using contour or response surface plots to obtain the desired property of juice which was found suitable for subsequent membrane based clarification processes. With these promising results, this strain could be an alternative to commercial strain for pectinase production.
Acknowledgement
Authors are grateful to Third World Academy of Sciences (TWAS) for supporting this work vide grant number 10-134 to MS.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Response surface optimization of conditions for clarification of carambola fruit juice using a commercial enzyme. J. Food Eng.. 2007;81:65-71.
- [Google Scholar]
- Optimization of pectinase production from Geotrichum candidum AA15 using response surface methodology. Pak. J. Bot.. 2019;51(2):743-750.
- [Google Scholar]
- Utilization of wheat bran for enhanced production of exo-polygalacturonase by Penicillium notatum using response surface methodology. Pak. J. Agric. Sci.. 2013;50:469-477.
- [Google Scholar]
- Endopolygalacturonase production from Kluyveromyces marxianus. I. Resolution, purification, and partial characterisation of the enzyme. Enzyme Microb. Technol.. 1990;12:891-897.
- [Google Scholar]
- Application of enzymes in fruit juice technology. In: Enzymes and Food Processing. Dordrecht: Springer; 1981. p. :129-147.
- [Google Scholar]
- Studies on kinetics and thermostability of a novel acid invertase from Fusarium solani. J. Agric. Food Chem.. 2006;54:4617-4623.
- [Google Scholar]
- Production and partial characterization of an endopolygalacturonase from Saccharomyces cerevisiae. Can. J. Microbiol.. 1994;40:974-977.
- [Google Scholar]
- Purification and characterization of a novel pectinase from Acrophialophora nainiana with emphasis on its physicochemical properties. J. Biotechnol.. 2006;123:33-42.
- [Google Scholar]
- Comparison between Adaptive filter Algorithms (LMS, NLMS and RLS) IJSETR. 2013;2:100-1103.
- [Google Scholar]
- Prickly palm cactus husk as a raw material for production of ligninolytic enzymes by Aspergillus niger. Food Sci. Biotechnol.. 2016;25:205-211.
- [Google Scholar]
- Statistical optimization of culture conditions and characterization for ligninolytic enzymes produced from Rhizopus sp. Using prickly palm cactus husk. Chem. Eng. Comm.. 2017;204:55-63.
- [Google Scholar]
- Statistical optimization of immobilization of yeast cells on corncob for pectinase production. Biocatal. Agric. Biotechnol.. 2018;14:450-456.
- [Google Scholar]
- Production, purification and partial characterization of an endopolygalacturonase from Cryptococcus albidus var. albidus. Antonie van Leeuwenhoek. 1985;51:139-150.
- [Google Scholar]
- Production and characterization of polygalacturonase from Geotrichum candidum. World J. Microbiol. Biotechnol.. 1992;8:480-482.
- [Google Scholar]
- Guava. Tropical fruit processing. USA: Academic Press Inc.; 1988.
- Clarification of orange juice by crude fungal pectinase from citrus peel. Nigerian Food J.. 2007;25:130-137.
- [Google Scholar]
- Production, characterization and application of a thermostable polygalacturonase of a thermophilic mould Sporotrichum thermophile Apinis. Bioresour. Technol.. 2004;94:239-243.
- [Google Scholar]
- Enzymes and their uses in the processed apple industry: a review. Process Biochem.. 1982;17:35-41.
- [Google Scholar]
- Influence of agro-waste amendment on soil microbial population in relation to plant growth response. J. Environ. Biol.. 2007;28:623-626.
- [Google Scholar]
- Production of alkaline pectinase by bacteria (Cocci sps.) isolated from decomposing fruit materials. J. Phytol.. 2012;4:1-5.
- [Google Scholar]
- Optimizing conditions for enzymatic clarification of banana juice using response surface methodology (RSM) J. Food Eng.. 2006;73:55-63.
- [Google Scholar]
- Effect of solid state fermentation of cocoa shell on the secondary metabolites, antioxidant activity, and fatty acids. Food Sci. Biotechnol.. 2018;27:107-113.
- [Google Scholar]
- Production of Pectinolytic enzymes by the yeast Wickerhanomyces anomalus isolated from citrus fruits peels. Biotechnol. Res. Int.. 2013;2013:1-7.
- [Google Scholar]
- Effect of environmental pH on enzyme activity and growth of Bacteroides gingivalis W50. Infect. Immun.. 1988;56:1096-1100.
- [Google Scholar]
- Studies on pH and thermal deactivation of pectinolytic enzymes from Aspergillus niger. Biochem. Eng. J.. 2003;16:57-67.
- [Google Scholar]
- A cold-active pectin lyase from the psychrophilic and basidiomycetous yeast Cystofilobasidium capitatum strain PPY-1. Biotechnol. Appl. Biochem.. 2005;42:193-196.
- [Google Scholar]
- Pectinolytic yeast isolates for cold-active polygalacturonase production. Innov. Food Sci. Emerg. Technol.. 2011;12:178-181.
- [Google Scholar]
- Pectinolytic enzymes-solid state fermentation, assay methods and applications in fruit juice industries: a review. 3 Biotech. 2018;8:199.
- [Google Scholar]
- Optimizing pectinase usage in pretreatment of mosambi juice for clarification by response surface methodology. J. Food Eng.. 2004;64:397-403.
- [Google Scholar]
- Clarification of apple juice by hollow fiber ultrafiltration: fluxes and retention of odor-active volatiles. J. Food Sci.. 1987;52:375-377.
- [Google Scholar]
- Use of pectinases produced by a new strain of Aspergillus niger for the enzymatic treatment of apple and blueberry juice. LWT-Food Sci. Technol.. 2013;51:469-475.
- [Google Scholar]
- Optimization of enzymatic clarification of sapodilla juice using response surface methodology. J. Food Eng.. 2006;73:313-319.
- [Google Scholar]
- Limiting permeate flux in the clarification of untreated starfruit juice by membrane ultrafiltration. Chem. Eng. J.. 1998;69:45-148.
- [Google Scholar]
- Crossflow microfiltration of passion fruit juice after partial enzymatic liquefaction. J. Food Eng.. 1999;42:215-224.
- [Google Scholar]
- Some pink yeasts associated with softening of olives. Appl. Microbiol.. 1969;18:771-775.
- [Google Scholar]
- Pectinolytic activity of Saccharomyces fragilis cultured in controlled environments. Biotechnol. Bioeng.. 1978;20:231-242.
- [Google Scholar]







