Translate this page into:
Characterization and cadmium detoxification dynamics of endophytic bacteria, isolated from rice plants
⁎Corresponding author. iullah@kau.edu.sa (Ihsan Ullah)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Over the past few decades, a special focus has been placed on plant endophytic bacteria's ability to improve plant responses to various stresses. We isolated bacteria from SWAT-1 rice variety and characterized them biochemically and morphologically. The isolates included seven from leaves, eight from roots, and five from stems. A total of 20 isolates were identified, of which four were gram-negative and sixteen were gram-positive. Eight of the isolates were catalase-positive, whereas twelve were catalase-negative. The highest production of indole-3-acetic acid (IAA) was recorded in IALIV (4.01 µg/ml), with the lowest production by IARIV (0.22 µg/ml). Similarly, IALIII and IASII produced the lowest (0.24 µg/ml) and highest (0.73 µg/ml) amylase respectively. IARVI and IASII showed maximum antimicrobial activity (27 mm) against Calcibacter and minimum activity (10 mm) against Xanthomonas. In terms of growth promotion, all isolates increased shoot length, root length, biomass production, and Cd uptake in plants. As compared to uninoculated plants, the inoculation of endophytes significantly improved (p < 0.05) the plant growth attributes in plants exposed to Cd stressors (7 mM and 12 mM). Generally, isolates have been shown to be useful as biocontrol agents and biofertilizers.
Keywords
Phytoremediation
Heavy metals
Abiotic stress
Antibacterial activity
Cadmium toxicity
1 Introduction
An array of metabolites, such as phytohormones, are synthesized by the bacterial endophytes in order to support plant growth and development (Barman et al., 2016). Phytohormones, antimicrobial compounds, and other nutrients are produced by endophytic bacteria, which play an important role in plant growth and development (Ardanov et al., 2012). They have been found in a wide variety of plant species, live in root systems and decline from leaves to stems, and can colonize a particular host (Singh and Singh, 2019). Endosymbiosis and co-evolution of endophytes and plants made an intimate niche that help bacteria in nourishment and protection and in the response, plants are protected from stresses. Plants can tolerate pathogens, salinity drought, and heavy metal stress conditions due to the endophytic bacteria found in their tissues. These bacteria support the ecological balance of natural systems (Mercado-Blanco and Bakker. 2007; Weyenset al., 2009). The endophytic population varies in different regions, plants and species, even in the same plant (Amaresan et al., 2020). Overall, the largest proportion of endophytes belong to the Firmicutes, Proteobacteria, and Actinobacteria. Endophytes have also been identified from Streptomyces, Stenotrophomonas, Burkholderia, Azoarcus, Bacillus, Pseudomonas, Serratia, and Gluconobacter phyla (Taghavi et al., 2009; Deng et al., 2011). Indole-3-acetic acid (IAA) provided by bacteria increases plant growth by enhancing root growth and nutrient availability due to the larger area of fertile soil occupied, causing the plants to grow more biomass and become more resistant to stressors (Khan et al., 2015).
Heavy metals contamination including cadmium (Cd) contamination in soil is a known environmental hazard and cause toxicity in plants and animals (Ghosh et al., 2018). These hazardous factors have resulted in the development of physiological and molecular mechanisms in plants (Weilharter et al., 2011). Cd contamination induces mutation, DNA damages and eventually causes cancer in animals. Cd interaction with plants initiates competition with elements causing a disturbance in the electron transport chain in mitochondria and chloroplast (Bertalan et al., 2009). It produces reactive oxygen species inhibiting enzymatic activity, protein denaturation, and non-functionalization of the lipid membrane (Naik et al., 2009). As a defense system, the plant produces antioxidant metabolites such as glutathione and polyphenols proteins like catalase enzymes (Ullah et al., 2019). The application of the useful microbes is among the most prominent strategy to detoxify the heavy metal contaminates (Bertalan et al., 2009). The interaction of plants with soil microorganisms can significantly influence the acclimatization of plants to metalliferous environments, and this can be explored further to improve plant tolerance to metals (Ryan et al., 2008). Through various mechanisms, microbes associated with plants reduce metal accumulation in plant tissues and soil bioavailability of metals (De-Mandal and Passari, 2021). Using a novel phytobacterial strategy, heavy metal tolerant bacteria have now been proven to increase stress tolerance and heavy metal remediation in plants (Malfanova et al., 2013).
