Translate this page into:
Characterization of cheese processed wastewater and treatment using calcium nanoparticles synthesised by Senna auriculata L flower extract
⁎Corresponding author. kalamravi@gmail.com (Balasubramani Ravindran)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objectives
The objective of this work was to develop a new pretreatment method to remediate processed cheese wastewater using calcium nanoparticles (CaNPs) synthesized from Senna auriculata L. flower extract.
Methods
Processed cheese effluent was collected from a typical dairy factory and characterized the physical and chemical features such as color, odor, pH, total solids, total dissolved solids (TDS), total suspended solids and chemical oxygen demand (COD). The effluent was subjected to treatment using (CaNPs) synthesized from S. auriculata L. flower extract as a reducing agent. The NPs were characterized by X-ray diffraction, Fourier transformation spectroscopy, and scanning electron microscopy with energy dispersive X-ray spectroscopy.
Results
The crystalline nature of the synthesized CaNPs was found to be amorphous with a grain sizes 56 nm. The characterization studies confirmed the formation of CaNPs. The optimal dosage of NPs was 0.08 g, which reduced TDS and COD in the effluent by 73% and 82% respectively, within 2 h. The outcome of the investigation confirmed the reducing and stabilizing properties of from CaNPs synthesized from S. auriculata L plant flower extract and its effluent treatment efficiency.
Conclusions
The results revealed that the treatment of processed cheese wastewater can be effective with the application of enhanced CaNPs. It can be also suggested as an eco-friendly material for wastewater remediation. Senna auriculata L plant flower extract is a good source for CaNPs synthesis, adopting eco-friendly methods. In future, biologically synthesized nanomaterials would be an extremely promising solution for food industry wastewater treatment.
Keywords
Cheese effluent
Senna auriculate L
Calcium nanoparticles
Total dissolved oxygen
Chemical oxygen demand
1 Introduction
Worldwide milk production (81% cow milk, 15% buffalo milk, and 4% goat, sheep and camel milk combined) increased by 1.3% in 2019 to approximately 852 Mt. Milk powder, condensed milk, cheese, yogurt, ice cream, butter and curd are widely manufactured milk -related food items prepared via chilling and pasteurization process. Various rapidly growing industries simultaneously have improved productivity but also release toxic contaminants into the environment there by posing health risks to all living organisms (Porwal et al., 2015; Al-Dhabi and Arasu, 2022; Al-Dhabi et al., 2020b).
The dairy industry is one among the main source of food processing wastewater. This industry produces a large amount of effluent with organic and inorganic substances such as soluble organics, suspended solids, organic nitrogen, protein, urea, nucleic acid, orthoactive phosphorus, polyactive phosphours, casein lactose, fats, inorganic salts detergents, sanitizers, germicides. (Al-Dhabi et al., 2021). Phosphorus compounds are mostly inorganic, phosphate (PO43–) and diphosphate (P2O74–), but in this dairy effluent present in organic form, and other chemicals (Gani et al., 2015). Dairy wastewater contains large amount chemical oxygen demand (COD), biological oxygen demand (BOD) and nutrients. The COD ranges from 80 mg/L to 95,000 mg/L and BOD ranges from 40 mg/L to 48,000 mg/L, which is a large load of suspended solids (Kushwaha et al., 2011; Vijayaraghavan et al., 2021). Dairy industry wastewater enters the aquatic and terrestrial ecosystem and deteriorates natural resources. Treatment and disposal of industrial contaminants by conventional method seems to be difficult for various industries owing to the high cost production of large volume of sludge, and ineffectiveness for certain pollutants, among the other factors. Cheese making occurs in three main stages: In the first stage, milk is moulded into solid curd and liquid whey by the coagulation of the milk protein, casein. The coagulation of casein is done through two complementary methods: acidification and proteolysis. Acidification occurs when lactic acid bacteria ferment the disaccharide lactose to produce lactic acid. Originally, it can be done by naturally occurring lactic acid bacteria in the milk but today, dairy industries usually standardize the process by the addition of domesticated bacterial cultures, including strains of Lactococcus lactis, Streptococcus thermophilus and Lactobacillus sp.
