Translate this page into:
Characterization, enzymatic and biochemical properties of endophytic bacterial strains of the medicinal plant Ajuga turkestanica (Rgl.) Brig (Lamiaceae)
⁎Corresponding authors. dilfuzajabborova@yahoo.com (Dilfuza Jabborova), Rahulmedcure@gmail.com (Rahul Datta)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
This research work aims to isolate endophytic bacteria from the Ajuga turkestanica plant that have plant growth promoting properties. The microscopic, enzymatic and biochemical properties of the isolated endophytic bacteria are described. Twelve isolates of endophytic bacteria were isolated by standard microbiological methods. Isolated endophytic bacteria were identified by (MALDI-TOF MS). Endophytic bacteria isolated from the plant were detected in MALDI-TOF MS. Bacterial isolates were dominated by endophytic bacteria belonging to the genus Bacillus, Pseudomonas, Siccibacter. When the growth-enhancing properties of cultivated plants used in sustainable agriculture were studied, the isolated bacterial strains showed positive results in siderophor, ammonia, indole acetic acid production, and nitrogen fixation properties. Numerous endophytic bacteria have been found to have the enzymatic activity of oxidase, catalase, amylase, lecithinase and lipase. The results showed that endophytic bacteria isolated from the plant A. turkestanica are have plant stimulating properties which can be shown as a candidate for the production of bioinoculants individually or as part of a consortium.
Keywords
A. turkestanica (Regel) Briq (Lamiaceae)
Endophytic bacteria
Siderophore production
Nitrogen fixation
Phosphate solubilization
IAA production activity
1 Introduction
In pharmaceuticals plant-derived substances are used in the treatment of cardiovascular diseases (myocardial infarction) (Egamberdieva and Jabborova, 2018; Egamberdieva and Jabborova, 2020). The plant extract has a wide range of antimicrobial and antifungal properties. The bioactive substances of the medicinal plant Ajuga turkestanica have antioxidant, anti-leukemic, cardiotonic and cytotoxic properties and are used in many clinics in Russia and Uzbekistan (Mamatkhanov et al., 1998; Zadeh and Kor, 2014).
A. turkestanica (Regel) Brig. (Lamiaceae) is rich in biologically active substances the leaves and stems contain up to 3.76% flavonoids, iridoids, phytoectisteroids and up to 0.1% of the plant leaves contain a compound called turkesterone (Baltaev 2000; Ramazanov 2005). The medicinal plant A. turkestanica contains biologically active substances with high pharmacological effects, such as ecdysterone, turkesterone, cyasterone, ajugasterone, ajugalactone, cyasterone-22 acetate and phytoecdysteroid used in the treatment of cancer, malaria, gastrointestinal, in cardiovascular problems (Mamatkhanov et al., 1998; Syrov, 2001; Abdukadirov et al., 2005; Shakhmurova et al., 2010).
In recent years, scientific hypotheses have been put forward that endophytic bacteria, which make up the plant microbiota, create optimal conditions for the normal growth and development of the host plant under adverse conditions (Singh and Padmavathy, 2014; Szilagyi-Zecchin et al., 2014, Selim et al., 2011; Kumar et al; 2016, Bezerra et al., 2012). Bio stimulants and foliar application is another way to get optimal growth of host plant (Abbas et al., 2020; Ullah et al., 2020; khan et al., 2020) Endophytic bacteria dissolve insoluble phosphate compounds in the soil by producing organic acids thereby stimulating plant growth (Sharma et al., 2013; Jabborova et al., 2015; Jabborova et al., 2020a). Soil properties changes with the land use (Marfo et al., 2019a; Marfo et al., 2019b). As the medicinal plant A. turkestanica contains pharmacologically important biologically active substances, it is important to analyze its endophytic microbiome. This study was performed to investigate characterization, enzymatic and biochemical properties of endophytic bacterial strains of the medicinal plant A. turkestanica.
2 Materials and methods
2.1 Sample collection
Ajuga turkestanica (Rgl.) Brig (Lamiaceae) grows on stony slopes of gypsum and red sandstone, soil and rock mixed areas at an altitude of 600–1000 m on the south-western slopes of the Pamir-Alay mountains of Boysun district of Surkhandarya region (Fig. 1).
Map of the area where the medicinal plant A. turkestanica is collected. Boysun district of Surkhandarya region Uzbekistan.
2.2 Surface sterilization of plant samples
Surface sterilization was performed using Miral and Verma methods to isolate endophytic microorganisms from the medicinal plant A. turkestanica (Verma et al., 2014). Plant samples prepared for surface sterilization were cut into small pieces using sterile tweezers. Plant parts prepared for surface sterilization were washed in running water for fifteen minutes. The washed plant parts were then washed with copious amounts of distilled water. In the next step, plant samples were transferred to a laminar box (Thermo scientific Maxisafe 2020 Germany) provided with aseptic conditions. Plant parts (roots, stems, leaves) were cut into 3–5 cm pieces to facilitate surface sterilization and sterilized for one minute in 70% absolute ethyl alcohol. At the stage of surface sterilization of plant samples are stored in a solution of hypochorite sodium (NaClO) five percent. Also, plant parts divided into small volumes are thoroughly washed in sterile deionized water. To determine how successful the sterilization process was, a pre-prepared Chapek doks agar medium was dripped from the last washed water using a sterile glass rod. If no microorganisms grow from the water droplet medium it confirms that the surface sterilization process was positive.
