Translate this page into:
Challenging multidrug-resistant urinary tract bacterial isolates via bio-inspired synthesis of silver nanoparticles using the inflorescence extracts of Tridax procumbens
⁎Corresponding authors. soona70@yahoo.com (Mysoon M. Al-Ansari), ranjitspkc@gmail.com (A.J.A. Ranjitsingh)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The increasing drug resistance pattern in bacterial pathogens promotes the need to find out alternative strategies to ensure human health. In the imperative lookout for effective drugs to combat multidrug-resistant bacteria, silver nanoparticles (AgNPs) are given priorities. Hence in the present approach, AgNPs were synthesized using the extract of the inflorescence of a medicinal plant, and its antibacterial activity against multidrug-resistant uropathogens was studied. For the synthesis of AgNPs, the inflorescence of a medicinal plant Tridax procumbens was subjected to a microwave irradiation technique. The characteristics of the synthesized nanoparticles (NPs) were analyzed by using UV–visible spectroscopy (UV–Vis), Dynamic light scattering device (DSL), Scanning electron microscope (SEM), Fourier-transform infrared (FTIR) spectroscopy and Zeta potential analyzer. The synthesized AgNPs were with unique optical morphology and semi-spherical shape having irregular contour with the size range 40.0–52.5 nm. The bacterial isolates Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Gram-positive Staphyloccocus saprophyticus from urinary tract infected persons that showed resistance to more than ten antibiotics were chosen for AgNPs impact analysis. The mean diameter of zone of inhibition (in mm) for the different isolates at the dose of 50 µg/mL concentration showed a maximum for S. saprophyticus (21.0 ± 1.7 mm)followed by P. aeruginosa (18.0 ± 1.3 mm), K. pneumoniae (18.0 ± 0.09 mm) and E. coli (17.0 ± 1.70). The MIC values for the isolates showed a minimum for S. saprophyticus (2.5 µg/mL) and a maximum for E. coli (55.5 µg/mL). The results show that the T. procumbens phytochemicals inspired silver nanoparticles can be explored further to develop useful antibiotics.
Keywords
Silver nanoparticles
Antibiotics
UTI
Drug resistance
Uropathogenic
Tridax procumbens
1 Introduction
Urinary tract infection (UTI) is a growing health issue among men and women. The women are primary victims of UTI due to their biological conditions (Miaomiao et al., 2019). UTIs cause a significant amount of morbidity and mortality. Of the different etiological agents, Enterobacteriaceae, particularly E. coli, is the most common pathogen that causes UTIs (Zeyaullah and Kaul, 2015). In the Kingdom of Saudi Arabia, 25% of all infections seen in the emergency department is because of community-acquired urinary tract infections (Alanazi et al., 2019). The UTIs constitute a significant burden on healthcare systems, and its prevalence ranges between 1.4% and 5.1%. Globally catheter-associated urinary tract infections (CAUTI) affect more than 150 million people annually (Ozturk and Murt, 2020). UTI bacterial isolates are mostly resistant to many drugs (Christy et al., 2019). Antibiotic-resistance caused by multidrug-resistant (MDR) microbes is becoming a severe threat to human health globally. MDR pathogens were the only significant reason identified for de-escalation failure on hospitalized patients (Alshareef et al., 2020). MDR caused by Gram-negative bacteria leads to higher mortality rates than Gram-positive bacteria (Gandra et al., 2019). The growing use and abuse of antibiotics has led to the emergence of antimicrobial-resistant microbes (AMR). As it is caused by hard-to-treat with an existing therapeutic agent, there is an imperative need to search alternative therapeutics to combat AMR (Thombre et al., 2019). Mostly the AMR resistance genes (ARG) are dispersed through horizontal gene transfer mediated by genetic elements (Thombre et al., 2016). Compounds from marine seaweeds, phytochemicals antimicrobial peptides, and proteins are reported to possess antibacterial properties (Dutta et al., 2007; Thombre et al., 2016). Marine natural products and their bioactive compounds are said to have the potential to be developed as effective drugs against infectious diseases in this AMR era (Thombre et al., 2019; Liu et al., 2019). Baptista et al. (2018) highlighted the importance of nano natural drugs for drug-resistant microbes. Giau et al. (2019) proposed the treatment of pathogenic infections using nano-drug delivery vehicles. Regí and González (2019) reported the importance of nanomaterials as an alternative to infection with resistant microbes. Weldrick et al. (2019) proposed the administration of antibiotics combining with nanocarriers to get better control over drug-resistant bacteria. Silver nanoparticles with unique optical, electrical, and thermal properties can be incorporated into a wide array of products to use it as carrier molecules. Nanoparticles synthesized using plant extracts are reported to be good antibacterial agents against drug-resistant microbes (AlSalhi et al., 2016’ Devanesan et al., 2020, 2018; Valsalam et al., 2019; Mariselvam et al., 2014). The well documented antimicrobial activity of silver nanoparticles has led to the development of novel applications which made them as an alternative to antibiotics (Durai et al., 2014; Devanesan et al., 2020).