Chemical treatment removes mostly colloids as well as soluble contaminants from milk processing effluents. It includes reagent oxidation or pH correction. For the duration cheese wastewater reaction with FeSO4/H2O2, up to 80 % of fat removal (initial concentration. Extreme pH values of dairy wastewaters below 6.5 and above 10 can increase the corrosion of pipes and be highly detrimental to microbiological assemblages in biological processes (Kolev Slavov, 2017; Al-Dhabi et al., 2020a).
Cheese whey is a byproduct of the dairy industry; it is a fluid produced when converting milk into cheese, explicitly from the agglomeration of casein micelles. Nanotechnology involves the synthesis of nanoscale (1–100 nm) materials with diverse application in medical field and environmental remediation. Many researchers have synthesized different types of metal nanoparticles (NPs) using microorganisms and plants extracts (Rao et al., 2015; Arasu et al., 2019; Gurusamy et al. 2019). Calcium nanoparticles (CaNPs) like other nanoparticles can act as nanofertilizer to supply nutrients such as Ca and make available some bound minerals via pH, organic matter and cation exchange capacity modification. Calcium is a macronutrient essential to plants for physiology, photosynthesis, plant cell wall, membranes, hormonal system, enzyme activities, antioxidant activity, chemical reactions, for delivering coupling messages responsible for extracellular signals and intracellular physiological responses in addition to reduction of soil salinity.
Calcium based chemical coagulants such as calcium oxide, calcium hydroxide are widely used for industrial effluent treatment. In this analysis, CaNPs werethe synthesized via the precipitation method using Senna auriculata L flower extract as an eco-friendly reducing agent. Senna auriculata L is a type of shrub belonging to the Fabaceae family that is distributed in world wide. S. auriculata (L.) Roxb. is found in wooden grasslands, grows up to a height of 500 m. It breeds wild in dry regions with annual precipitation of 300 mm in 15–28 °C. It grows well in areas and needs full sun for its growth.
2 Materials and methods
2.1 Collection and characterization of processed cheese wastewater
Fresh processed cheese wastewater used in this study was collected from a typical dairy industry. Physical and chemical feature such as color, odor, pH, total solids (TS), total dissolved solids (TDS) total suspended solids (TSS) and chemical oxygen demand (COD) were analyzed as according to the standard protocols (APHA, 2012).
2.2 Collection and preparation of S. auriculata L. flower extract
Healthy and fresh flowers of the S. auriculata L. plant were collected, washed with distilled water and dried at 40℃. The dried flower sample was ground well and used for extract preparation. The aqueous extract was prepared by mixing 5 g of flower powder with 100 mL of deionized water, which was boiled at 60 °C for 10 min. The boiled extract was filtered through Whatman No.1 filter paper and cooled at 27 ℃. The obtained filtrate was used for the synthesis of CaNPs.
2.3 Synthesis and purification of calcium nanoparticles (CaNPs)
For the synthesis of calcium nanoparticles, calcium carbonate was used as a precursor material and plant extract as a reducing agent. The calcium nanoparticles were prepared by mixing flower extract with 100 mL of 5 mM calcium carbonate solution in the ratio 1:1 at room temperature under continuous stirring. The purification process was carried out with continuous washing with distilled water by centrifuging with the speed of 3000 rpm for 20 min at 4 °C. The pellet was resuspended with 10 mL of sterile distilled water and again centrifuged to eliminate any tainting plant material. Finally, the pellet was kept in hot air oven for 24 h at room temperature for drying. The dried pellet was stored and used for further analysis.
For CaNP synthesis, calcium carbonate was used as a precursor material and plant extract was used as a reducing agent. The CaNPs were prepared by mixing flower extract with 100 mL of 5 mM calcium carbonate solution a ratio of 1:1 at room temperature under continuous stirring. The purification process was conducted with continuous washing with distilled water and centrifuging a speed of 3000 rpm for 20 min at 4 °C. The pellets were re suspended with 10 mL of sterile distilled water and again centrifuged to eliminate plant material contamination. Finally, the pellets were stored in a hot air oven for 24 h at room temperature to dry. The dried pellets were stored and used for further analysis.
2.4 Characterization of green synthesized of CaNPs
The dried CaNP pellets were characterized by X-ray diffraction (Bruker D8 ADVANCE Mother Teresa Women’s University, Kodaikanal, Tamilnadu, India. Fourier transform infrared spectroscopy (FTIR) (Perkin Elmer Spectrum Frontier), and scanning electron microscopy with energy dispersive X-ray spectroscopy (SEM-EDX) techniques. The average crystallite size was determined by the Debye Scherrer equation (Eq. 1).