2.3 Isolation and identification of endophytic bacterial strains
In order to isolate endophytic bacteria from plant specimens the pieces which were initially cut into small pieces, were transferred to Petri dishes containing sterile tweezers Tryptic Soy Agar medium (NaCl 5.0 g;Dextrose 2.5 g; K2HPO4 2.5 g; Agar 15.0 g). Plant specimens were incubated at 28° C for ten to fifteen days after transfer to medium. After incubation, morphologically diverse bacterial colonies were selected and their morphological microscopic and biochemical properties were examined. The isolated bacterial isolates were grown overnight in the Tryptic Soy Broth medium. Cultured endophytic bacterial cultures are centrifuged at a rate of 15,000 revolutions per minute. The supernatant portion of the culture fluid was drained and a precipitate was removed for processing in subsequent stages. The separated precipitate was first washed using 250 µl of sterile deionized water and then 800 µl of ethyl alcohol. The resulting clutoral fluid was again centrifuged at a rate of 15,000 beats per minute to remove the supernatant portion. The precipitate obtained was then dried in a laminar cabinet under aseptic conditions for 5–7 min. The precipitate was then mixed by adding 25 μL of formic acid and acetonitrile. The resulting mixture was then centrifuged again and a total of 1 μL of supernatant was placed on a 96-point tablet and dried. One microliter of MALDI MS solution was then poured into the samples and allowed to dry under aseptic conditions. MALDI TOF MS mass spectra were analyzed using bacterial identification system MALDI Biotyper®. Each sample was analyzed three times to confirm the reliability of the results. The control was carried out on the relevant spectra of existing strains in the collection of sanitary and epidemiological control of the Main Department of Medicine under the Presidential Administration of the Republic of Uzbekistan. According to the results obtained, the bacterial identification scores are as follows: ≥2000 species-level identification means 1700 1999 species-level identification. If the score is <1700, it means that the isolate is not identified.
2.4 Morphological characterization of endophytes
After incubation, the endophytic bacteria isolated from plant parts were purified according to their phenotypic properties. The morphology of endophytic bacterial isolates (color of bacterial colonies, gram staining, shape of colony margins, growth dynamics and nature of colonies) was studied according to Berge’s (Bergey and Holt, 2000, Sayed et al., 2019). The gram method was used for morphological analysis of endophytic bacteria. Reagents used in staining of bacterial isolates by Gram reaction: (crystal violet (basic dye), iodine solution (purple color stabilizer in cell wall, color decolorization of samples (ethanol 70%) and distilled water).
2.5 Biochemical properties of endophytic bacterial isolates
Endophytic bacteria were grown in Simmons citrate agar medium and incubated for 24 h at 28 ± 2 °C. If the bacterial isolates have the property of assimilating citrate the medium color will change from green to blue. Such a change by endophytic bacteria is a positive reaction.
The carbohydrate fermentation property of the isolated bacterial isolates was determined by cultivation in Triple Sugar Iron agar medium (3.0 g beef extract; 20.0 g peptone; 3 g yeast extract; 10.0 g sugar; 10.0 g lactose; 1.0 g dextrose; 0.2 g ferrous sulfate; 5.0 g sodium chloride, 0.3 g sodium thiosulfate; 0.024 g phenol red and 12.0 g agar). Bacterial strains were incubated at 30 ± 2 °C for one day in TSI (Triple Sugar Iron agar) medium. The medium in which endophytic bacteria are grown exhibits an alkaline / acidic state. As a result, if the slope of the medium inside the test tube is red and the bottom is yellow, it means that the bacteria have only carried out dextrose fermentation. When endophytic bacteria grow in a medium acidic state, the slope and bottom of the medium turn yellow. This indicates that the bacteria carried out the fermentation of dextrose, lactose and sucrose.
When endophytic bacteria form a medium alkaline medium in which they grow, the slope and bottom of the medium inside the test tube remain unchanged. This result indicates that carbohydrate fermentation has not taken place. The blackening of the medium inside the solution indicates that hydrogen sulfide was produced by the bacterial isolates. The division of the medium and its rise in the solution indicates that the endophytic bacteria have formed a gas (Patel et al., 2016, Singh and Dubey, 2020).
Bacterial strains were incubated in Glucose Phosphate Broth (Buffered peptone 7.0 g; dextrose glucose 5.0 g; dipotassium phosphate 5.0 g; final pH 6.9 ± 0.2) for two days at 28 °C. At the end of the incubation period, 1 mL of bacterial fluid was transferred to a fresh sterile solution. Then 1–2 drops of methyl red solution were added to the solution. The rapid formation of a red color in the bacterial fluid when a methyl red solution was added was taken as a positive reaction.
2.6 Screening of plant growth promoting
Endophytic bacteria have been isolated from various parts of the A. turkestanica plant which is listed as an endemic species of flora of Uzbekistan. These endophytic bacteria have been found to have plant-enhancing properties used in sustainable agriculture.
The ability of endophytic bacterial strains to break down phosphate salts was studied in the Pikovskaya agar medium (C6H12O6 10.0 g; Ca3(PO4)2 5.0 g; yeast extract 0.5 g; (NH₄)2SO₄ 0.5 g; KCl 0.2 g; NaCl 0.2 g; MgSO4 0.1 g; MnSO4·H2O, 0.002 g; FeSO4·7H2O, 0.002 g agar 15 g; distilled water 1 L). Bacterial strains were stored for ten days at a temperature of 28 ± 2 °C after planting in agar medium. Bacterial colonies were monitored during incubation. Bacteria break down phosphate compounds by producing organic acids into the medium. The yellow color formed around the bacterial colony indicates that the phosphate compounds are broken down (Ogale et al., 2018; Jain et al., 2017).
Endophytic bacteria were grown in King’s B medium (Proteose peptone 20.0 g; K2HPO4 1.5 g; MgSO4‧7H2O 1.5 g; distilled water 1 L) in an incubator at a temperature of 28 °C in a shaker with a rotational speed of 170 times per minute for 72 h The bacterial fluid was then taken in 1.5 mL small-volume solutions and centrifuged at 6000 rpm for ten minutes. Take 1 mL of the liquid from the sepernatant portion and add 2% FeCl3 from the aqueous solution. The appearance of orange and reddish-brown color in the solutions indicates that endophytic bacteria have synthesized siderophores.
Ammonia-synthesizing properties of bacterial strains were screened in a peptone medium. Bacterial cells were inoculated with peptone water without control. To assess the ammonia production of bacterial strains, 1 mL of Nessler reagent was added to a liquid medium with peptone. A change in color to light yellow indicates that minimal amounts of ammonia have been produced. A change from orange to brown indicates that the maximum amount of ammonia has been produced (ALKahtani et al., 2020; George et al., 2020).
