Changes in major flavonols and quercetin glycosides upon sprouting in onion cultivars
⁎Corresponding author at: Dept. of Food Technology and Nutrition, Lovely Professional University, Punjab, India. ishratmajid89@gmail.com (Ishrat Majid)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objective
This study was carried out to demonstrate the effect of sprouting on the major flavonols and quercetin glycosides in onion powders developed from four onion cultivars (Punjab White, Punjab Naroya, PRO-6 and Commercial) of Indian Origin.
Methods
The major flavonols summing up total flavonoids and quercetin glucosides comprising up to the total quercetin glucoside concentration were determined by High performance liquid chromatography and Fourier transform infrared (FTIR) spectroscopy.
Results and conclusions
The major flavonols were quercetin, myricetin and kaempferol, which were found to be significantly higher (p < 0.05) in all sprouted powders. Quercetin gylcosides also increased with sprouting, while non-significant decrease was found in Punjab Naroya and Commercial onion powders. FTIR analysis revealed the presence of functional groups with O–H and C–H bonds and the structural similarities and differences between onion powders was found following sprouting which were evident by the presence of certain sharp bands in sprouted samples. Quantitative analysis of flavonoids by HPLC showed that sprouted onion powders contained more phytochemicals than raw onion powder. This research suggests the potential of sprouting in onion for product development with enhanced bioactive compounds.
Keywords
Onion cultivar
Quercetin glycosides
Sprouting
High performance liquid chromatography
FTIR
Fingerprint
1 Introduction
The wide spread consumption of vegetables as a source of flavonoids (quercetin and quercetin glycosides) is due to their well established health claims. Amongst all the vegetables, onion is the widely consumed across the globe as the species is the richest source of quercetin and its conjugates (Majid et al., 2018a). The flavonoids reported in onion include majorly the flavonols viz, kaempferol, quercetin, isorhamnetin, and their glycosides as well as anthocyanins like, cyaniding glycosides, peonin and cyanidin (Majid et al., 2018b). The concentration of these non-nutrient antioxidants and secondary metabolites greatly varies in onion, depending on the variety, storage conditions, climatic conditions, and geographical origin (Majid and Nanda, 2017a). These variables bring about changes in flavonols, anthocyanins (Cools et al., 2010) and flavor-associated compounds (Uddin and MacTavish, 2003). The reported health benefits of quercetin and its glycoside conjugates includes the suppression of growth of cancerous cells as a result of their antioxidant capacity, chelating power and free-radical scavenging ability. Further, in context to understand the fate of flavonoids from foods it has been reported that inside human gut the fecalases hydrolyzes the corresponding quercetin glycosides to quercetin (Yoo et al., 2010). The principal flavonol quercetin present in the form of quercetin 4-monoglucoside and quercetin 3,4-diglucoside has been found to be effective against cardiovascular diseases (Caridi et al., 2007). The principal classes of flavonoids include flavonols and anthocyanins which are mostly present in glycosylated forms (Rodrigues et al., 2011). The chief flavonoid quercetin (3,3,4,5,7-pentahydroxyflavone; Q) is majorly present as quercetin glucoside (QG) with only a small fraction as aglycone (free) quercetin (Q). The quercetin components which constitute upto 80% of total quercetin (TQ) content are quercetin-3,4-diglucosde (Q3,4 G) and,quercetin-4-glucoside (Q4G) (Perez-Gregorio et al., 2014). Further, seventeen different compounds constitute upto 15% of the total quercetin concentration and out of that isorhamnetin-glucoside and quercetin-3-glucoside (Q3G) are major components (Grzelak et al., 2009). The extraction and quantification of the flavonoid components in plant extracts (onion) is thus of great interest and high-performance liquid chromatography (HPLC) coupled with UV detection is considered to be the method of choice (Merken and Beecher, 2000). The above mentioned studies clearly demonstrated the nutritional and health benefits of flavonoids in onion, justifying the increased interest on their usage in recent years. Numerous studies related to the presence of flavonoid compounds in onion has been reported (Caridi et al., 2007; Perez-Gregorio et al., 2011, 2010, 2014; Cools et al., 2010; Grzelak et al., 2009; Rodrigues et al., 2011; Merken and Beecher, 2000; Ramos et al., 2006) and limited literature related to the fate of these compounds due to sprouting is available (Majid et al., 2016, Majid and Nanda, 2017b). However, no single study has been reported so far related to the fate of the flavonoid components especially their glycosidic forms in sprouted food products. Therefore, the aim of the present study was undertaken to investigate the changes in flavonoid components especially their glycosidic forms in sprouted onion powders so as to enhance the utilization of sprouted onions for the development of new food products.