Phytoremediation involves removing heavy metals using plants. However, some plants are well-known for taking up heavy metals, but they have shown poor phytoremediation due to limited production of biomass for selective metals and a slower growth rate (Braud et al., 2006). Heavy metals in contaminated soil are detoxified by bacteria and hence accelerating plant growth (Khan et al., 2015). We isolated and characterized endophytic bacteria from rice plants in the present study. Using rice plants inoculated with various concentrations of Cd (7 mM and 12 mM), bacterial isolates were evaluated for Cd uptake, tolerance, and accumulation. The isolates were also tested for an antibacterial activity so they can be exploited as biofertilizers and phytoremediators in agriculture (Bulgarelli et al., 2012).
2 Materials and methods
2.1 Sample collection and surface sterilization
SWAT-1 rice (Oryza sativa) was used to isolate endophytes bacteria, and G4R1 rice was used to evaluate the Cd toxicity on plant growth. Plant samples were sterilized using 1% NaOCl and then were repeatedly washed with deionized autoclaved water to remove any residue (Sun et al., 2008).
2.2 Isolation of endophytes
Sterilized pieces were transferred to LB agar plates and incubated at 37 °C for colony formation. The colonies were selected based on their growth patterns and morphology (color, shape, size). Fresh medium was smeared with the selected pure cultures and incubated at 37 °C until colonies developed. The pure culture was used to separate the bacteria based morphological and biochemical characteristics (Zinniele et al., 2002).
2.3 Morphological biochemical characterization
The morphological characterization was carried out via color, colony size, texture and structure of the colonies following the Ma et al., (2015). Furthermore, the bacterial isolates were subjected to gram staining folowing Dharni (et al., 2014). The activity of catalase was assessed using hydrogen peroxide (H2O2) according to the Sona et al. (2011). Amylase activity was examined according to Wind et al. (1994).
2.4 Indole acetic acid production test
Salkowski reagent was made in 1000 ml of H2SO4 (7.9 M) by mixing 12 g FeCl3 to screen bacteria for IAA production. The supernatants from culture broth were mixed with Salkowski reagent at the ratio of 2:1. The mixture was then incubated in dark 30 min at 25 °C. A pink to red coloration, after incubation was indication of IAA which was measured at 535 nm using spectrophotometer. The standard curve of IAA was made with commercially available IAA from Sigma-Aldrich. After comparing it with the IAA standard curve, the concentration of IAA was determined.
2.5 Antimicrobial activity
Antibacterial activities of the extract of isolates were assessed through disc fusion method according to the Barman et al., (2017), against Escherichia coli, Citrobacter freundii, Calcibacter, Bacillus cereus, Xanthomonas campestri. At 37 °C, the plates were incubated to get the zones of inhibitions. The diameters of the zones of inhibitions were measured.
2.6 Bacterial effects on plant growth under cadmium stress
The G4R1 rice seeds were surface sterilized and kept on wet filter for germination. Seeds were transferred to disinfected sand pots after germinating. The pots were treated with cadmium nitrate [Cd(NO3)2] of different concentrations i.e. 7 mM, 12 mM, and isolates, and compare with control (untreated). Total plant length (shoot and root) and total biomass (fresh and dry weight) were noted down. Plant tissues were crushed in liquid nitrogen and digested in a mixture of HClO4 and HNO3 to measure Cd concentration. As a result of inductively coupled plasma spectroscopy, Cd in digested samples was determined (ICP, Optima 79000DV, PerkinElmer, USA).
2.7 Statistical analysis
To analyze the data statistically, we used GraphPad Prism (Version 5.0; USA). We compared the mean values using Duncan's multiple ranges tests at P < 0.05 (SAS, USA).