The potential for IAA synthesis of bacterial strains was determined in the tryptophan Nutrient Broth medium. Bacterial strains were grown in a shaker incubator for 7 days at a temperature of 28 ± 2 °C after inoculation in the medium. At the end of the storage period, the bacterial fluid in which the strains were grown was centrifuged for ten to fifteen minutes at a speed of 6000 rpm. In the next step, the supernatant was isolated and 1 drop of Salkowski reagent (35 mL of 35% HCIO4 and 1 mL of 0.5 M FeCl3·6H2O) was added to it. After the reagent was added to the supernatant, it was kept in a dark place for thirty minutes. The gradual development of cherry red in the supernatant indicated that bacterial strains synthesized indole acetic acid (Ogale et al., 2018).
Nitrogen fixation properties of endophytic bacteria were determined in Jensen’s medium (Sucrose 20 g;K2HPO4 1.0 g; MgSO4‧7H2O 0.5 g; NaCI 0.5 g; FeSO4‧7H2O 0.1 g; Na2MoO4 0.005 g; CaCO3 2.0 g; Agar 15.0 g). Bacteria cultured in Jensen’s medium were incubated for 7 days at 28 ± 2 °C. The appearance of endophytic bacterial colonies in Petri dishes during incubation indicates that bacterial cultures have the property of nitrogen fixation.
Endophyte bacterial strains were cultured in Nutrient Broth medium to test the activity of ACC deaminase. The cultured bacterial cultures were centrifuged at 8000 rpm for 8 min. Bacterial sediment was isolated and washed with sterilized 0.1 M Tris-HCl (pH 7.5). Bacterial cells were transplanted into a medium of minimal saline agar. A medium with the addition of 0.2 percent ammonium sulfate was used for positive control. Non-nitrogen source DF medium was used as a negative control. The cultures were incubated for 36 h at 28° ± 2. The occurrence of endophytic bacterial colonies in DF minimal saline medium was evaluated as a positive control (Sánchez-Cruz et al., 2019; Ngoma et al., 2013; Mintoo et al., 2019).
To determine the amylolytic activity of endophytic bacteria, starch agar was incubated for 48 h at 28 ± 2 °C. At the end of the incubation period of bacteria, a solution of potassium iodide was poured into Petri dishes and soon drained. In the presence of amylase activity in endophytic bacterial strains, a colorless transparent zone is formed around the bacterial colonies (Ntabo et al., 2018; Malleswari and Bagyanarayana, 2013).
The protease activity of endophytic bacteria was determined in sterile Skim Milk Agar medium (Casein 5.0 g; glucose 1.0 g; yeast extract 2.5 g; Agar 15.0 g; distilled water 1 L, added 7% skim milk). Bacterial strains were stored for two days at a temperature of 28 ± 2 °C after inoculation on the medium. Degradation of proteolytic activity around bacterial colonies after incubation was detected. A transparent zone around the bacterial colonies indicated that the substrate was broken down by proteases (Ntabo et al., 2018).
Lipase and lecithinase activity of bacterial strains was determined by growing in Egg Yolk Agar. Bacterial strains were inoculated and stored at a temperature of 30 ± 2 °C for two to three days.The formation of light zones around bacterial colonies after incubation indicates that lecithinase is synthesized by bacteria. The lipase activity of endophytic bacteria was determined as follows. A saturated solution of copper (II) sulfate was poured into the petri dishes and kept for 10–15 min and the excess reagent was discarded (Malleswari and Bagyanarayana, 2013). A greenish-blue color is formed around bacterial colonies that have lipase activity. This condition is considered a positive result.
A drop of endophytic bacterial culture grown in a nutrient broth medium was instilled into a glass plate and 3% hydrogen peroxide (H2O2) was added. Cultures with catalase activity form bubbles.
Whatman №1 filter paper was cut into 10 mm circles and immersed in a freshly prepared 1% solution of tetramethyl paraphenyldiamine dihydroxychloride (C10H18Cl2N2) and dried. A small amount of endophytic bacterial colony was then instilled into the filter paper with a sterile Petri loop. The formation of a strong dark purple color in five to ten seconds was accepted as a positive reaction of the oxidase test. Whatman №1 filter paper color change was taken as a negative reaction.
Urea is a product of the decarboxylation of amino acids. Hydrolysis of urea results in the formation of ammonia and carbon dioxide. The formation of ammonia in the process alkalizes the medium and a change in pH (6.8–8.1) turns the light yellow to pink. When bacteria have urease activity, the medium turns light yellow to pink within 24 h. (Patel et al., 2016). Bacteria were cultured in Christensen medium to determine the urease enzyme activity of endophytic bacteria. Urea decomposes at high temperatures that are not resistant to heat. Therefore, a 40% solution of urea under aseptic conditions was prepared. 2 g urea was filtered in 5 mL of distilled water in a 0.22 nm millipore paper filter. After sterilization, the medium was cooled to 45–50° and 5 mL sterile 40% urea solution was added under aseptic conditions and poured into Petri dishes. Bacterial strains were transplanted into medium and stored in a thermostat at 28 ± 2 °C for 24 h. When bacterial strains have urease activity the urea in the medium is broken down. The ammonia formed was determined by changing the color of the medium from light yellow to pinkish red.
The property of gelatin hydrolysis of bacterial isolates was determined in Nutrient gelatin (Nutrient agar 23 g/L, gelatin 8 g/L) agar medium. Bacterial strains were grown for three to seven days at a temperature of 30 ± 2 °C in a gelatin agar medium. After incubation, bacterial isolates were poured into saturated Petri dishes from a saturated solution of ammonium sulfate (NH4)2SO4 and kept for 8–10 min, then discarded. Ammonium sulfate precipitates gelatin that has not been hydrolyzed by bacterial isolates and zones are formed around the bacterial colonies (Gamalero et al., 2020).