T. procumbens L. Taxonomic Serial No.: 38,575 can be weedy according to the authenticate with a taxonomist. Tridax procumbens L. plants widely distributed with different names [National Plant Data Center]. The plant T. procumbens and its parts are reported to have an excellent antibacterial activity, antioxidant activity, antitumor activity and several other pharmacological properties (Jachak et al., 2017; Beck et al., 2018). T. procumbens L. leaf extract plays a significant role in reducing the gene expressions (TNF-α and COX-2) in induced inflammation sites in Swiss albino mice (Berlin-Grace et al., 2019). AgNPs synthesized using T. procumbens showed an excellent antibacterial activity by increasing protein leakage at the cell level and nucleic acid degradation (Rani et al., 2020). Hence in the present study, the inflorescence extract T.procumbens was used to synthesis silver nanoparticles. The prepared nanoparticles were tested for antibacterial activity against the bacterial isolates having resistance to more than ten antibiotics. The multidrug-resistant bacteria isolated from patients with urinary tract infection were used. It is decided to test whether the silver nanoparticles prepared by using the medicinal plant T. procumbens extracts can be of any use to inhibit the growth of the UTI inhabiting multidrug-resistant bacteria that pose a severe problem for treatment.
2 Materials and methods
2.1 Plant extract preparation
The chosen plant T. procumbens was collected from Western Ghats regions in Tirunelveli belt of Tamil Nadu South India. The inflorescence of T. procumbens were removed using a sterilized knife and washed several times in running tap water and then followed with deionized water. Two hundred grams of the T. procumbens inflorescence, and 400 mL deionized water was taken in a 1 L Erlenmeyer flask. The mixture was heated in at 100˚C for 2 h. The mix of the solution filtered with the help of the Whatman No.1 filter paper. The pure extract was retained of the synthesis process. The extract and its application in silver nanoparticles preparation was made as explained earlier, (Devanesan et al., 2018).
3 Synthesis of AgNPs using Tridax procumbens extract
The inflorescence of the medicinal plant T. procumbens was used to prepare the extract. Twenty-five milliliter of the extract of the inflorescence was taken in a 100 mL flask, and 25 mL of AgNO3 (10 mM) solution was added to it. The solution was kept in an ultrasonic bath for 30 min, and it was subjected to 360 W microwave irradiation for 5 min. The mixture was then centrifuged for 20 min at 12000 rpm, and the organic residues were removed. The pellets were washed three times with ethanol. The pellets were powdered to get nanoparticles, and the powdered nanoparticles were lyophilized and kept at room temperature for further work.
4 Characterization of silver oxide nanoparticles
The formed Ag-NPs were confirmed using Ultraviolet–visible (UV–Vis) (ThermoScientificMultiscanSpectrum1500, USA) study with a range of 200–800 nm. The functional groups on AgNP were further studied with FTIR spectroscopy using in the field of 400–4000 cm (Shimadzu, Japan). The morphology and properties of AgNPs were measured by SEM (Shimadzu, Japan), Dynamic light scattering device (DSL), and Zeta potential analyzer (Nano ZS-90 Malvern Instruments, England).