2.5 Effect of CaNPs on TDS and COD removal from cheese effluent
The processed cheese effluent treatment efficiency of green synthesized NPs was assessed by taking 100 mL of effluent in a series of five conical and adding varying dosages such as 0.02, 0.04, 0.06, 0.08, and 0.10 g of CaNP powder. The content was mixed thoroughly and kept under static conditions for 2 h. The treated effluent samples were centrifuged at 3000 rpm for 15 min. The reduction of TDS and COD was noted every 30 min during the treatment process.
3 Results
3.1 Physical and chemical characteristics processed cheese wastewater
The physical and chemical properties of processed cheese wastewater were analyzed and are summarized in Table 1. The effluent had a whitish hue with an unobjectionable odor. The effluent was acidic with pH of 5.6. TS, TDS, and TSS concentrations were 4030, 3820, and 210 mg/L, respectively. COD is a core parameter for detecting the pollution level of industrial effluents. The sample had a COD concentration of 7000 mg/L owing to the presence of milk constituents such as casein, lactose, fat, inorganic salts, and other chemical substances used for milk processing.
Properties
Result
Colour
Whitish
Odour
Unpleasant
pH
5.6
TS
4030 mg/L
TDS
3820 mg/L
TSS
210 mg/L
COD
7000 mg/L
3.2 Extraction of Cassia auriculata flower
Fresh Cassia auriculata L. flower was collected from a commercial garden. Then the flower was dried and ground to create a fine powder with a high speed electrical stainless steel blender. The C. auriculata flower powder was mixed with 500 mL of distilled water and refluxed at 80 °C for approximately 1 h. The extract was then cooled and filtered through Whatman No. 1 filter paper.
3.3 CaNP synthesis
The mixture of flower extracts of S. auriculata L. and calcium carbonate solution (1:1) was stirred constantly. After 30–45 min of stirring, the yellow colored solution changed in to a brownish color, which indicated the formation of CaNPs (Fig. 1). Synthesized CaNPs were characterized by XRD, FTIR, and SEM-EDX.
Calcium nanoparticles synthesized using S. auriculata L flower extract.
3.4 Characterization of CaNP
3.4.1 XRD analysis
XRD analysis is the preliminary tool used for the characterization of CaNPs. The XRD pattern of green synthesized CaNPs is given in Fig. 2. The pattern showed intense peaks at the 2θ angle regions of 29.54°, 36.10°, 39.44°, 43.28°, 47.64°, and 48.60° corresponding to crystal plane miller indices (0 1 2), (1 0 4), (1 1 0), (1 1 3), (2 0 2) and (0 1 0) of the calcite phase respectively, which matched the JCPDS File No (00-005-0586). The XRD pattern showed the formation of maximum purity CaNPs, and no contamination peaks were identified.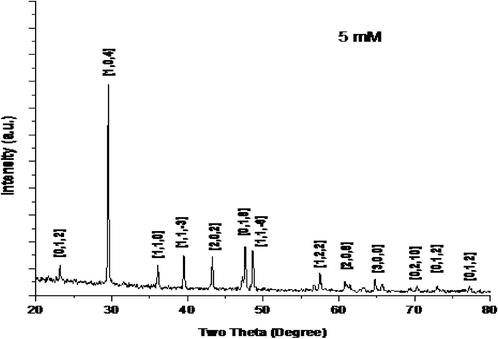
XRD patterns of CaNP.