3 Results
3.1 Morphological properties of bacterial strains
Twelve strains of bacteria were isolated from the medicinal plant A. turkestanica (root − 7, stem − 3, leaf − 3). Endophytic bacteria are white, yellow, whitish, yellowish in color and consist of flat and ascending colonies 1–2 mm in size. The morphological and microscopic appearance of endophytic bacteria is shown in Fig. 2, the morphological characteristics in Table 1. + Positive; −negative.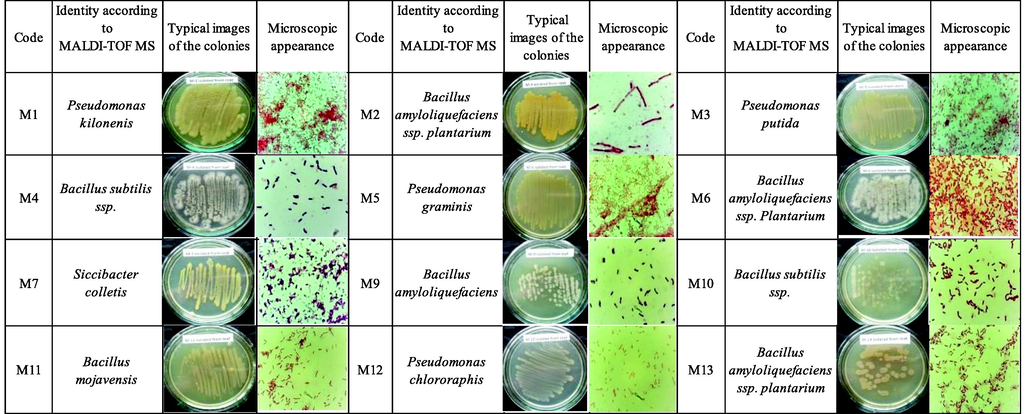
Macroscopic and microscopic view of endophytic bacterial strains.
Isolates
Identity according to MALDI-TOF MS
Colour
Texture
Margins of colony
Degree of growth
Nature
Gram staining
Size
M1
Pseudomonas kilonensis
yellowish
raised
undulate
profuse
creamy
−ve
bacilli
M2
Bacillus amyloliquefaciens ssp. plantarum
yellow
flat
undulate
profuse
creamy
−ve
bacilli
M3
Pseudomonas putida
yellowish
flat
undulate
profuse
creamy
−ve
bacilli
M4
Bacillus subtilis ssp.
white
flat
entire
profuse
discrete
−ve
bacilli
M5
Pseudomonas graminis
yellowish
flat
entire
profuse
creamy
−ve
bacilli
M6
Bacillus amyloliquefaciens ssp. plantarum
whitish
flat
entire
profuse
discrete
−ve
bacilli
M7
Siccibacter colletis
yellowish
raised
entire
profuse
creamy
+ve
bacilli
M9
Bacillus amyloliquefaciens
white
flat
entire
scanty
discrete
−ve
bacilli
M10
Bacillus subtilis ssp.
whitish
raised
entire
scanty
discrete
−ve
bacilli
M11
Bacillus mojavensis
yellowish
flat
entire
profuse
creamy
−ve
bacilli
M12
Pseudomonas chlororaphis
whitish
flat
undulate
profuse
creamy
−ve
bacilli
M13
Bacillus amyloliquefaciens ssp. plantarum
yellowish
flat
entire
scanty
discrete
−ve
bacilli
3.2 Biochemical and plant growth promoting properties of endophytic bacteria
The ability to ferment carbohydrates and enzymatic activities (amylase, lipase, lecithinase, caseinase, protease, gelatinase, urease, catalase, oxidase) and plant growth promoting traits (phosphate solubility property, ACC production, siderophore production, indole acetic acid production) were studied. A citrate test is used to determine the ability of microorganisms to use citrate as a carbon source. Pseudomonas kilonensis, Bacillus amyloliquefaciens ssp. bacterial strains of plantarum, Pseudomonas putida, Pseudomonas graminis, Siccibacter colletis, Pseudomonas chlororaphis showed positive results in citrate use test (Table 2 and Fig. 3).
Isolates
Identity according to
MALDI-TOF MSPlant parts
Results (slant/butt)
Interpretation
M1
Pseudomonas kilonensis
root
red and yellow
Glucose fermentation and peptone catabolism
M2
Bacillus amyloliquefaciens ssp. plantarum
root
yellow and yellow
Fermentation of glucose, lactose and sucrose
M3
Pseudomonas putida
stem
yellow and yellow
M4
Bacillus subtilis ssp.
leaf
red and yellow
Glucose fermentation and peptone catabolism
M5
Pseudomonas graminis
root
yellow and yellow
Fermentation of glucose
M6
Bacillus amyloliquefaciens ssp. plantarum
stem
red and yellow
Glucose fermentation and peptone catabolism
M7
Siccibacter colletis
root
yellow and yellow
Fermentation of glucose, lactose and sucrose
M9
Bacillus amyloliquefaciens
leaf
yellow and yellow
M10
Bacillus subtilis ssp.
stem
red and yellow
Glucose fermentation and peptone catabolism
M11
Bacillus mojavensis
leaf
yellow and yellow
Fermentation of glucose, lactose and sucrose
M12
Pseudomonas chlororaphis
root
yellow and yellow
M13
Bacillus amyloliquefaciens ssp. plantarum
root
yellow and yellow
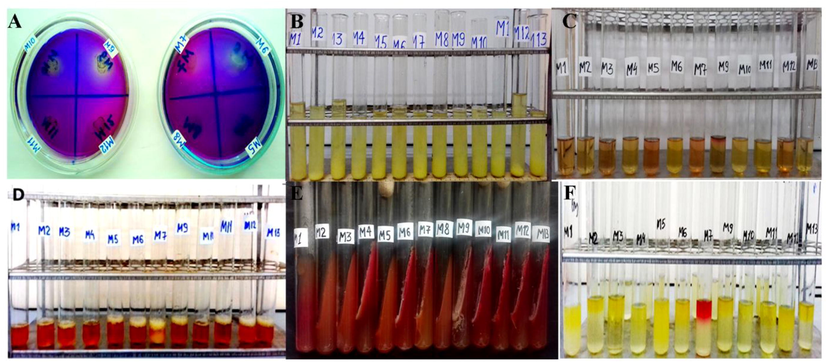
Plant Growth promoting properties of the bacterial strains. A – Detection of phosphate solubilisation. B – Detection of ammonia production. C – Detection of IAA production. D – Detection of siderophore production. E – Detection of carbohydrate fermentation. F Detection of methyl red test.