2 Materials and methods
2.1 Chemicals and reagents
The HPLC-grade acetonitrile was purchased from Sigma Aldrich (Missouri U.S.) and the standards quercetin, myricetin, kaempferol and quercetin glucosides from Acros Organics, New Jersey, USA.
2.2 Development of sprouted onion powders
The onion cultivars viz, Punjab Naroya, PRO-6, Punjab white, and Commercial obtained from P.A.U Ludhiana, India after storing them for three weeks at an average temperature of 15.22 °C, relative humidity of 80.2% and light intensity of 64 Lumen got sprouted. The sprouted onion cultivars were then utilized for the development of onion powders by freezing onion tissues at −20 °C, and then freeze drying to obtain powder (Majid et al., 2018a).
2.3 High-performance liquid chromatography (HPLC) analysis of major flavonols and quercetin glucosides
The major three flavonols, quercetin, myrecetin and kaempferol and quercetin glucosides from powder sample (2.5 g) of each variety were extracted by 60% ethanol (10 ml) for 24 h at room temperature. The extract was kept under refrigerated conditions after filtering through PTFE membrane filter till chromatographic analysis. The HPLC analysis of the extract was performed using the method described by Majid et al. (2016). The flavonoids were detected at 360 nm using HPLC set with a quaternary pump and coupled with UV absorbance detector. The samples were injected at the rate of 20 µl using mobile phase of concentration: acetonitrile (A) and 0.5% formic acid in water (B): 0–10 min, 20% B; 10–15 min, 20–80% B; 15–22 min, 80–20% B. The standards were used to identify and quantify the flavonols based on their retention time and spectral characteristics.
2.4 FTIR analysis
FTIR spectroscopy was used to assess the effects of sprouting on the infrared spectra of onion powders. FTIR spectra were obtained using an Agilent Technologies Cary 660 FTIR spectrometer (Ettlingen, Germany). The spectra of raw and sprouted onion powders were recorded in absorbance mode from 4000 to 500 cm−1, co-adding 128 scans at 8 cm−1 resolution. The anhydrous potassium bromide was used to prepare potassium bromide (KBr) pellets in order to obtain spectra of onion powder samples. The fine onion powder samples taken in the proportion of 1:15 w/w of KBr and pressed under hydraulic pressure were placed into pellet-forming mould. The IR spectrum of thin film of powder samples was recorded at 1800–800 cm−1 at room temperature (Correia et al., 2008).
3 Statistical analysis
The significant statistical effects of sprouting and variety on determined properties were verified by two way ANOVA. The average values of data were analyzed by Duncan's multiple range test using Statistica.v.12. Each parameter was determined thrice and significant differences among the mean values were considered at a 0.05 confidence level.
4 Results and discussion
Onion variety as well as sprouting treatment was found to significantly (P < 0.05) effect the content of major flavonols and individual glucosides except Isorhamnetin 3,4ꞌ-diglucoside content in all the onion powders as depicted by the two way analysis of data.