3.4.2 FTIR analysis
The biogenic CaNPs were analyzed using FTIR. The FTIR spectrum of calcium CaNPs is shown in Fig. 3. The functional groups and capping molecules of the flower extract were determined by FTIR analysis. The FTIR spectrum was created by the absorption of radiation and was observed at a frequency of 400–4000 cm−1 (Joshi et al., 2018). The FTIR spectrum of green synthesized NPs showed broad peaks at the 712 cm−1, 876 cm−1, 1100 cm−1, 1420 cm−1, 1658 cm−1, 2936 cm−1, and 3398 cm-1regions corresponding to the functional groups plane bending with C–H bending, third overtone N–H stretching, second overtone C–H stretching, first overtone amide (N–H), primary amide C = stretching, asymmetric hydrogen bound groups (O–H stretching) with a carboxylic group and primary amide asymmetric stretching, respectively. The bands of out-of-plane bending (874 cm−1) and in-plane bending (710 cm−1) were credited to calcite.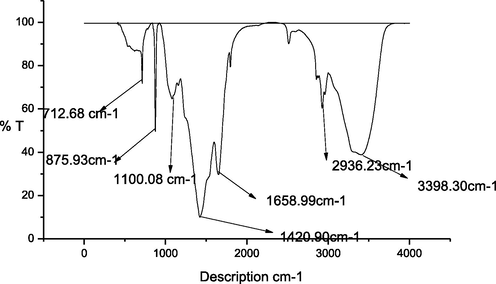
FTIR spectrum of CaNP.
3.4.3 SEM-EDX analysis
The morphological features and elemental analysis were studied using SEM-EDX analysis and the results are presented in Fig. 4(a and b). The SEM image clearly confirmed the synthesized NPs. The SEM analysis confirmed the rhombic distorted cubes and rhombohedra structure of CaNPs synthesized using S. auriculata L. flower extract. Calcium carbonate usually exists in both the calcite and aragonite form. Calcite and aragonite have various crystal natures and patterns. The EDX spectrum of CaNPs confirmed the presence of elemental CaNPs.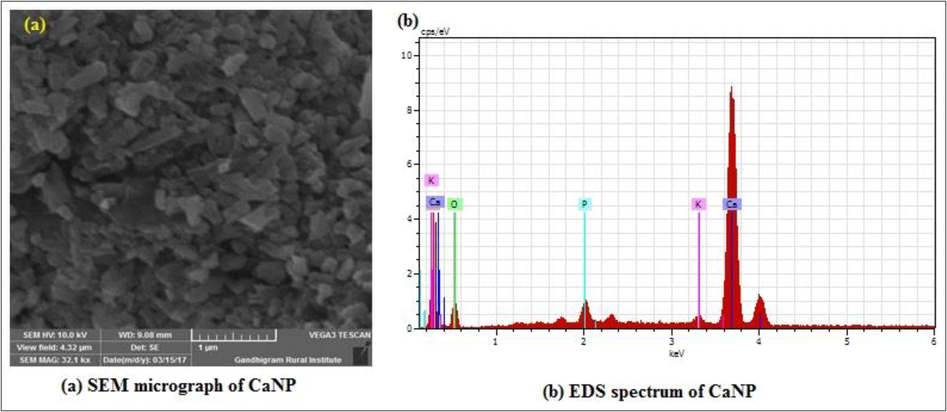
(a,b). SEM – EDX analysis of CaNP.
3.4.4 TDS and COD removal efficiency of CaNP
The processed cheese effluent collected from the dairy industry was subjected to treatment using different dosages of green synthesized CaNPs. Maximum reduction was observed with dosage of 0.08 g, which removed 73% of TDS and 82% of COD from the effluent after 2 h of treatment. The overall TDS removal efficiency improved over time from 22.7% to 20.2% after 2 h. The COD removal efficiency of CaNPs was higher than that of TDS. Figs. 5 and 6 indicate that a higher removal rate of COD and TDS occurred at the initial time of the process up to approximately 30 min. The elimination percent endured to enhance however with simplest variation and after one hundred twenty a discount occurred. The COD and TDS removal rates for the studied concentration were 0.02–0.01 g/mL, which followed the same behavior. Furthermore, the COD and TDS removal rates were inversely proportional to the increase in the initial value when the other experimental attribute were unchanged.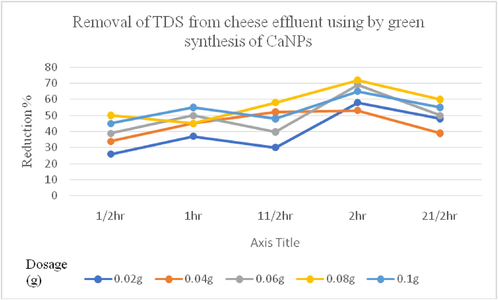
Graphical representation of TDS reduction from cheese effluent by CaNP.