5 Antibacterial study
5.1 Bacterial isolation and characterization
The bacterial isolates that showed maximum resistance were isolated and were inoculated on Nutrient agar, blood agar, and Mac Conkey agar and incubated at 37 °C for 24 h. After the incubation period, the isolated colonies were further purified and sub cultured. To select the pure colonies of the isolates nutrient agar, and 5% sheep blood agar, were used. After the incubation period preliminary identification of each isolate was carried out based on morphological, cultural and biochemical characteristics. Gram-staining, capsule staining and motility test were performed for morphological characterization The growth pattern characteristic for each isolate was traced using different culture media including Nutrient agar, Bismuth Glycine Glucose Yeast agar, MacConkey agar, Eosine Methylene Blue agar, Citrimide agar, Mannitol Salt agar, and Blood agar base with 5% sheep blood supplementation. For the identification, the biochemical characteristics of each isolate was measured using the biochemical parameters viz., sugar fermentation (lactose, glucose, mannitol, maltose, sucrose and xylose), TSI, IMViC (indole, MR, VP, citrate) oxidase, catalase and nitrate tests Further for E. coli identification, selective media such as Eosin methylene blue agar (EMB) was used After the preliminary identification the bacterial isolates identified specifically using automated bacterial identification unit. This was performed in Vivek clinical laboratory and Research centre, Nagercoil Tamilnadu, India using with the Vitek 2 compact (bioMérieux Inc. USA) system using GP ID REF21342 (identification-Gram-positive bacteria) and GN ID REF21341 (identification-Gram-negative bacteria) cards. The manufacturer’s instructions were strictly followed in all test protocol. For the experiments, four bacterial strains that showed resistance to more than ten antibiotics were selected. The isolated strains are E. coli, K. pneumoniae, S. saprophyticus, and P. aeruginosa. Of the four isolates except for S. saprophyticus all the other three are gram negative bacteria. The isolates were recruited from early morning midstream urine of males diagnosed with UTI. The urine sample of infected patients was collected from the midstream urine, and the bacteria were isolated, identified, and sensitivity study was made using more than sixteen antibiotics. One strain from each selected bacterial species that showed resistance to a maximum number of ten antibiotics were identified for further antibacterial potency testing using the T. procumbens bio-inspired silver nanoparticles. For antimicrobial study, five different doses of Ag-NPs viz., 10, 20, 30, 40, and 50 µg/mL were used. The inoculum of UTI bacterial isolates identified were grown overnight in the Mueller-Hinton medium at 37 °C experimental protocol was followed (Alharbi and Alarfaj, 2020). The suspension of Ag-NPs and standard antibiotics were loaded into sterile disks and incubated for 24 h at 37 °C. The antibacterial activity of Ag-NPs was measured using inhibition assay. The antimicrobial activity of Ag nanoparticles was tested separately for each UTI isolates, E. coli, S. saprophyticus, K. pneumoniae, and P.aeruginosa. The mean zone of inhibition for five disks for each dose of Ag-NPs was calculated. For comparison, the standard antibiotic Ciprofloxacin was used.
5.2 Minimum inhibitory concentrations (MICs)
The MICs were determined by the standard broth microdilution method (CLSI, 2008) using a 96-well titer plate, as suggested by Baker et al. (2017). The isolated bacteria, E. coli, S. saprophyticus, K. pneumoniae, and P. aeruginosa were incubated in Mueller Hinton broth medium at 37 °C for 4 h. Two-fold serial dilution of AgNPs at concentrations [100, 50, 25, 12.5, 6.25, 3.12, 1.6, 0.78 and 0.39 µg/mL] were done using Mueller-Hinton Broth medium. Then, ten μL of the log phase bacterial culture was added to each well to achieve a final inoculum size of 105 CFU/mL, followed by incubation at 37 °C for 18 to 20 hrs. After incubation, the MIC values were determined. The test tubes were incubated for 24 h. At 37 °C. The lowest concentration that inhibited the microbial growth was taken as MIC. All tests, and their respective control samples, were performed in triplicate.