3 Results
3.1 Isolation and morphological characterization of bacteria
Endophytic bacterial colonies were obtained from different tissues of the plants on agar plates (Fig. 1). Eight bacteria i.e., IARI, IARII, IARIII, IARIV, IARV, IARVI, IARVII and IARVIII from the roots, seven bacteria i.e., IALI, IALII, IALIII, IALIV, IALV, IALVI, IALVII from the leaves and five bacteria i.e., IASI, IASII, IASIII, IASIV, IASV, IASVI and IASVII from the stem were isolated. Depending on the colony colors, shape, and size, the bacterial colonies were classified. Colors, including yellow, red, and off-white, and shapes, including cocci and rods, were seen in endophytic bacterial colonies. Four of the isolates were gram-negative, and sixteen were gram-positive.
Endophytic bacterial growth on agar plates.
3.2 Antimicrobial activity of bacterial isolates
Using the agar disc diffusion assay, Bacillus cereus, Calcibacter, Xanthomonas campestris, Citrobacter freundii, and Escherichia coli were used for antimicrobial activity. The antimicrobial activity of several isolates was ranged from 10 mm to 27 mm diameter of zone of inhibition, while others showed no antimicrobial activity. IASII showed the lowest activity (10 mm) against Xanthomonas and IARVI showed the highest activity (27 mm) against Calcibacter (Table 1). *NA stands for no activity.
Diameter of zone of inhibition (mm)
Isolates
E. coli
B. cereus
Calcibacter sp.
C. freundii
X. campestri
STREPTOMYCIN
19 ± 2
25 ± 4
23 ± 3
35 ± 5
21 ± 4
IARI
17 ± 3
NA
NA
NA
NA
IARII
17 ± 3
19 ± 4
16 ± 3
NA
18 ± 3
IARIII
16 ± 2
16 ± 3
NA
NA
14 ± 4
IARIV
14 ± 4
23 ± 2
21 ± 2
NA
14 ± 3
IARV
17 ± 4
17 ± 3
25 ± 3
16 ± 2
23 ± 4
IARVI
NA
24 ± 3
27 ± 3
19 ± 4
24 ± 4
IARVII
16 ± 2
22 ± 3
23 ± 2
14 ± 3
19 ± 5
IARVIII
18 ± 3
NA
19 ± 3
NA
19 ± 3
IASI
NA
NA
18 ± 2
18 ± 4
12 ± 3
IASII
NA
NA
14 ± 3
12 ± 2
10 ± 3
IASIII
NA
16 ± 2
14 ± 2
14 ± 3
14 ± 4
IASIV
NA
17 ± 4
19 ± 3
16 ± 3
16 ± 3
IASV
NA
NA
NA
18 ± 4
18 ± 3
IALI
NA
NA
14 ± 3
NA
16 ± 2
IALII
NA
15 ± 2
17 ± 2
19 ± 3
17 ± 3
IALIII
NA
15 ± 2
NA
NA
NA
IALIV
NA
16 ± 3
NA
15 ± 4
14 ± 2
IALV
17 ± 4
17 ± 3
16 ± 4
NA
NA
IALVI
NA
NA
15 ± 5
NA
14 ± 3
IALVII
NA
18 ± 4
NA
NA
NA
3.3 Catalase test and amylase activities of the bacterial isolates
Catalase-positive isolates produced bubbles when hydrogen peroxide (H2O2) was added and the results showed that twelve out of twenty isolates were catalase-positive and other eight were catalase-negative (Fig. 2A, B). Bacterial isolates produced varying amounts of amylase enzyme (Fig. 3). The highest amylase production was observed in IASII (0.73 g/ml), whereas IALIII (0.24 g/ml) produced the lowest amylase.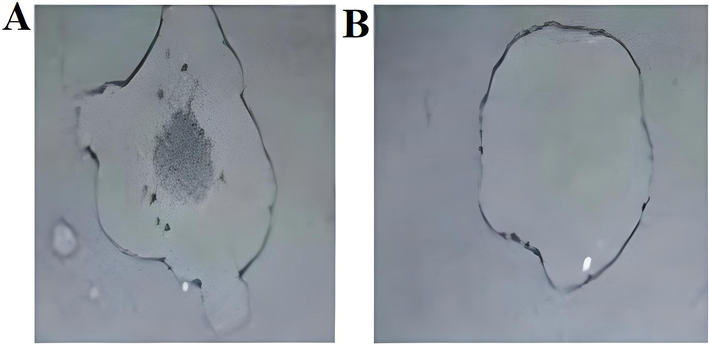
(A). Bubble formation after the addition of hydrogen peroxide. (B). No bubble formation after the addition of hydrogen peroxide.