4.1 Major flavonols
The concentration of major flavonols of both raw and sprouted powders is presented in Table 1, and the HPLC graph showing the presence of major flavonols for sprouted and raw PRO-6 powder is presented in (Fig. 1 a & b). The quercetin content in raw as well as sprouted onion powders was found in the following decreasing order of PRO-6, Punjab Naroya (PN), Punjab White (PW) and commercial (CM). While comparing the raw and sprouted powder samples the quercetin content showed significant increase in powder from Punjab Naroya (185.02–201.01 mg/kg) and PRO-6 (203.02–259.02 mg/kg) with non significant increase in Commercial (65.09–68.08 mg/kg) and non significant decrease in Punjab White onion powder (122–06-119.02 mg/kg). The kaempferol content was reported to be highest in PRO-6 onion powder followed by Commercial, Punjab Naroya and Punjab White among raw powders. While in case of sprouted powders the kaempferol content was also highest in PRO-6 onion powder followed by Commercial, Punjab White and Punjab Naroya powder. The kaempferol content with sprouting was also found to significantly increase in Punjab White (3.46–5.16 mg/kg) and PRO-6 (11.79–15.87 mg/kg) onion powder while significant decrease in Punjab Naroya (9.56–4.11 mg/kg) and non significant decrease was found in Commercial onion powder (9.81–8.67 mg/kg). The myricetin content in both raw and sprouted powders followed the same order as PRO-6 onion powder followed by Punjab Naroya (PN), Punjab White (PW) and Commercial (CM) onion powder. The change in myricetin content as a result of sprouting was significant increase in Punjab Naroya (28.15–32.75 mg/kg), PRO-6 (31.18–37.46 mg/kg) and Commercial (1.74–2.13 mg/kg) onion powder with non significant decrease in Punjab White onion powder (26.18–25.84 mg/kg). The concentration of total flavonoids was found in the decreasing order of PRO-6 onion powder followed by Punjab Naroya, Punjab White and Commercial onion powder in raw as well as sprouted onion powders. The total flavonoid content was found to significantly increase in Punjab Naroya (222.73–237.87 mg/kg) and PRO-6 (245.99–312.35 mg/kg) onion powder with non significant increase in Commercial onion powder (76.64–78.88 mg/kg) and non significant (151.70–150.02 mg/kg) decrease in Punjab White. From the above results it is obvious that significant differences (P < 0.05) among onion powders was found in their individual flavonol as well as total flavonoid content. The difference in the content of flavonol as well as total flavonoid content also with in a particular variety was irregular that is statistically significant (P < 0.05) increase or decrease was noticed which is consistent with the studies reported by Majid et al. (2016). The results are in accordance with those reported in onion powders in which increase in flavonoid components was observed due to sprouting (Majid and Nanda, 2017b). The change in the flavonoid profile amongst sprouted and raw powders is ascribed to the extent of differences in the metabolism during the sprouting process probably because of varietal differences (Arora et al., 2010; Majid and Nanda, 2017a, 2017c).
| Punjab White | Punjab Naroya | PRO-6 | Commercial | |||||
|---|---|---|---|---|---|---|---|---|
| Flavonoids (mg/kg) | Raw | Sprouted | Raw | Sprouted | Raw | Sprouted | Raw | Sprouted |
| Quercetin | 122.06 ± 2.40cP | 119.02 ± 0.64CP | 185.02 ± 1.68bQ | 201.01 ± 2.08BP | 203.02 ± 1.96aQ | 259.02 ± 1.12AP | 65.09 ± 1.99dP | 68.08 ± 1.47DP |
| Kaempferol | 3.46 ± 0.31cQ | 5.16 ± 0.32CP | 9.56 ± 0.72bP | 4.11 ± 0.16CQ | 11.79 ± 0.87aQ | 15.87 ± 1.15AP | 9.81 ± 1.14bP | 8.67 ± 0.40BP |
| Myricetin | 26.18 ± 1.43bP | 25.84 ± 0.94CP | 28.15 ± 0.92bQ | 32.75 ± 1.42BP | 31.18 ± 1.49aQ | 37.46 ± 0.86AP | 1.74 ± 0.07cQ | 2.13 ± 0.22DP |
| Total Flavonoids | 151.70 ± 1.38cP | 150.02 ± 0.64CP | 222.73 ± 1.10bQ | 237.87 ± 1.22BP | 245.99 ± 1.44aQ | 312.35 ± 1.04AP | 76.64 ± 1.07dP | 78.88 ± 0.69DP |
Results are expressed as mean values ± standard deviations. Means in a row with same superscripts (a,b,c,d) for raw onion powder and (A,B,C,D) for sprouted onion powder are not significantly different (P < 0.05). Means in a row with same superscripts (P,Q) within a particular variety are not significantly different (P < 0.05).