6 Results and discussion
The combination of T. procumbens extract and ten mM of AgNO3 solution mixture turned a color change from colorless to brown-orange. The reaction indicated the formation of nanoparticles from a particular reaction mixture. The color changes took place to the interaction of Ag+ and plant extract constituents, particularly proteins, amino acids, enzymes, polysaccharides, etc. these are involved as a stabilizing and reducing agents to formation Ag+ to Ag0. The formed. UV–visible absorption spectra of AgNPs of T. procumbens recorded with a scanning range of 200–800 nm. The stiff prominent absorption peak was recorded at 420 nm in Fig. 1. The peak was associated with surface plasmon resonance (SPR) for AgNPs.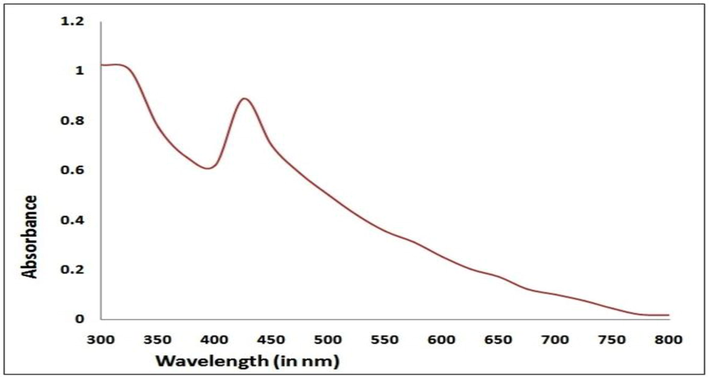
UV–Vis Spectra of AgNPs synthesized using T. procumbens.
SEM measurement was used to categorize the surface morphology and evaluate the size distribution of the synthesized AgNPs (Fig. 2). The NPs were aggregated and spread with uniform shapes. AgNPs look like semi-spherical and non-uniform contours with 40.0–52.5 nm average diameters. The zeta potential, an indicator of surface charge potential, helps to understand the stability of nanoparticles in Aqueous suspensions (Erdogon et al., 2019). Zeta potential values of AgNPs were measured −31.4 ± 0.7 mV, and the produced nanoparticles are negatively charged on their surface (Fig. 3). FTIR spectrum exhibit the characteristic formation existence of AgNPs (Fig. 4). The band seen between of 3490–3500 cm−1 is due to O–H stretching H-bonded alcohols and phenols. The peak found around 1500–1550 cm−1 indicates C–H bond, peak around 1450–1500 cm−1 is due to N–H. The stretch for Ag- NPs is found around 500–550 cm−1. FTIR study suggests the presence of proteins, flavonoids, and terpenoids around nanoparticles. Amino acid residues and proteins can bind metal and promote capping of silver nanoparticles to prevent agglomeration and thereby stabilize the medium (Marslin et al., 2018; Alfuraydi et al., 2019). The reducing sugars present in the plant products reduces the metal ions and facilitate the formation of corresponding metal nanoparticles. The terpenoids are reported to reduce metal ions by oxidation of aldehyde groups in the molecules to carboxylic acids (Erdogan et al., 2019). The vibrant presence of flavonoids in the inflorescence of T. procumbens as per FTIR observation confirms its potency to facilitate NPs formation and equipping the formed NPs with mechanisms to target microbes and tumor cells. GNPs surface chemistry is helpful in the advancement of biomedical applications. The surface of green synthesized NPs with functionalized surfaces and sticky capping agents promotes advanced biological applications (Marslin et al., 2018).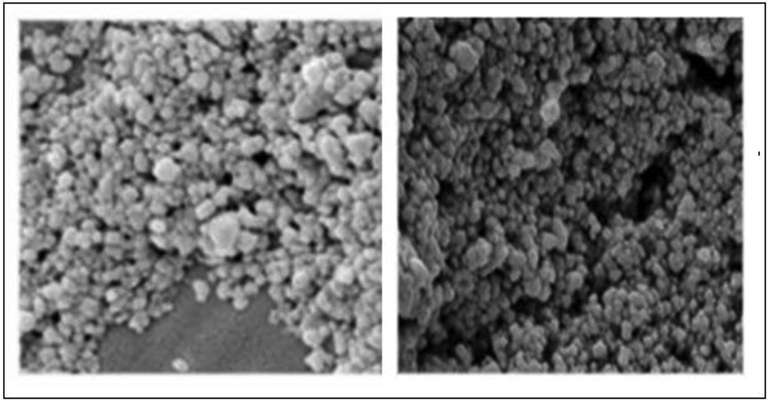
SEM images AgNPs formed using T. procumbens.