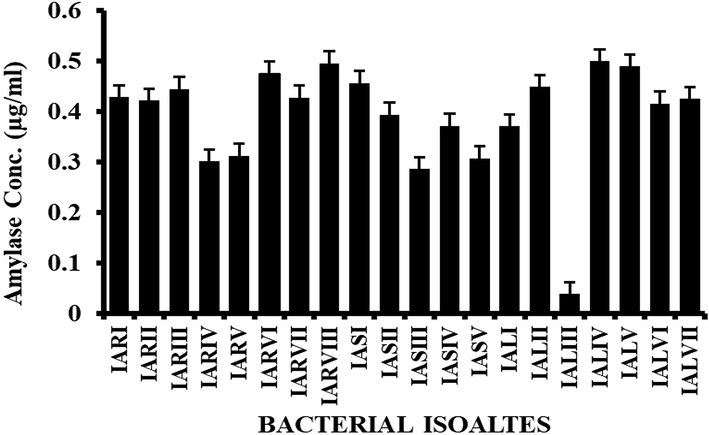
Different concentrations of amylase enzyme produced by all the selected isolates. The X-axis represents the selected isolates while Y-axis represents the concentration of amylase.
3.4 Indole acetic acid (IAA) assessment in bacteria
The pink to red color of the bacterial extract after mixing with Salkowski’s reagent was indication of IAA which was measured at 535 nm via spectrophotometer. The results revealed that all bacterial isolates produced IAA. However, the results also showed that IALIV produced higher (4.01 µg/ml) concentration of IAA while IARIV produced the lowest concentration of (0. 22 µg/ml) of IAA (Fig. 4).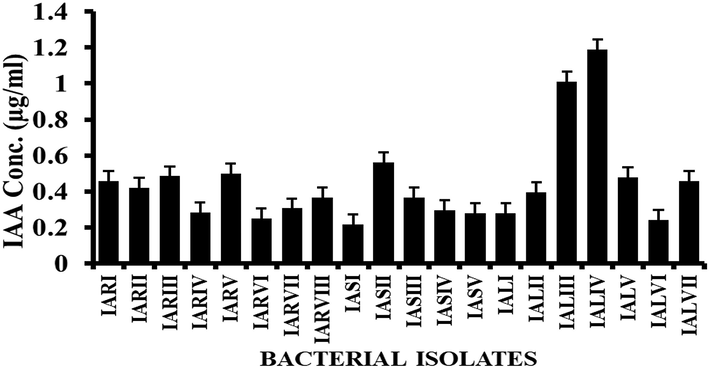
Different concentrations of indole acetic acid (IAA) produced by isolates. Error bars represent the standard deviation.
3.5 Bacterial effects on plant growth under cadmium stress
Plants stressed with 7 mM and 12 mM Cd concertation and inoculated with bacterial isolates were subjected to measurement of growth dynamics e.g., total plant length (Shoot + root) and total biomass (fresh + dry weight; Fig. 5A, B). The plant growth was significantly reduced under the 7 mM and 12 mM Cd stress compared to control. However, application of endophytic bacteria, plant growth was significantly increased in plants contaminated with both 7 mM and 12 mM of Cd in comparison with uninoculated plants. Inoculation significantly (p < 0.05) enhanced biomass (fresh + dry weight) the plants contaminated with 7 mM and 12 mM Cd in comparison with uninoculated plants. Inoculated plants also showed significant increase (p < 0.05) in roots length and shoot length under 7 mM and 12 mM Cd concentration in comparison with uninoculated control (Table 2). Cd concentration was calculated on the basis of the dry weight (DW). Values in each column represent themean ± SD.Mean values of the control and the treatments using different Cd concentrations (mg kg − 1 dry sand) in each column denoted by the different letters are significantly different at p < 0.05. DW = Dry Weight, FW = Fresh weight, RL = Root length, SL = Shoot Length.