The following results were obtained when studying the carbohydrate fermentation properties of endophytic bacteria. Bacterial endophytes P. kilonensis, B. subtilis ssp, B. amyloliquefaciens ssp. plantarum performed only glucose fermentation. Bacillus amyloliquefaciens ssp. plantarum, P. putida, P. graminis, S. colletis, B. amyloliquefaciens, B.subtilis ssp, B. mojavensis, P. chlororaphis, B.amyloliquefaciens ssp. plantarum endophytic bacterial isolates have been found to carry out the fermentation of glucose, lactose and sucrose (Table 2).
The acid production potential of microorganisms during glucose fermentation is determined by methyl red test screening. Some endophytic bacteria synthesize the final products of glucose when they use glucose. The results showed a positive result of S. colletis bacterial endophyte. Other bacterial isolates presented a negative result. The following results were obtained when the amylolytic activity of endophytic bacteria was examined. Bacterial endophytes P.putida, B. subtilis ssp, P. graminis, B. amyloliquefaciens ssp. plantarum, S. colletis, B. amyloliquefaciens, B. subtilis ssp., B. mojavensis and P. chlororaphis were found to have high amylolytic activity. The results obtained showed high protease (caseinase) activity of B. subtilis ssp., B. amyloliquefaciens, B. subtilis ssp.and B. mojavensis bacterial endophytes. Enzymatic screening revealed B. subtilis, B.amyloliquefaciens, B. mojavensis strains with lipase and lecithinase activity. (Table 3 and Fig. 4).
Biochemical tests
Bacterial endophytes
Pseudomonas kilonensis
Bacillus amyloliquefaciens ssp. plantarum
Pseudomonas putida
Bacillus subtilis ssp.
Pseudomonas graminis
Bacillus amyloliquefaciens ssp. plantarum
Siccibacter colletis
Bacillus amyloliquefaciens
Bacillus subtilis ssp.
Bacillus mojavensis
Pseudomonas chlororaphis
Bacillus amyloliquefaciens ssp. plantarum
Test for enteric bacteria
Methyl red test
+
+
+
+
+
+
+++
+
+
+
+
+
Citrate utilization
+
+
+
–
+
–
+
–
–
–
+
–
Enzyme assay
Oxidase
+
+
+
+
+
+
+
+
+
+
+
+
Catalase
–
+
+
+
+
–
+
–
–
+
+
+
Amylase
+
+
+
+
+
+
+
+
+
+
+
+
Lesitinase
–
–
–
+
–
+
+
+
+
+
–
–
Lipase
–
–
–
+
–
+
+
+
+
+
–
–
Protease
–
–
–
+
–
–
–
+
+
+
–
–
caseinase
–
–
–
+
–
–
–
+
+
+
–
–
urease
+
+
–
–
–
–
–
–
–
+
+
–
PGPB properties
Phosphate solubilization
–
–
–
–
–
+
+
+
+
+
+
–
Siderophore
production+
+
+
+
+
+
+
+
+
+
+
+
ACC deaminase production
+
–
+
–
–
–
+
–
–
–
+
–
Ammonia production
+
+
+
+
+
+
+
+
+
+
+
+
IAA production
+
+
+
–
+
–
+
+
–
–
+
–
Nitrogen fixation
+
+
+
+
+
+
+
+
+
+
+
+
Gelatin hydrolysis
–
+
–
–
–
+
–
+
+
+
–
+
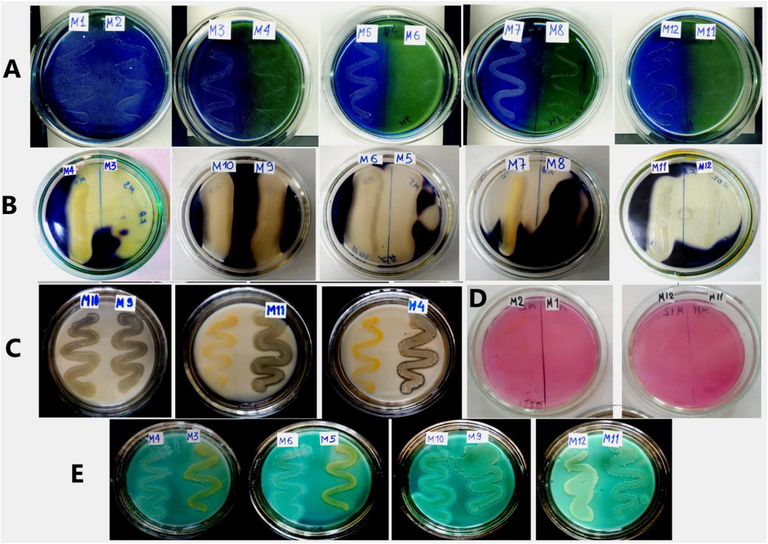
Enzymatic activity of the bacterial strains. A – Detection of citrate utilization. B – Detection of amylase activity. C – Detection of proteolytic activity. D – Detection of urease activity. E – Detection of lecithinase and lipase activities.
All bacterial endophytes of the medicinal plant A.turkestanica showed a positive oxidase test result. The results of the scientific research showed that the bacterial endophytes P. kilonensis, B. amyloliquefaciens ssp. plantarum, B. mojavensis and P.chlororaphis exhibit urease enzyme activity. Gelatin hydrolysis is a two-step process. The enzyme gelatinase hydrolyzes gelatin and results in the formation of polypeptides. Polypeptides are converted into amino acids and used by the cell during metabolism. When the properties of gelatin hydrolysis of endophytic bacteria were studied B. amyloliquefaciens ssp. plantarum, B. amyloliquefaciens, B. subtilis ssp., B. mojavensis and B. amyloliquefaciens ssp. plantarum gave a positive result on gelatin hydrolysis (Table 3).
Among these bacterial endophytes yellow zones were formed in medium B. amyloliquefaciens ssp. plantarum, S. colletis, B.amyloliquefaciens, B. subtilis ssp., and B. mojavensis. This result confirmed that the above five bacterial endophytes have phosphate dissolving properties. Bacterial strains in the DF saline medium were found to synthesize the enzyme ACC deaminase. The results showed that the bacterial isolates P. kilonensis, P.putida, S. colletis and P.chlororaphis have significantly higher property of forming the enzyme ACC deaminase (Table 3 and Fig. 4).