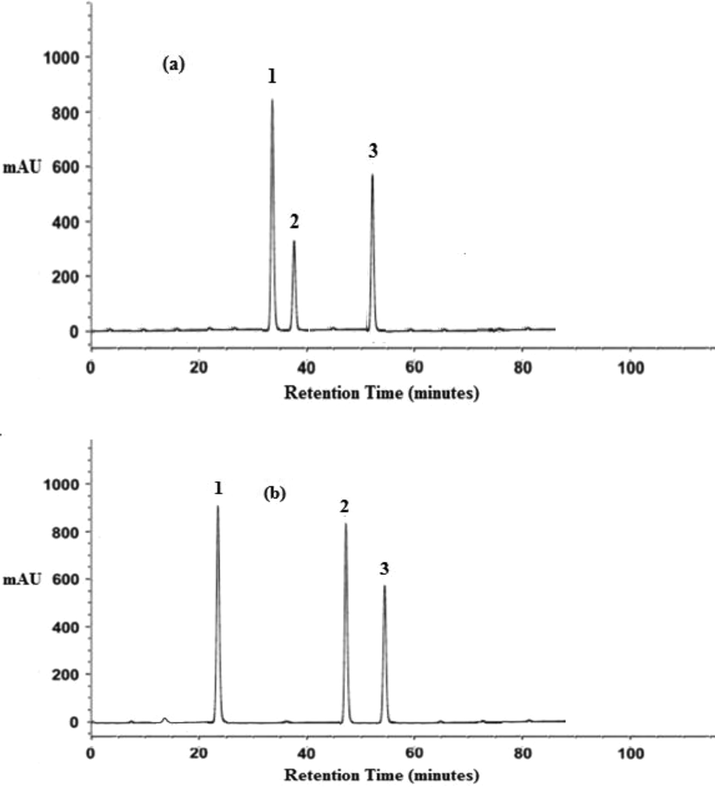
- Typical HPLC chromatograms depicting the change in major flavonols with sprouting in (a) PRO-6 Raw onion powder (b) PRO-6 Sprouted onion powder (1: Quercetin 2: Myricetin 3: Kaempferol).
4.2 Quercetin glycosides
The concentration of glycosidic forms of major flavonols in sprouted and raw onion powders is presented in Table 2, and the HPLC graph showing the presence of glycosidic forms for sprouted and raw PRO-6 onion powder is presented in (Fig. 2 a & b). The concentration of quercetin 3- glucoside was found highest in PRO-6 onion powder followed by Punjab Naroya, Commercial and Punjab White powder both in raw as well as sprouted onion powders. The quercetin 3- glucoside was found to be significantly higher in sprouted PRO-6 (3.91 mg/kg) and Commercial onion powder (2.03 mg/kg) than their corresponding raw PRO-6 (2.63 mg/kg) and Commercial (1.81 mg/kg) powder samples. While in Punjab White powder it non significantly increased (0.81 mg/kg) in sprouted powder than raw onion powder (0.75 mg/kg) and in Punjab Naroya powder it non significantly decreased (2.54 mg/kg) in sprouted powder than raw onion powder (2.61 mg/kg). The quercetin 4- glucoside in raw onion powders was highest in PRO-6 onion powder followed by Punjab White (PW), Punjab Naroya (PN) and Commercial (CM) onion powder. In case of sprouted onion powders the trend in the decreasing order was like PRO-6 onion powder followed by Punjab Naroya (PN), Punjab White (PW) and Commercial (CM) onion powder. The quercetin 4- glucoside was found significantly higher in sprouted PRO-6 onion powder (81.45 mg/kg) than PRO-6 raw onion powder (74.91 mg/kg) and in sprouted Punjab White powder quercetin 4- glucoside was found significantly lower (34.67 mg/kg) than Punjab White raw onion powder (49.38 mg/kg). The quercetin 3,4-diglucoside in raw onion powders was highest in PRO-6 onion powder followed by Punjab Naroya (PN), Punjab White (PW) and Commercial (CM) onion powder and in sprouted onion powders it was also highest in PRO-6 onion powder followed by Punjab White (PW), Punjab Naroya (PN) and Commercial (CM) onion powder. The quercetin 3,4-diglucoside was significantly higher in sprouted Punjab White (154.69 mg/kg) and PRO-6 (221.74 mg/kg) onion powder than their counter raw Punjab White (136.28 mg/kg) and PRO-6 (205.91 mg/kg) powder samples. In Punjab Naroya onion powder (158.35–147.64 mg/kg) and Commercial onion powder (96.70–44.37 mg/kg) a significant decrease in quercetin 3,4-diglucoside was found. The quercetin 7,4-diglucoside in raw as well as sprouted powder samples followed the trend of decreasing order as PRO-6 onion powder followed by Punjab Naroya (PN) , Punjab White (PW) and Commercial (CM) onion powder. The quercetin 7,4-diglucoside in all the onion powders was significantly higher in sprouted samples than raw powder samples. The increased concentrations of both total quercetin and quercetin glucosides in sprouted onion powders was attributed to the increase in concentrations of