(A) plant growth with inoculation of isolates (B) growth of uninoculated rice plants under cadmium stress.
7 mM of Cd concentration 12 mM of Cd concentration 7 mM of Cd concentration 12 mM of Cd concentration
Treatments
Dry W (g)
FW (g)
DW (g)
FW (g)
RL (cm)
SL (cm)
RL (cm)
SL (cm)
Control
0.02 ± 0.003a
0.01 ± 0.002a
0.02 ± 0.003a
0.03 ± 0.001a
3.74 ± 0.32a
4.23 ± 1.21a
3.64 ± 1.65a
4.2 ± 1.62a
IARI
0.06 ± 0.005b
0.03 ± 0.005b
0.08 ± 0.007b
0.04 ± 0.002b
8.73 ± 0.22b
7.33 ± 1.61b
7.66 ± 2.65b
9.3 ± 2.65b
IARII
0.05 ± 0.02c
0.03 ± 0.005b
0.09 ± 0.007c
0.03 ± 0.001c
7.76 ± 1.22c
5.16 ± 2.45c
8.13 ± 3.64c
9.16 ± 3.37b
IARIII
0.07 ± 0.02d
0.03 ± 0.003b
0.08 ± 0.002b
0.04 ± 0.002b
6.3 ± 1.34d
9.63 ± 2.67d
8.56 ± 2.55c
8.2 ± 2.43c
IARIV
0.09 ± 0.01e
0.05 ± 0.004c
0.08 ± 0.004b
0.03 ± 0.001c
8.56 ± 2.12e
7.83 ± 3.74b
9.86 ± 4.66d
8.66 ± 2.22c
IARV
0.05 ± 0.01c
0.03 ± 0.005b
0.07 ± 0.003d
0.03 ± 0.001c
9.53 ± 2.32f
7.16 ± 3.04b
10.7 ± 3.22e
6.7 ± 1.35d
IARVI
0.05 ± 0.005c
0.03 ± 0.005b
0.06 ± 0.007d
0.02 ± 0.001d
8.83 ± 1.82e
4.5 ± 0.77a
9.43 ± 3.15d
6.4 ± 2.21d
IARVII
0.07 ± 0.002d
0.03 ± 0.003b
0.08 ± 0.007b
0.03 ± 0.003c
8.36 ± 1.24e
7.5 ± 2.36b
5.23 ± 1.13f
4.83 ± 2.33e
IARVIII
0.06 ± 0.51d
0.03 ± 0.003b
0.09 ± 0.007b
0.05 ± 0.002d
6.96 ± 2.25d
5.3 ± 1.53c
11.2 ± 3.35 g
8.23 ± 2.45c
IASI
0.05 ± 0.005c
0.04 ± 0.005d
0.08 ± 0.007b
0.03 ± 0.002c
5.6 ± 1.43f
3.4 ± 0.08e
6.2 ± 2.55 h
4.7 ± 2.45e
IASII
0.06 ± 0.02f
0.05 ± 0.004c
0.08 ± 0.007b
0.05 ± 0.001d
8.66 ± 1.52b
10.0 ± 3.82f
8.9 ± 2.63c
7.7 ± 2.35f
IASIII
0.09 ± 0.02e
0.04 ± 0.005d
0.09 ± 0.007b
0.03 ± 0.001c
4.96 ± 2.62 g
3.26 ± 1.02e
9.06 ± 3.45d
4.4 ± 1.60a
IASIV
0.10 ± 0.01 g
0.03 ± 0.005b
0.09 ± 0.007b
0.04 ± 0.001b
5.86 ± 1.32f
3.6 ± 1.94e
7.2 ± 2.56b
7.83 ± 2.34f
IASV
0.11 ± 0.005 h
0.03 ± 0.005b
0.11 ± 0.007e
0.03 ± 0.002c
6.26 ± 1.22d
3.3 ± 1.30e
8.5 ± 2.32c
3.9 ± 1.63a
IALI
0.11 ± 0.02 h
0.03 ± 0.002b
0.08 ± 0.007b
0.03 ± 0.002c
7.4 ± 1.52c
4.63 ± 1.57a
7.0 ± 2.35b
8.0 ± 2.45c
IALII
0.05 ± 0.01c
0.04 ± 0.003d