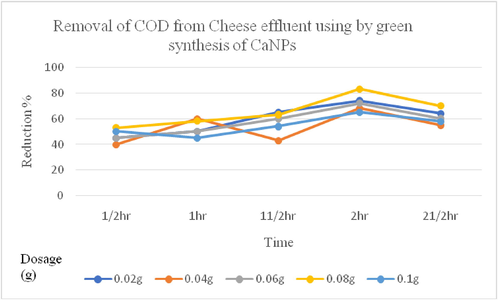
Graphical representation of COD reduction from cheese effluent by CaNP.
4 Discussion
The basic features of processed cheese wastewater were whitish and grayish color (Table 1). Unpleasant odor might have been due to the decomposition of organic and aromatic compounds released during milk processing and microbial activities. The effluent was acidic with a pH of 5.6. The acidic nature might have been attributed to the decomposition or conversion of lactose to lactic acid by microbial activities (Tikariha and Sahu, 2014). Azza and Mostafa (2013) reported the acidic pH of dairy wastewater contains a higher concentration of dissolved solids indicated the presence of pollutants such as inorganic salts, fats, and organic compounds in colloidal form. The discharge of effluent with an elevated level of suspended solids into the environment can affect the quality of water bodies and cause severe detrimental effects on aquatic flora and fauna (Shivsharan et al., 2013; Vinodhini and Soundhari, 2019). These compounds can contribute to the increase in COD and BOD in the effluent, thereby leading to oxygen depletion in polluted water bodies (Chavda and Rana, 2014). The processed cheese wastewater contained high concentrations of TDS and COD. Thus, the effluent was subjected to treatment using green synthesized CaNPs.
In this study, flower extract of S. auriculata L. was used to synthesis CaNPs (Fig. 1). Yao et al. (2013). The rich source of phenolic acid and flavonoids in a fruit wastes extract might be responsible for reduction of metal ions and efficient stabilization of synthesized nanoparticles. S. auriculata flower has alkaloids, glycosides, saponins, polyphenols, tannins, phloro-tannins, terpenoids, triterpenes, carbohydrates, proteins, amino acids, anthraquinone, aloe-emodin, and sitosterols. S. auriculate, has the adsorption properties and are low in cost. Once the coagulant is introduced in to the water, the individual colloids must cumulative and grow bigger so that the impurities can be settled down at the bottom of the beaker and separated from the water suspension. The coagulant maybe synthetic material or natural coagulant with the properties of coagulant having +ve charge, these positive charge proteins would bind to the -ve charged particles in the solution that cause turbidity. Water and wastewater treatment is the removal of suspended and colloidal particles, untreated matter, microbes and other substances that are deleterious to life, in search of lowest cost deployment, operation, maintenance, and reduced environmental impacts to the contiguous (Roopan et al., 2019). Synthesized nanosized calcium carbonate particles using various fruit extracts. This species of plant has commonly been used for the preparation of silver NPs (Muthu and Priya, 2017; Thirumurugan et al., 2019). The CaNPs were studied using XRD, FTIR, and SEM-EDX, XRD is an important analytical tool for identifying the structure of crystalline subject.
The basic principal of XRD technique is that, the X-rays make a diffraction on the subjects (powder or crystal particles) and make a diffraction pattern, then calculate the diffracted intensity is stand for with spotted two theta (2q) angles. The XRD-pattern of Ca NPs is represented in Fig. 2 and all the identified peaks are create in good conformity with standard JCPDS data. The XRD pattern of green synthesized calcium nanoparticle is given in Fig. 2. The pattern showed intense peaks at 2θ angle regions 29.54°, 36.10°, 39.44°, 43.28°, 47.64° and 48.60° corresponding to crystal plane miller indices (0 1 2), (1 0 4), (1 1 0), (1 1 3), (2 0 2) and (0 1 0) planes of calcite phase respectively, which matched with JCPDS File No: (00-005-0586). Calcite is a crystallized type of calcium carbonate like aragonite and vaterite (Guowei et al., 2009). The characteristic diffraction peak 36.10° appeared at 2θ attributed (1 0 4) plane strongly indicates the presence of calcite compound (Yao et al., 2013). According to Debye Scherrer’s equation, the average crystallite size was calculated as 56 nm.