quercetin, kaempferol and myricetin (Majid and Nanda, 2017a; Majid et al., 2016). The Isorhamnetin 4-diglucoside content among raw onion powders was highest in powder from Punjab Naroya (0.82 mg/kg) and then followed by PRO-6 (0.76 mg/kg), Punjab White (0.73 mg/kg) and Commercial (0.51 mg/kg) onion powder. In sprouted powder samples it was also highest in Punjab Naroya powder (1.64 mg/kg) but sprouted onion powders from both PRO-6 (1.16 mg/kg) and Punjab White (1.16 mg/kg) powder had similar content of Isorhamnetin 4-diglucoside followed by Commercial (0.77 mg/kg) onion powder. The change in the content of Isorhamnetin 4-diglucoside due to sprouting was observed to be significant increase in Punjab White (0.73–1.16 mg/kg), Punjab Naroya (0.82–1.64 mg/kg) and PRO-6 (0.76–1.16 mg/kg) onion powders with only non significant increase in Commercial onion powder (0.51–0.77 mg/kg). The Isorhamnetin 3,4- diglucoside was highest in PRO-6 onion powder followed by Punjab Naroya, Commercial and Punjab White among raw onion powder samples. However in case of sprouted powder samples it was found to be highest in Punjab Naroya powder followed by PRO-6, Commercial and Punjab White sprouted powders. The Isorhamnetin 3,4- diglucoside was found to have significantly increased after sprouting in powders from Punjab White (1.75–1.97 mg/kg), Punjab Naroya (2.61–5.28 mg/kg) and Commercial (1.96–2.37 mg/kg) onion powders with only non significant increase in PRO-6 onion powder (4.15–4.92 mg/kg). The total glycosides concentration in case of raw as well as sprouted onion powders were documented highest in PRO-6 onion powder followed by Punjab Naroya onion powder, Commercial onion powder and Punjab White onion powder respectively. The concentration of total glycosides ranged from 189.60 to 290.99 in raw onion powders where as in sprouted onion powders it ranged from 84.07 to 316.83 mg/kg. The variation in the content of different flavonoid compounds is influenced by the various extrinsic factors including varietal variations, storage and exposure to post harvest conditions (sprouting) as reported by Ko et al. (2015). While comparing the raw and sprouted onion powders the total glycoside concentration was significantly found to increase in PRO-6 onion powder (290.99–316.83 mg/kg) with non significant increase in Punjab White onion powder (189.60–194.24 mg/kg). However, non-significant decrease in Punjab Naroya onion powder (202.64–198.10 mg/kg) and significant decrease in Commercial onion powder (134.95–84.07 mg/kg) was found. The change in individual as well as total glycosides concentration is attributed to the process of sprouting. The pattern of inconsistent change that is increase and decrease in concentration is further justified by the difference in metabolic pathways due to their varietal differences. The onion powder from red onions contained more total quercetin glucoside concentration as compared to onion powder from White onion variety which is also claimed in previous studies (Kwak et al., 2017).