0.11 ± 0.007e
0.04 ± 0.003b
9.03 ± 1.22 h
9.1 ± 2.45d
5.9 ± 1.65f
4.1 ± 1.26a
IALIII
0.06 ± 0.03f
0.03 ± 0.003b
0.06 ± 0.007d
0.04 ± 0.003b
6.8 ± 1.22d
10.0 ± 3.12f
7.1 ± 2.65b
4.83 ± 1.25a
IALIV
0.09 ± 0.02e
0.04 ± 0.002d
0.09 ± 0.007c
0.04 ± 0.003b
10.2 ± 1.52i
7.5 ± 2.33b
6.76 ± 2.25 h
4.16 ± 2.25a
IALV
0.08 ±.031i
0.03 ± 0.002b
0.08 ± 0.007b
0.05 ± 0.002d
6.3 ± 1.52d
4.53 ± 0.87a
10.0 ± 3.25e
5.96 ± 1.62 g
IALVI
0.08 ± 0.012i
0.04 ± 0.002d
0.09 ± 0.007c
0.02 ± 0.002e
9.56 ± 1.72f
9.23 ± 2.33d
5.2 ± 2.21f
2.63 ± 1.22 h
IALVII
0.08 ± 0.30i
0.05 ± 0.005c
0.08 ± 0.007b
0.04 ± 0.002b
7.73 ± 1.22c
9.56 ± 2.33d
9.13 ± 3.75d
9.7 ± 3.15b
3.6 Endophytic bacteria enhance cadmium uptake in plants
Results showed an increase in Cd uptake by rice plants after inoculation with endophytic bacteria compared to control (Fig. 6). Inoculation of almost all bacterial isolates significantly increased in the Cd concentration in plant. However, inoculation of IALIII, IARII induced the Cd accumulation in plant significantly (p < 0.05) as compared to the inoculation of other isolates.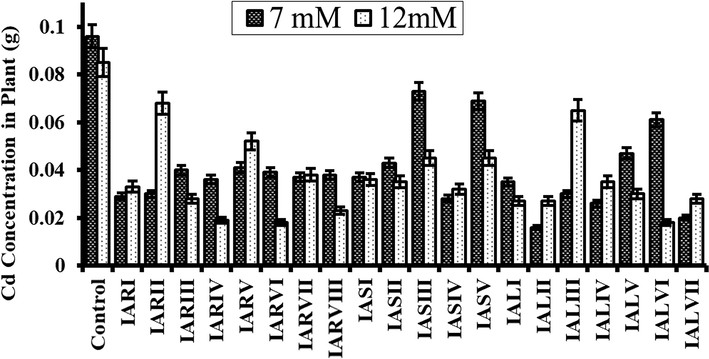
Enhanced uptake of 7 mM and 12 mM cadmium by rice inoculated plant as compared to control which was uninoculated. Error bars represent the standard deviation.
4 Discussion
Twenty isolates of endophytic bacteria were obtained based on the morphological characteristics of stems, leaves, and roots of the swat 1 rice variety. These isolates were tested on two levels of cadmium stress to see how they affected the growth of G4R1 rice. Biochemical characterization of selected isolates was performed. All isolates were screened for extracellular enzymes like amylase and catalase (Germaine et al., 2004). Eight isolates were catalase-positive and twelve were catalase-negative, whereas six endophytic bacteria isolates were catalase-positive (Hardoim et al., 2008). Various concentrations of the enzyme amylase were produced by all isolates, which converts starch to maltose.