Infra-red spectroscopy is a major analytical technique to offer a fundamental idea on vibration of molecules and different rotational ways of molecules in the respective compounds. FT-IR basically shows maximum resolution spectrum. This technique works on the combination of many IR rays and gives the data about various functional groups in the synthesized nanoparticle. These peaks indicate the presence of –N–H, -O–H (Carboxylic), –CN, C=O, C=N, C=C, C-O, C–C, Ca-O (linkages) etc in the calcium nanoparticle. The functional groups and capping molecules of floral extract can be determined by FTIR analysis. In one study similar to our work show that, FTIR spectrum was develop IR radiation in the frequency ranges from 400 to 4000 cm−1 (Joshi et al., 2018), it almost closer to our values. The FTIR observations of the calcium NPs showed the existence of C-O and hydroxyl functionalities on the NP surface. The obtained results are coincided with outcome reported by Yugandhar and Savithramma (2013). Different types of phytochemical constituents such as protein, carbohydrate, alkaloids, phenol, flavonoids and tannins are functioning as a reducing, stabilizing as well as capping agents for the development of calcium nanoparticles (Devi et al., 2006; Raj et al., 2012). The band at 1620 cm-1 is for the fruit extract. These peaks are dependable for the inhibit the over-growth of nanoparticles of the CaNPs (Peternela et al. 2018). These findings revealed that the synthesized calcium NPs are stabilized by biomolecular compounds present in the extract of S. auriculata. Flowers of S. auriculata shows a significant amount of alkaloids, glycosides, saponins, phenols, tannins, phloro-tannins, phenols terpenoids, triterpenes carbohydrates, proteins, and amino acid and also revealed the presence of anthraquinone, aloe-emodin, and sitosterols.
The observed images indicate a rhombic distorted cubes and rhombohedra structure of calcium nanoparticles synthesized by using the S. auriculata L floral extract. The morphological features and elemental analysis were studied by SEM-EDX analysis and the results are presented in Fig. 4(a,b). The SEM analysis confirmed the rhombic distorted cubes and rhombohedra structure of calcium nanoparticles synthesized using S. auriculata L floral extract. Moreover, the plate like structure of nanoparticles might be due the carbonation rate as well as the dissolving rate of CO2 at different conditions and also lead to changes in morphological characteristics of nanoparticles (Wen et al., 2003; Hariharan et al., 2014). EDX spectrum of CaNP confirmed the presence of elemental calcium nanoparticles. These results are similar to those of previous studies in which green synthesized Ca NPs (Hussain et al., 2016; Fahmy et al., 2021).
The cheese processed effluent collected from dairy industry was subjected to treatment using different dosage of powdered green synthesized calcium nanoparticles. Maximum reduction was observed in the dosage 0.08 g and it removed 73% of TDS as well as 82% of COD from the effluent after two hours of treatment process. The removal mechanism of TDS and COD from cheese effluent by nanoparticles might be attributed by adsorption mechanism or surface precipitation of contaminants (Wang et al., 2006). Nassar et al. (2014). Different types of phytochemical constituents such as protein, carbohydrate, alkaloids, phenol, flavonoids and tannins are functioning as a reducing, stabilizing as well as capping agents for the development of calcium nanoparticles, reported that the dosage of nanoparticles is higher, the removal of pollutants from the effluent also increased due to the larger surface area of the nanoparticles. For applying Pseudomonas Sp. in the reduction process of TDS, one of the study reported 68.8% of TDS in rubber processing effluent was reduced (Shruthi et al., 2012). Gaikwad et al. (2014) had also made a study on by using microbial consortia of various bacterial species for the reduction of TDS and they find out a maximum of 74.36% reduction in TDS. Increase the concentration of TDS reduces the efficacy of water and it makes lots of environmental issues. Similarly, the reduction in COD of the dairy wastewater (99.9%) studied by Cosa, 2014 and they used consortium of two marine species. Bacterial species Neisseria and Citrobacter were used as a reduction agent of COD in diary wastewater and they got a better results such as 69.1% and 45.3% reduction (Chatterjee and Pugaht, 2013). Dairy is one of the leading industries, producing excess COD containing wastewater (Harush et al., 2011). Bacteria in the cheese processing wastewater was isolated and studied to reuse this bacterium for the reduction of COD rate in wastewater from the dairy industry (Maghsoodi, 2007).