The synthesis of IAA in endophytic bacterial strains was studied. P. kilonensis, B. amyloliquefaciens ssp. plantarum, P. putida, P. graminis, S. colletis, B. amyloliquefaciens and P. chlororaphis showed a positive result. When 2–3 drops of Salkovsky reagent were added to the culture fluid in which endophytic bacteria were grown, a cherry red color was formed in the culture fluid. The bacterial strains identified during our scientific study were found to have the property of assimilating free nitrogen in the air (Table 3).
4 Discussion
Endophytic bacteria and plant growth promoting bacteria play an important role in increasing the plant growth (Jabborova et al., 2020b; Jabborova et al., 2021; Egamberdieva et al., 2013; Jabborova et al., 2013; Egamberdieva and Jabborova, 2013), plant nutrition (Egamberdieva et al., 2017; Egamberdieva et al., 2018; Jabborova et al., 2020), and nodule formation of different plants (Jabborova et al., 2013; Jabborova and Davranov, 2015; Jabborova et al., 2018).
Scientific studies on isolated endophytic bacteria have yielded the following results. Three of the endophytic bacterial strains were found to synthesize indole three acetic acid. (Table 3). Regulation of hormonal processes in root, stem and leaf development is closely related to indole acetic acid (IAA). Indole acetic acid (IAA), synthesized by endophytic microorganisms, is actively involved in all stages of the plant development process. This bioactive compound regulates biological processes such as tissue and cell formation in mechanisms that take place at the cellular level. These physiological processes play an important role in the formation of xylem and root tissue of plants.
The indole three acetic acid synthesized by the studied bacterial strains has a positive effect on the root system of plants and ensures that the root penetrates deep into the soil layers. Indole triacetic acid (IAA) acts in two directions on plants. The first is involved in the rapid division and proliferation of cells. The second effect plays a smoother role in the specialization of cells. Bacteria in the rhizosphere have the property of synthesizing 80% indole acetic acid, and the use of such microorganisms in agricultural plants increases the chances of high yields in plants. IAA synthesized by rhizobacteria affects the root system of plants. Plants increase weight, the number of branches on the branches, and the surface area of the root in contact with the soil. In rhizosphere bacteria, IAA metabolism is mainly dependent on L-tryptophan-dependent and independent pathways. Most rhizospheric bacteria have chosen the L-tryptophan pathway as the basis for IAA synthesis. Most root rhizosphere bacteria use L-tryptophan, which is secreted from root exudate, a precursor to IAA production (Ogale et al., 2018; Jabborova et al., 2020c).
Rhizosphere bacteria have the ability to dissolve complex phosphate compounds and mineralize organic phosphate in the soil. For example, these bacteria convert organic phosphate into an inorganic form that plants can assimilate. Endophyte bacterial strains break down phosphate compounds by forming organic acids. The phosphate compounds present in the soil are not dispersed in a form suitable for plant nutrition. The endophytic bacterial species that colonize the plant body play an important role in this process. Endophytic bacterial strains break down phosphate salts in the soil through the production of organic acids to form nutrients for the plant. That is, in the form of insoluble, sedimentary, plant roots cannot directly absorb this mineral compound. Endophytic microorganisms that colonize the plant body break down these insoluble phosphate compounds by producing organic acids into a form that the plant can assimilate. These endophytic microorganisms, which make up the plant microbiome, exhibit beneficial properties for their host plant. Among phosphorus-soluble microorganisms in the soil, the proportion of endophytic bacteria or rhizobacteria (PSB and PSR) and the phosphorus dissolution potential is 1–50%, while in phosphorus-soluble fungi it is 0.1–0.5%. Effective phosphate-soluble bacteria for plants include Pseudomonas, Bacillus. Phosphate-soluble bacteria dissolve inorganic forms of phosphate through organic acids, phosphate-bound cations or by lowering the pH of the rhizosphere (proton formation – with bicarbonate release). In our study six bacterial endophytes (B.amyloliquefaciens ssp. plantarum, S. colletis, B. amyloliquefaciens, B. subtilis ssp., B. mojavensis) were found to have the property of dissolving phosphorus compounds in the soil (Table 3). Therefore, the solubility and mineralization of phosphate in the soil may be present in the same bacterial strains (Ogale et al., 2018). Similar investigations indicated that Li et al. (2018) reported endophytic bacteria with the same phosphate solubilization trait.
Endophytic bacteria prevent the growth of various pathogenic fungi in the rhizosphere by producing siderophores that bind to Fe3+ ions around the plant roots where they are located. It has been observed that endophytic bacteria belonging to the genus Pseudomonas, which form siderophores, destroy various pathogens found in the soil. Experiments have shown that wild-type siderofor-producing strains are more effective in preventing diseases caused by various pathogenic fungi in the rhizosera environment than non-siderofor-producing mutant strains.
The word siderophore means iron carrier. This compound is produced by endophytic bacteria that colonize the plant body in the rhizosphere (Cornelis, 2010; Ogale et al., 2018; Firdous et al., 2019). Endophyte bacteria increase the movement of micronutrients from the soil to the root, which plants assimilate through the roots through sedophores synthesized by them. There are species such as Bacillus, Rhizobium, Pseudomonas, Agrobacterium, E. coli, endophytic bacteria that have the ability to synthesize such siderophores. All of the endophytic bacteria studied in our scientific study were found to synthesize siderophores. Bacillus sp., which has a similar property in scientific studies and Pseudomonas sp endophytic bacteria were detected.