Several isolates showed antibacterial activities ranging from 10 mm to 27 mm diameter zone of inhibition. IARVI showed the highest antimicrobial activity against Calcibacter, while IASII showed the lowest antimicrobial activity against xanthomonas. Jang et al. (2020) tested bacterial extract against various bacterial strains and found very significant antibacterial activities. There might be differences in the antibacterial activity of different bacterial extracts owing to their antibacterial components. Due to the chemical contents present in their extracts, endophytic bacteria play a significant role in traditional and commercial medicine. Studies have shown that the extracts of bacteria contain chemical constituents that are very effective against pathogenic bacteria (Ullah et al., 2020).
IAA, as signaling molecules played the main role in plant growth and development and helped nutrients uptake by improved roots length. Phytohormone production is slower in plants under stresses, hindering optimal growth of plant (Deveau et al., 2007). In the present study, almost all isolates were found positive for IAA production. Khan et al., (2015) reported different concentrations of IAA by five endophytic bacterial isolates and reported that that IAA induced the Cd uptake in plant tissues. Addition of exogenous IAA and miRNAs expression has been investigated to regulate the Cd, As and Cu and plants showed improved growth under heavy metal stress (Deveau et al., 2007).
Biotic and abiotic stresses affect the cereal crops inhibiting the quality and quantity of the crop. To maintain the physiology and metabolism, plants produce defensive proteins to control stress condition (Naik et al., 2009). Endophytic bacteria aided plant growth and development under biotic and abiotic stresses (Ullah et al., 2019). All twenty isolates were found growth promoters, showing increased shoots length, roots length, dry weight, and fresh weight, under different concentrations of Cd stress. The inoculation of bacteria was improved by accumulating Cd through intracellular and extracellular precipitation of Cd, and the metals were thereby decontaminated and immobilized in the bacterial cell body (Ma et al., 2015). Khan et al. (2015) also reported significant increase in root and shoot length, as well as dry and fresh weight when endophytic bacteria RSC-14 was inoculated to the plant exposed to Cd contamination. Studies have shown that endophytic bacteria inhibit metal phytotoxicity by a variety of mechanisms, including extracellular precipitation, intracellular sequestration, and accumulation of toxic metal ions. (Guo et al., 2010; Luo et al., 2011).
5 Conclusion
Twenty endophytic bacterial isolates were assessed for Cd accumulation and plant growth promotion against G4R1 rice plant grown under Cd stress (7 mM-12 mM). The enzymatic analysis showed the amylase and catalase activities in bacterial isolates. The bacterial isolates such as IASII and IARVI showed antibacterial activities ranging from 10 mm to 27 mm diameter of inhibition zone respectively. Inoculation of endophyte also improved plant growth and biomass in plants exposed to Cd as compared to uninoculated plants.
Acknowledgements
All types of support for this study was provided by the IBGE, The University of Agriculture, Peshawar, Pakistan
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Methylobacterium-induced endophyte community changes correspond with protection of plants against pathogen attack. PLoSOne.. 2012;7(10):e46802
- [Google Scholar]
- Assessment of Antimicrobial Activity of Endophytic Fungi Isolated from Justicia adhatoda L. UGB J. Plant Biol. Biotechnol.. 2016;1:19-23.
- [Google Scholar]
- Longterm storage of post-packaged bread by controlling spoilage pathogens using Lactobacillus fermentum C14 isolated from homemade curd.. 2017;12:e0184020
- Complete genome sequence of the sugarcane nitrogen- fixing endophyte Gluconacetobacter diazotrophicus Pal5. BMC Genomics. 2009;10:450-461.
- [Google Scholar]
- Changes in extract- ability of Cr and Pb in a polycontaminated soil after bioaugmentation with microbial producers of biosurfactants, organic acids and siderophores. Water Air Soil Pollut.. 2006;6(3):e4
- [Google Scholar]
- Bulgarelli, D., Rott, M., Schlaeppi, K., Ver Loren van, Themaat, E., Ahmadinejad, N., Assenza, F., Rauf, P., Huettel, B., Reinhardt, R., Schmelzer, E., Peplies, J., Gloeckner, F.O., Amann, R., Eickhorst, T., Schulze-Lefert, P., 2012. Revealing structure and assembly cues for Arabidopsis root-inhabiting bacterial microbiota. Nature 488 (7409):91-95.