5 Conclusion
In this study, the calcium nanoparticle was synthesized using Senna auriculata L flower extract and characterized by XRD, FTIR and SEM-EDX analytical methods and confirmed the formation of calcium nanoparticles. The outcome of the investigation confirmed, the reducing and stabilizing property of S. auriculata L plant flower extract for nanoparticle synthesis and its effluent treatment efficiency. The results of this study have clearly indicated that CaNP is a useful tool for the treatment of dairy wastewater. Thus, it is also concluded that the green synthesized Ca nanoparticles can be used as an alternative source of expensive adsorbents for the treatment of waste water especially cheese processed effluent.
Acknowledgment
This work was carried out with the support of ‘Cooperative Research Program for Agriculture Science and Technology Development (Project No. PJ01529302)’ Rural Development Administration, Republic of Korea.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Effective degradation of Chlortetracycline using dual bio catalyst. Environ. Res.. 2022;204:112115
- [Google Scholar]
- Removal of nitrogen from wastewater of date processing industries using a Saudi Arabian mesophilic bacterium, Stenotrophomonas maltophilia Al-Dhabi-17 in sequencing batch reactor. Chemosphere. 2021;268:128636.
- [Google Scholar]
- Sustainable conversion of palm juice wastewater into extracellular polysaccharides for absorption of heavy metals from Saudi Arabian wastewater. J. Cleaner Prod.. 2020;277:124252.
- [Google Scholar]
- Effective degradation of tetracycline by manganese peroxidase producing Bacillus velezensis strain Al-Dhabi 140 from Saudi Arabia using fibrous-bed reactor. Chemosphere. 2021;268:128726.
- [Google Scholar]
- One step green synthesis of larvicidal, and azo dye degrading antibacterial nanoparticles by response surface methodology. J. Photochem. Photobiol., B. 2019;190:154-162.
- [Google Scholar]
- APHA. American Public Health Association (2012) Standard methods for the examinations of water and wastewater. American Public Health Association. 22nd edition. Washington DC. New York.
- Treatment of cheese processing wastewater by physicochemical and biological methods. Int. J. Microbiol. Res.. 2013;4(3):321-332.
- [Google Scholar]
- Performance evaluation of effluent treatment plant of dairy industry. Pratiksinh Chavda Int. J. Eng.. 2014;4(9):37-40.
- [Google Scholar]
- Bioflocculant production by a consortium of two bacterial species and its potential application in industrial wastewater and river water treatment. Pol. J. Environ. Stud.. 2014;23:689-696.
- [Google Scholar]
- Treatment of dairy wastewater using aerobic biodegradation and coagulation. Int. J. Environ. Sci. Res.. 2011;1:23-26.
- [Google Scholar]
- Antidiabetic and hypolipidemic effect of Cassia auriculata in alloxan induced diabetic rats. Int. J. Pharamacol.. 2006;2:601-607.
- [Google Scholar]
- Green synthesis of platinum and palladium nanoparticles using Peganum harmala L. seed alkaloids: biological and computational studies. Nanomaterials. 2021;11:965.
- [Google Scholar]
- Chin Ming Er. Phycoremediation of dairy wastewater by using green microlgae: Botryococcus Sp. Appl. Mech. Mater.. 2015;773–774:1318-1323.
- [Google Scholar]
- Development of microbial consortia for the effective treatment of complex wastewater. J. Bioremed. Biodeg. 2014;5:4.
- [Google Scholar]
- The crystallization behaviorof calcium carbonate in ethano water solution containing mixed nonionic anionic surfactants. Powder Technol.. 2009;1920(1):58-64.
- [Google Scholar]
- Environmental friendly synthesis of TiO2-ZnO nanocomposite catalyst and silver nanomaterials for the enhanced production of biodiesel from Ulva lactuca seaweed and potential antimicrobial properties against the microbial pathogens. J. Photochem. Photobiol., B. 2019;193:118-130.
- [Google Scholar]
- Synthesis and characterization of CaCO3 (calcite) nano particles from cockle shells using Chitosan as precursor. Int. J. Sci. Res. Publ.. 2014;4:1-5.
- [Google Scholar]
- Green synthesis of nanoparticles and its potential application. Biotechnol. Lett.. 2016;38(4):545-560.