| QG (mg/kg) | Punjab White | Punjab Naroya | PRO-6 | Commercial | ||||
|---|---|---|---|---|---|---|---|---|
| Raw | Sprouted | Raw | Sprouted | Raw | Sprouted | Raw | Sprouted | |
| Quercetin 3- glucoside | 0.75 ± 0.08cP | 0.81 ± 0.09CP | 2.61 ± 0.18aP | 2.54 ± 0.15BP | 2.63 ± 0.19aQ | 3.91 ± 0.59AP | 1.81 ± 0.10bQ | 2.03 ± 0.08DP |
| Quercetin 4- glucoside | 49.38 ± 1.78bP | 34.67 ± 1.91CQ | 36.58 ± 1.42cP | 38.82 ± 1.83BP | 74.91 ± 1.41aQ | 81.45 ± 0.95AP | 33.02 ± 2.68cP | 33.40 ± 1.75CP |
| Quercetin 3,4-diglucoside | 136.28 ± 8.24cq | 154.69 ± 7.38Bp | 158.35 ± 3.65 bp | 147.64 ± 7.47 Bq | 205.91 ± 1.92bq | 221.74 ± 0.58Ap | 96.70 ± 1.96dp | 44.37 ± 0.85Cq |
| Quercetin 7,4-diglucoside | 0.71 ± 0.10dq | 0.94 ± 0.05Cp | 1.67 ± 0.12bq | 2.18 ± 0.08Bp | 2.63 ± 0.11aq | 3.65 ± 0.19Ap | 0.92 ± 0.06cq | 1.13 ± 0.02Cp |
| Isorhamnetin 4- diglucoside | 0.73 ± 0.07aq | 1.16 ± 0.35Bp | 0.82 ± 0.10aq | 1.64 ± 0.24Ap | 0.76 ± 0.15aq | 1.16 ± 0.08Bp | 0.51 ± 0.09 bp | 0.77 ± 0.15Cp |
| Isorhamnetin 3,4- diglucoside | 1.75 ± 0.15bq | 1.97 ± 0.18Cp | 2.61 ± 0.32bq | 5.28 ± 0.13Ap | 4.15 ± 1.13ap | 4.92 ± 0.29Ap | 1.96 ± 0.02bq | 2.37 ± 0.12Bp |
| Total quercetin glucoside concentration | 189.60 ± 6.40cp | 194.24 ± 7.19Bp | 202.64 ± 6.44 bp | 198.10 ± 3.67Bp | 290.99 ± 7.17aq | 316.83 ± 1.72Ap | 134.95 ± 2.85dp | 84.07 ± 1.71Cq |
Results are expressed as mean values ± standard deviations. Means in a row with same superscripts (a,b,c,d) for raw onion powder and (A,B,C,D) for sprouted onion powder are not significantly different (P < 0.05). Means in a row with same superscripts (P,Q) within a particular variety are not significantly different (P < 0.05).
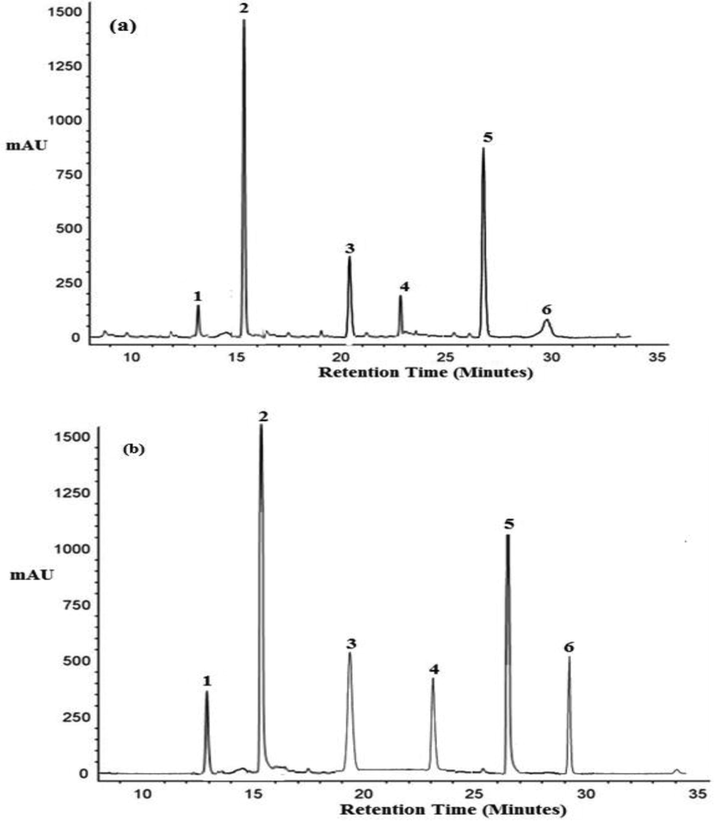
- Typical HPLC chromatograms depicting the change in Quercetin glucosides with sprouting in (a) PRO-6 Raw onion powder (b) PRO-6 Sprouted onion powder (1. Quercetin 7,4-diglucoside, 2. quercetin 3,4-diglucoside 3. Isorhamnetin 3,4- diglucoside 4. Quercetin 3- glucoside 5. Quercetin 4- glucoside, 6. Isorhamnetin 4- diglucoside).