The presence of atmospheric nitrogen uptake by bacteria is one of the important factors in the process of plant nutrition. Nitrogen assimilated by bacterial strains plays an important role in plant life activities and nutrition processes. Three main routes of nitrogen-fixing endophytic bacteria entering the root are described: 1) through root holes (caused by mechanical damage) and randomly emerging roots; 2) through the root hairs; 3) through undamaged epidermal cells [Cocking, 2003]. We know that there is enough nitrogen in the air, but this nitrogen cannot be absorbed by plants. Plants assimilate nitrogen (NH4+) and NO3–. The absorption of NO3– occurs with the flow of protons, the absorption of NH4+ occurs with the release of protons. According to this process, it leads to saturation of the rhizosphere with hydroxides and acids and affects rhizosphere processes (Dekas et al., 2009). When living organisms die, organic matter is consumed by soil heterotrophs and the decomposition process takes place. In this process, nitrogen in the dead body is converted by microorganisms into a form that is important for plants. The process of dinetrification of nitrite and nitrate nitrogen compounds is carried out by endophytic bacteria belonging to the genus Pseudomonas, Bacillus. Twelve endophytic bacterial strains isolated from the medicinal plant A. turkestanica were found to have ammonia-synthesizing properties (Li et al., 2018).
5 Conclusion
The results of our scientific research show that the medicinal plant Ajuga turkestanica which lives in a high mountainous region where naturally rocky and constant winds blow, is a microbiome inhabited by endophytic bacteria of different generations. Bacterial strains isolated from the medicinal plant A.turkestanica, which is the object of our study, were found to be dominated by Bacillus, Pseudomonas, Siccibacter genera. It has been found that these bacterial generations have many properties that enhance the growth of cultivated plants used in the development of sustainable agriculture. Compounds that stimulate and enhance plant growth synthesized by these endophytic bacteria, whose properties have been studied, increase soil fertility and create normal conditions for the host plant. Due to the fact that endophytic bacteria isolated from the plant A. turkestanica have the property of enhancing plant growth, they can be used to produce bioinoculants individually or in consortium. However, since studies on endophytic bacteria have been conducted in vitro, it is important to apply them to field conditions to prove their beneficial properties.
Acknowledgements
The study was funded by the Institute of Microbiology of the Academy of Sciences of the Republic of Uzbekistan. This project was supported by Researchers Supporting Project number (RSP-2022/257) King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Effect of seaweed extract on productivity and quality attributes of four onion cultivars. Horticulturae. 2020;6:28.
- [Google Scholar]
- Ecdysterone and turkesterone in Ajuga turkestanica determined by HPLC. Chem. Nat. Compd.. 2005;41(4):475-476.
- [Google Scholar]
- Isolation and characterization of plant growth promoting endophytic bacteria from desert plants and their application as bioinoculants for sustainable agriculture. Agronomy. 2020;10(9):1325.
- [Google Scholar]
- Phytoecdysteroids: structure, sources, and biosynthesis in plants. Russ. J. Bioorganic. Chem.. 2000;26:799-831.
- [Google Scholar]
- Bergey, D.H., Holt, John, G.,2000. Bergey's manual of determinative bacteriology Publisher: Philadelphia: Lippincott Williams & Wilkins, 2000.
- Richness of endophytic fungi isolated from Opuntia ficus-indica Mill. (Cactaceae) and preliminary screening for enzyme production. World J. Microbiol. Biotechnol.. 2012;28(5):1989-1995.
- [Google Scholar]
- Endophytic colonization of plant roots by nitrogen-fixing bacteria. Plant Soil. 2003;252(1):169-175.
- [Google Scholar]
- Iron uptake and metabolism in pseudomonads. Appl. Microbiol. Biotechnol.. 2010;86(6):1637-1645.
- [Google Scholar]
- Deep-sea archaea fix and share nitrogen in methane-consuming microbial consortia. Science. 2009;326(5951):422-426.
- [Google Scholar]
- Isolation and characterization of endophytic bacteria from ginger (Zingiber officinale Rosc.) Annals of. Phytomedicine. 2020;9(1):116-121.
- [Google Scholar]
- Improvement of cotton production in arid saline soils by beneficial microbes. In Crop Yields: Production, Management Practices and Impact of Climate Change; 2013. p. :109-122.
- Vegetation of Central Asia and Environs. Cham: Springer International Publishing; 2018. p. :211-237.
- Plant microbiome: source for biologically active compounds. Biodiversity and Biomedicine. Elsevier Inc.; 2020. p. :1-9.
- Egamberdieva, D., Jabborova, D., 2013. Improvement of cotton production in arid saline soils by beneficial microbes. In: Crop Yields: Production, Management Practices and Impact of Climate Change, Editors: Lijuan Huang and Qiao Zhao, Nova Publisher, USA.109–122.
- Plant Microbe Symbiosis: Fundamentals and Advances. New Delhi: Springer India; 2013. p. :291-303.
- Interaction of magnesium with nitrogen and phosphorus modulates symbiotic performance of soybean with Bradyrhizobium japonicum and its root architecture. Front. Microbiol.. 2018;9(1)
- [Google Scholar]
- Coordination between Bradyrhizobium and Pseudomonas alleviates salt stress in soybean through altering root system architecture. J. Plant Interact.. 2017;12(1):100-107.
- [Google Scholar]
- Endophytic bacteria and their potential application in agriculture: a review. Indian J. Agric. Res.. 2019;53(1):1-7.
- [Google Scholar]
- Screening of bacterial endophytes able to promote plant growth and increase salinity tolerance. Appl. Sci.. 2020;10:57-67.
- [Google Scholar]
- Isolation, Identification and Characterisation of Endophytic Bacteria in Biophytum sensifivum (L.) DC. J. Pure Appl. Microbiol.. 2020;14(1):64-655.
- [Google Scholar]
- Effect of phosphorus and nitrogen concentrations on root colonization of soybean (Glycine max L.) by Bradyrhizobium japonicum and Pseudomonas putida. Int. J. Adv. Biotechnol. Res.. 2015;16(3):418-424.
- [Google Scholar]
- Salt tolerant Pseudomonas strain improved growth, nodulation and nutrient uptake of soybean grown under hydroponic salt stress condition. XVII international plant nutrition colloquium and boron satellite meeting proceedings book. 2013:260-261.
- [Google Scholar]
- Plant growth promoting bacteria Bacillus subtilis promote growth and physiological parameters of Zingiber officinale Roscoe. Plant Science Today. 2021;8(1):66-71.
- [Google Scholar]
- Effect of Bacillus subtilis 1 strain on the growth and development of wheat (Triticum aestivum L.) under saline condition. Bulgarian J. Agric. Sci.. 2020;26(4):744-747.