- Complete genome sequence of Bacillus subtilis BSn5, an endophytic bacterium of Amorphophallus konjac with antimicrobial activity for the plant pathogen Erwinia carotovora subsp. carotovora. J. Bacteriol.. 2011;193:2070-2071.
- [Google Scholar]
- The mycorrhiza helper Pseudomonas fluorescens BBc6R8 has a specific priming effect on the growth, morphology and gene expression of the ectomycorrhizal fungus Laccaria bicolor S238N. New Phytol.. 2007;175:743-755.
- [Google Scholar]
- Colonization of poplar trees by gfp expressing bacterial endophytes. FEMS Microbiol Ecol.. 2004;48:109-118.
- [Google Scholar]
- Biological Activities of Alternaria Sp. Rl4 –A Potent Endophytic Fungus Associated with Rauvolfia Serpentina L. Benth. Asian J. Pharm. Clin. Res.. 2018;11:178-182.
- [Google Scholar]
- Properties of bacterial endophytes and their proposed role in plant growth. Trends Microbiol.. 2008;16:463-471.
- [Google Scholar]
- Jang, E.K., Jung, B. K., Park, G.-S., Khan, A. R., Hong, S.J., Park, Y.J, Kim, W.C., Shin, J.H., Al-Ghamdi, K.M.S., Al-Johny, B.O., Anwar, Y., Siddiqui, M. F., Ullah, I., 2020. Cloning and expression of the insecticidal toxin gene “tccB” from Photorhabdus temperata M1021 in Escherichia coli expression system. journal of Asia pacific entomol. 1-7.
- Improvement in phytoremediation potential of Solanum nigrum under cadmium contamination through endophytic-assisted Serratia sp. RSC-14 inoculation. Environmental Science and Pollution Research. 2015;22(18):14032-14042.
- [Google Scholar]
- Serpentine bacteria in uence metal translocation and bioconcentration of Brassica juncea and Ricinus communis grown in multi-metal polluted soils. Front. Plant Sci.. 2015;5:757.
- [Google Scholar]
- Malfanova, N., Lugtenberg, B., Berg, G., 2013. Bacterial endophytes: who and where, and what are they doing there? In: Molecular Microbial Ecology of the Rhizosphere; F. J de Bruijn, Ed. ch 36, Wiley- Blackwell, N. J Hoboken, USA. pp. 393-403.
- Interactions between plants and beneficial Pseudomonas spp.: exploiting bacterial traits for crop protection. Antonie Van Leeuwenhoek.. 2007;92:367-389.
- [Google Scholar]
- Naik, B. S., Shashikala, J., L Krishnamurthy, Y., 2009. Study on the diversity of endophytic communities from rice (Oryza sativa L.) and their antagonistic activities in vitro. Microbiological Research, 164(3) 290-296.
- Bacterial endophytes: recent developments and applications. FEMS microbiology letters. 2008;278(1):1-9.
- [Google Scholar]
- Genome survey and characterization of endophytic bacteria exhibiting a beneficial effect on growth and development of poplar trees. Appl. Environ. Microbiol.. 2009;75:748-757.
- [Google Scholar]
- Exploring the Insect Control and Plant Growth Promotion Potentials of Endophytes Isolated from Calotropis procera Present in Jeddah KSA. Natural Product and Communication.. 2020;15:1-9.
- [Google Scholar]
- Endophytic bacteria isolated from Solanum nigrum L., alleviate cadmium (Cd) stress response by their antioxidant potentials, including SOD synthesis by sodA gene. Ecotoxicology and Environmental Safety.. 2019;174:197-207.
- [Google Scholar]
- Complete genome sequence of the plant growth-promoting endophyte Burkholderia phytofirmans strain PsJN. J. Bacteriol.. 2011;193:3383-3384.
- [Google Scholar]
- Phytoremediation: plant-endophyte partnerships take the challenge. Curr Opin Biotechnol.. 2009;20:248-254.
- [Google Scholar]
- Characterization of a new Bacillus stearothermophilus isolate: a highly thermostable α-amylase producing strain. Appl Microbiol Biotechnol.. 1994;41:155-162.
- [Google Scholar]