4.3 FTIR analysis
FTIR analysis in association with HPLC analysis was used to determine sprouting effect on onion powder composition. The characteristic absorption spectrum is obtained at particular frequencies and it is possible to acquire same spectrum for two different molecules. The difference in spectrum is usually found within in the frequency range of 1600–600 cm−1 that is represented as the finger print. The study of spectra of raw and sprouted onion powders was used to compare them especially in their finger print region. The representative Infrared spectra of PNR raw and sprouted onion powder sample presented in Fig. 3 (PNR & PNS) revealed the presence of functional groups with O–H and C–H bonds. A comparison of Infrared spectra of raw (PWR, PNR, PRO-6R, CMR) and sprouted (PWS, PNS, PRO-6S, CMS) onion powders determined the structural similarities and differences between them. The structural changes in sprouted as well as raw onion powders found by FTIR analysis is in coherence with the structural changes observed by scanning electron microscopy in onion powders as reported by Majid et al. (2018c). FTIR spectrum of the sprouted onion powders showed structural changes which is evidenced by the presence of absorption peaks in sprouted Punjab Naroya powder at 17744.449 and 619.042 cm−1. The same pattern of changes has been found in sprouted onion powders from other three onion varieties and is well supported by the findings of Sreelekshmi et al. 2018. This suggests that the structural degradation during sprouting probably enhanced the bioactive potential of onion powders. Nevertheless broader peaks in both sprouted and raw powder samples were prominent in the spectrum. The analysis of infrared spectra of sprouted and raw onion powders depicted the presence of O–H bonds as strong peak was observed for Punjab White raw powder at 3414.567 cm−1, Punjab White sprouted powder at 3413.217 cm−1, Punjab Naroya raw powder at 3393.335 cm−1, Punjab Naroya sprouted powder at 3407.290 cm−1, PRO-6 raw powder at 3416.645 cm−1, PRO-6 sprouted powder at 3414.261 cm−1, Commercial raw powder at 3431.078 cm−1 and Commercial sprouted onion powder at 3400.760 cm−1. The compounds containing C–H bonds were present in both the types of powders (sprouted and raw) in the region of 2927.128 cm−1 for Punjab White raw onion powder, 2925.703 cm−1 for Punjab White onion sprouted powder, 2926.259 cm−1 for Punjab Naroya raw onion powder, 2926.500 cm−1 for Punjab Naroya sprouted onion powder, 2925.803 cm−1 for PRO-6 raw onion powder, 2928.685 cm−1 for PRO-6 sprouted onion powder, 2932.621 cm−1 for Commercial raw onion powder and 2926.701 cm−1 for Commercial sprouted onion powder. The raw and sprouted powder samples also contained amines 1 band in the region of 1600–600 cm−1. The spectral analysis of onion powders revealed the changes in signals of both bending and stretching vibrations due to sprouting. These findings are consistent with the HPLC results that evidenced the change in functional components in both sprouted and raw onion powders.

- The representative infra red spectra of: PNR (Punjab Naroya Raw Onion Powder) & PNS (Punjab Naroya Sprouted Onion Powder).
5 Conclusion
In the present study the combination of HPLC and FTIR spectroscopy were efficient methods to examine the changes in major flavonols and quercetin glycosides due to sprouting in onion powders. The sprouting process was found to make functional components more extractable and more accessible thereby changing the concentrations was confirmed. HPLC technique was used to extract and separate six quercetin glycosides in sprouted and raw onion powder samples. The difference in the concentration of major flavonols as well as quercetin glycosides amongst onion powders was found. A distinctive pattern in total as well as quercetin glycosides were obtained between onion powders which is ascribed to the process of sprouting. The findings of this study suggested the use of sprouted onion products as a potential source of antioxidants on daily basis for humans. This is also of paramount importance in current functional food market, where improved functional components is desired. Thus sprouted onion based functional foods can be developed by inexpensive and suitable method at domestic and industrial levels.
Acknowledgments
The authors extend their appreciation to the Researchers supporting project number (RSP-2020/193) King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Effect of germination and probiotic fermentation on nutrient composition of barley based food mixtures. Food Chem.. 2010;119:779-784.