- [Google Scholar]
- Utilization of waste leaves biomass of Myrica esculenta for the removal of Pb (II), Cd (II) and Zn (II) ions from waste waters. Oriental J. Chem.. 2018;34(5):2548-2553.
- [Google Scholar]
- Green synthesis, characterization and catalytic activity of silver nanoparticles using Cassia auriculata flower extract separated fraction. J. Saa. 2017;S1386–1425(17):30120-30128.
- [Google Scholar]
- Dairy Wastewaters: General characteristics and treatment possibilities of dairy wastewater–A review. Food Technol. Biotechnol.. 2017;55(1):14-28.
- [CrossRef] [Google Scholar]
- An overview of various technologies for the treatment of dairy wastewaters. Crit. Rev. Food Sci. Nutr.. 2011;51(5):442-452.
- [Google Scholar]
- Treatment of olive mill based wastewater by means of magnetic nanoparticles: decolourization, dephenolization and COD removal. Environ. Nanotechnol. Monitor. Manage.. 2014;1-2:14-23.
- [Google Scholar]
- Synthesis and impregnation of copper oxide nanoparticles. On activated carbon through green synthesis for water pollutant removal. Mater. Res.. 2018;21:e20160460
- [Google Scholar]
- Biodegradation of dairy effluent by using microbial isolates obtained from activated sludge. Water Resour. Ind.. 2015;9:1-15.
- [Google Scholar]
- Chemical compounds investigation of Cassia auriculata seeds: a potential folklore medicinal plant. Asian J. Plant Sci. Res.. 2012;2:187-192.
- [Google Scholar]
- Eco-friendly synthesis of MgO nanoparticles from orange fruit waste. Int. J. Adv. Res. Phys. Sci.. 2015;2(3):1-6.
- [Google Scholar]
- Assessment of physico-chemical parameters of dairy wastewater and isolation and characterization of bacterial strains in terms of COD reduction. Int. J. Sci. Environ. Technol.. 2013;2:395-400.
- [Google Scholar]
- Physicochemical characterization of dairy effluents. Int. J. Life Sci. Biotechnol. Pharma Res.. 2013;2(2):182-191.
- [Google Scholar]
- Bioremediation of rubber processing industry effluent by Pseudomonas sp. Int. J. Res. Environ. Sci. Tech.. 2012;2:27-30.
- [Google Scholar]
- Study of characteristics and treatments of dairy industry waste water. J. Appl. Environ. Microbiol.. 2014;2(1):16-22.
- [Google Scholar]
- Samadi, Akbar, Ghobadi, Zahra Iran, Biodegradation of effluents from dairy plant by bacterial isolates. Iran. J. Chem. Chem. Eng.. 2007;26:55-59.
- [Google Scholar]
- Effective removal of pharmaceutical impurities and nutrients using biocatalyst from the municipal wastewater with moving bed packed reactor. Environ. Res.. 2021;200:111777.
- [Google Scholar]
- CuO/C nanocomposite: synthesis and optimization using sucrose as carbon source and its antifungal activity. Mater. Sci. Eng.. 2019;101:404-414.
- [Google Scholar]
- Phytomediated synthesis of silver nanoparticles using L: Evaluation Cassia auriculata of antibacterial and antifungal activity. Asian J. Pharm. Pharmacol.. 2019;5(2):326-331.
- [Google Scholar]
- Phytoremediation of dairy effluent by using microalgal consortium. Pharma Innovat. J.. 2019;8(9):128;133.
- [Google Scholar]
- Synthesis of nanosized calcium carbonate (aragonite) via polyacrylamide inducing process. Powder Technol.. 2006;163(3):134-138.
- [Google Scholar]
- Synthesis of plate-like calcium carbonate via carbonation route. Mater. Letter. 2003;57:2565-2571.
- [Google Scholar]
- Green Synthesis of Calcium Carbonate with unusual morphologies in the presence of fruit extract. J. Chil. Chem. Soc.. 2013;58:4.
- [Google Scholar]
- Green synthesis of calcium carbonate nanoparticles and their effects on seed germination and seedling growth of Vigna mungo (L.) Hepper. Int. J. Adv. Res... 2013;1(8):89-103.
- [Google Scholar]