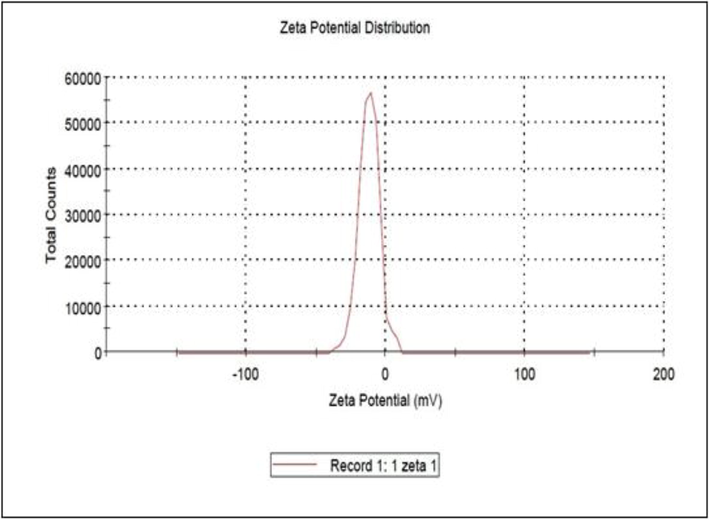
Zeta potential of silver nanoparticles synthesized using T. procumbens extracts.
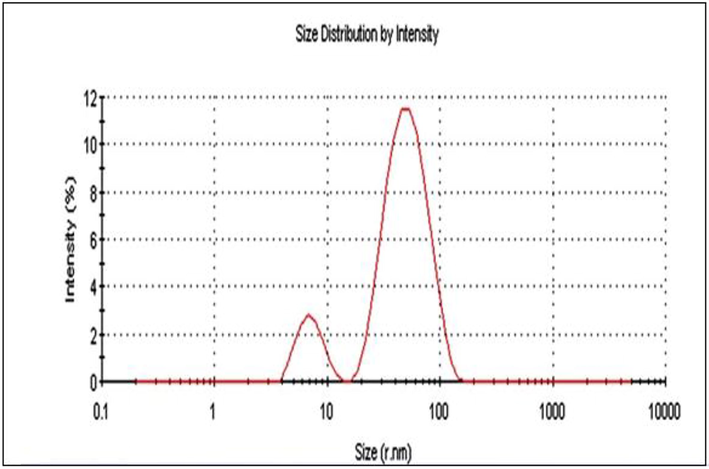
DL S graph of AgNPs synthesized using T. procumbens.