- [Google Scholar]
- Profiling and quantifying quercetin glucosides in onion (Allium cepa L.) varieties using capillary zone electrophoresis and high performance liquid chromatography. Food Chem.. 2007;105:691-699.
- [Google Scholar]
- Fate of flavonoids in the outer skins of onion (Allium cepa L.) throughout curing. J. Agric. Food Chem.. 2010;58:11709-11715.
- [Google Scholar]
- Protein profile and malt activity during sorghum germination. J. Sci. Food Agric.. 2008;88:2598-2605.
- [Google Scholar]
- Content of quercetin glycosides and fructooligosaccharides in onion stored in a cold room. Eur. Food Res. Technol.. 2009;228:1001-1007.
- [Google Scholar]
- Effect of different exposed lights on quercetin and quercetin glucoside content in onion (Allium cepa L.) Saudi J. Biol. Sci.. 2015;22:398-403.
- [Google Scholar]
- Variation of quercetin glycoside derivatives in three onion (Allium cepa L.) varieties. Saudi J. Biol. Sci.. 2017;24:1387-1391.
- [Google Scholar]
- Impact of sprouting on the degradation kinetics of color and vitamin C of onion powder packaged in different packaging materials. J. Food Process. Preserv. 2018
- [CrossRef] [Google Scholar]
- Moisture sorption isotherms and quality characteristics of onion powder during storage as affected by sprouting. J. Food Measur. Charact. 2018
- [CrossRef] [Google Scholar]
- Rheological, thermal, micro structural and functional properties of freeze dried onion powders as affected by sprouting. Food Bio Sci.. 2018;22:105-112.
- [Google Scholar]
- Majid, I., Nanda, V., 2017a. Instrumental texture and flavonoid profile of paste developed from sprouted onion varieties of Indian origin. Intl. J. Food Prop. 11, 2511-2526.
- Majid, I., Nanda, V., 2017b.Total phenolic content, antioxidant activity and anthocyanin profile of sprouted onion powder. Acta Alimentaria, An Intl. J. Food Sci. doi.org/10.1556/066.2017.0006.
- Effect of sprouting on physical properties, morphology and flowability of onion powder. J. Food Measur. Charact.. 2017;11:2033-2042.
- [Google Scholar]
- Effect of sprouting on physico-chemical, antioxidant and flavonoid profile of onion varieties. Intl J. Food Sci. Technol.. 2016;51:317-324.
- [Google Scholar]
- Measurement of food flavonoids by high-performance liquid chromatography: a review. J. Agric. Food Chem.. 2000;48:577-599.
- [Google Scholar]
- Changes in antioxidant flavonoids during freeze-drying of red onions and subsequent storage. Food Control.. 2011;22:1108-1113.
- [Google Scholar]
- Identification and quantification of flavonoids in traditional cultivars of red and white onions at harvest. J. Food Comp. Anal.. 2010;23:592-598.
- [Google Scholar]
- Increasing the added-value of onions as a source of antioxidant flavonoids: a critical review. Crit. Rev. Food Sci. Nutr.. 2014;54:1050-1062.
- [Google Scholar]
- Antibacterial and antioxidant activities of quercetin oxidation products from yellow Onion(Allium cepa) skin. J. Agric. Food Chem.. 2006;54:3551-3557.
- [Google Scholar]
- Effect of meteorological conditions on antioxidant flavonoids in Portuguese cultivars of white and red onions. Food Chem.. 2011;124:303-308.
- [Google Scholar]
- Production of coconut sprout wine using Saccharomyces cerevisiae and its physico-chemical analysis. MOJ Food Process. Technol.. 2018;6:445-449.
- [Google Scholar]
- Controlled atmosphere and regular storage induced changes in S-alk(en)yl cysteine-l-sulfoxides and alliinase activity in onion bulbs (Allium cepa L. cv. Hysam) Postharvest Biol. Technol.. 2003;28:239-245.
- [Google Scholar]
- Quantification of quercetin glycosides in 6 onion cultivars and comparisons of hydrolysis–HPLC and spectrophotometric methods in measuring total quercetin concentrations. J. Food Sci.. 2010;75:160-165.
- [Google Scholar]