- [Google Scholar]
- Improvement of seedling establishment of soybean using IAA and IAA producing bacteria under saline conditions. Soil Water J.. 2013;2(2):531-539.
- [Google Scholar]
- Co-inoculation of rhizobacteria and biochar application improves growth and nutrients in soybean and enriches soil nutrients and enzymes. Agronomy. 2020;8:1142.
- [Google Scholar]
- Effect of co-inoculation with Bradyrhizobium japonicum and Pseudomonas putida on root morph-architecture traits, nodulation and growth of soybean in response to phosphorus supply under hydroponic conditions. Bulgarian J. Agric. Sci.. 2018;24(6):1004-1011.
- [Google Scholar]
- Effect of phosphorus and nitrogen concentrations on root colonization of Soybean (GLYCINE MAX L.) by Bradyrhizobium japonicum and Pseudomonas putida. Int. J. Adv. Biotechnol. Res.. 2015;6(3):418-424.
- [Google Scholar]
- Isolation, characterization and application of endophytic bacteria isolated from medicinal plants. Int. Res. J. Nat. Appl. Sci.. 2017;4:2349-4077.
- [Google Scholar]
- Phosphorus Nutrient Management through Synchronization of Application Methods and Rates in Wheat and Maize Crops. Plants. 2020;9:1389.
- [Google Scholar]
- Isolation and characterization of bacterial endophytes of Curcuma longa L. 3. Biotech. 2016;6(1):60.
- [Google Scholar]
- Synergistic plant–microbe interactions between endophytic bacterial communities and the medicinal plant Glycyrrhiza uralensis F. Antonie Van Leeuwenhoek. 2018;111(10):1735-1748.
- [Google Scholar]
- Plant growth-promoting activities and molecular characterization of rhizobacterial strains isolated from medicinal and aromatic plants. J. Pharm. Biol. Sci.. 2013;6:30-37.
- [Google Scholar]
- Marfo, T.D.; Datta, R.; Pathan, S.I.; Vranová, V., 2019a. 2019b.Ecotone Dynamics and Stability from Soil Scientific Point of View. Diversity 2019. (11) 53.
- Marfo, T.D.; Datta, R.; Vranová, V.; Ekielski, A. Ecotone Dynamics and Stability from Soil Perspective: Forest-Agriculture Land Transition. Agriculture. (9) 228.
- Isolation of turkesterone from the epigeal part of Ajuga turkestanica and its anabolic activity. Chem Nat Comds.. 1998;34(2):150-154.
- [Google Scholar]
- Mintoo, M.N., Mishra, S., Dantu, P.K., 2019. Isolation and characterization of endophytic bacteria from Piper longum. Proceedings of the National Academy of Sciences, India Section B: Biological Sciences 89 (4), 1447–1454.
- Isolation and characterization of beneficial indigenous endophytic bacteria for plant growth promoting activity in Molelwane Farm, Mafikeng. South Africa. African journal of Biotechnology. 2013;12(26)
- [Google Scholar]
- Enzymatic activity of endophytic bacterial isolates from selected mangrove plants in Kenya. The Open Microbiology Journal. 2018;12(1)
- [Google Scholar]
- Screening of endophytic bacteria from the pharmacologically important medicinal plant Gloriosa superba for their multiple plant growth promoting properties. J Pharm Innov.. 2018;7(1):208-214.
- [Google Scholar]
- Isolation and Characterization of lipase producing bacteria from vegetable oil spillage site. International Journal of Current Microbiology and Applied Sciences. 2016;5(8):214-232.
- [Google Scholar]
- Phytoecdysteroids and other biologically active compounds from plants of the genus Ajuga. Chem Nat Compd.. 2005;41:361-369.
- [Google Scholar]
- Isolation and characterization of endophytes from nodules of Mimosa pudica with biotechnological potential. Microbiol. Res.. 2019;218:76-86.
- [Google Scholar]
- A. Sayed E.A., Hassan, E.A.E.T., El-Tobgy, K.M. K., Ramadan, E.M. Characterization of endophytic bacteria associated with some medicinal plants Arab Universities Journal of Agricultural Sciences 27 5 2019 2513 2526.
- Biodiversity and antimicrobial activity of endophytes associated with Egyptian medicinal plants. Mycosphere. 2011;2(6):669-678.
- [Google Scholar]
- Immunomodulating and antistress activity of ecdysterone and turkesterone under immobilization-induced stress conditions in mice. Pharm Chem J.. 2010;44(1):7-9.
- [Google Scholar]
- Phosphate solubilizing microbes: sustainable approach for managing phosphorus deficiency in agricultural soils. SpringerPlus. 2013;2(1):1-14.
- [Google Scholar]
- Isolation, screening and characterization of endophytic PGPR actinomycetes present commonly in Neem and Tulsi leaves-in vitro study (Tomato) Int J Recent Sci Res.. 2014;5(3):574-579.
- [Google Scholar]
- Isolation and characterization of a new endophytic actinobacterium Streptomyces californicus strain ADR1 as a promising source of antibacterial, antibiofilm and antioxidant metabolites. Microorganisms. 2020;8(6):929.
- [Google Scholar]
- Comparative experimental investigations of anabolic activity of phytoecdysteroids and steranabol. Pharm. Chem. J.. 2001;34:193-197.
- [Google Scholar]
- Identification and characterization of endophytic bacteria from corn (Zea mays L.) roots with biotechnological potential in agriculture. Amb Express.. 2014;1:1-9.
- [Google Scholar]
- Impact of Seed Dressing and Soil Application of Potassium Humate on Cotton Plants Productivity and Fiber Quality. Plants. 2020;9:1444.
- [Google Scholar]
- Impact of environmental variables on the isolation, diversity and antibacterial activity of endophytic fungal communities from Madhuca indica Gmel. at different locations in India. Annals of microbiology. 2014;64(2):721-734.
- Physiological and pharmaceutical effects of Ginger (Zingiber officinale Roscoe) as a valuable medicinal plant. European journal of experimental biology. 2014;4(1):87-90.
- [Google Scholar]