The size of nanoparticle is an important indicator of product quality and performance. It promotes the accumulations in the target sites through the vascular gap of the capillary, which was reported to be in size up to 400–600 nm (Perez et al., 2016). Such enhanced permeability and retention (EPR) effect, provides higher accumulations of nanoparticles with a size less than 400 nm into the tumor (Patil et al., 2018). DLS image (Fig. 5) shows that the particle sizes of AgNPs were found in the range 40.0–67.7 nm. In the green synthesis of AgNPs, it is possible to get a particle with a size lower than 100 nm.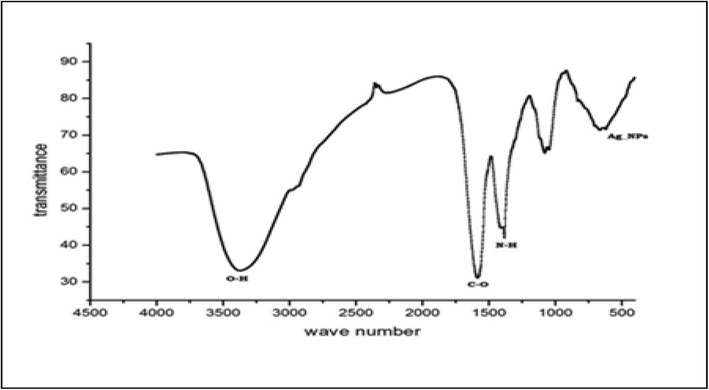
FTIR image of nanoparticles synthesized.
These characteristics help drug internalization into the cancer cells and attach with bacterial cells to a greater extent (Silva et al., 2019). In addition to potential zeta report is an assessment of the stability of synthesized AgNPs. The surface charge of the NPs concludes the stability of the products and also avoids the aggregation of each particle (Devanesan et al., 2020). In the current study, the zeta potential value of AgNPs was determined −32.3 ± 0.8 mV. The negative values are considered to be stable in the aqueous dispersion medium (Dos Santos et al., 2012). These findings highlighted that the presently synthesized AgNPs could preserve their structure over the long-term and aid in the successful cellular uptake of AgNPs.
In the present study, Ag-NPs prepared were tested against four multidrug-resistant uropathogens isolated from UTI patients. The following bacteria viz., E. coli, K. pneumoniae, P. aeruginosa, and gram-positive S. saprophyticus were isolated from UTI patient's urine sample in South India. The study proved that botanically synthesized Ag-NPs were able to inhibit the growth of the uropathogens more effectively than synthetic antibiotics Fig. 6 and Table 1(a-d). The mean diameter of zone of inhibition (in mm) for the different isolates at a dose of 50 µg/mL concentration showed a maximum for S. saprophyticus (21.0 ± 1.7 mm) followed by P. aeruginosa (18.0 ± 1.3 mm), K. pneumoniae (18.0 ± 0.09 mm) and E. coli (17.0 ± 1.70). The present study confirms the previous report of Rani et al. (2020) that the AgNPs synthesized by them using T. procumbens extract also inhibited the growth of two MDR strains, P. aeruginosa (11.00 ± 1.00 mm) and E. coli (15.33 ± 0.58 mm)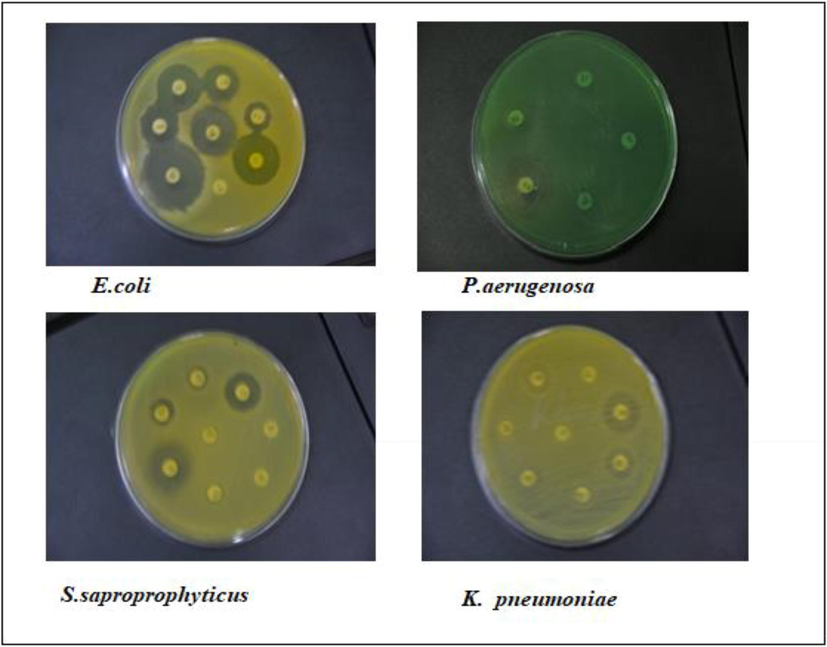
Sensitivity of bacterial isolates to plant extract inspired AgNPs is shown as a zone of inhibition.
A
Name of the organismConcentration of Ag nanoparticle (µg/mL)
Mean diameter of Zone of inhibition (in mm) 5 discs with SD
Green Ag-NPs
Standard antibiotics(Ciprofloxacin)
Escherichia coli
10
12 ± 1.3
10 ± 0.4
20
13 ± 1.2
10 ± 0.4
30
14 ± 1.4
10 ± 0.4
40
15 ± 1.3
10 ± 0.4
50
17 ± 1.7
10 ± 0.4
B
Name of the organismConcentration of Ag nanoparticle(µg/mL 0
Mean diameter of zone of inhibition (in mm) 5 discs with SD)
Green Ag-NPs
Standard antibiotics (Ciprofloxacin)
Klebsiella pneumoniae
10
14 ± 0.9
12 ± 0.5
20
15 ± 1.4
12 ± 0.5
30
16 ± 1.7
12 ± 0.5
40
17 ± 1.1
12 ± 0.5
50
18 ± 0.9
12 ± 0.5
C
Name of the organismConcentration of Ag nanoparticle(µg/mL)
Mean diameter of Zone of inhibition (in mm) 5 discs with SD
Green Ag-NPs
Standard antibiotics (Ciprofloxacin)
Staphyllococcus saprophyticus
10
13 ± 1.0
11 ± 0.1
20
14 ± 1.4
11 ± 0.1
30
17 ± 1.5
11 ± 0.1
40
21 ± 1.3
11 ± 0.1
50
21 ± 1.7
11 ± 0.1
D
Name of the organismConcentration of Ag nanoparticle(µg/mL)
Mean diameter of Zone of inhibition (in mm) 5 discs with SD
Green Ag-NPs
Standard antibiotics (Ciprofloxacin)
Pseudomonas aeruginosa
10
13 ± 1.4
11 ± 0.8
20
15 ± 1.0
11 ± 0.8
30
16 ± 1.6
11 ± 0.8
40
17 ± 1.4
11 ± 0.8
50
18 ± 1.3
11 ± 0.8
The MIC values for the isolates showed a minimum for S. saprophyticus (2.5 µg/mL) and a maximum for E. coli (55.5 µg/mL) (Table 2). Rani et al. (2020) reported a MIC value in a range between 11.43 and 102.8 µg/ml. For AgNPs synthesized using the extracts of T.procumbens tested against MDR resistant P. aeruginosa and E. coli. Also, it has been shown that the cellular membrane of bacteria has a negative charge (Van Der Wal et al., 1997). As antimicrobial resistance has become a severe threat to human health, it is necessary to overcome it with the help of alternative medicine if synthetic drugs fail. Therefore, there is an increased involvement in the investigation of plants as well as plants reduced NPs as a source to inhibit human microbial diseases. As there is an urgent need to combat multidrug-resistant bacteria, new generation antibiotics are to be developed. In this search, attention can be focused on AgNPs synthesized using T. procumbens.
Bacteria
MICs (µg/mL)
Escherichia coli (UTI multidrug-resistant strain)
53.5
Klebsiella pneumonia (UTI multidrug-resistant strain)
21.6
Pseudomonas. aeruginosa (UTI multidrug-resistant strain)
4.5
Staphyllococcus saprophyticus (UTI multidrug-resistant strain)
2.6
In the current study, the AgNPs were found to inhibit the growth of the drug-resistant UTI isolates more effectively than standard antibiotics. AgNPs with a small size (20–40 nm) easily binds with bacterial surface in comparison to larger AgNPs. The smaller sized particles have a large contact area and easy reachability to cellular organelles (Alharbi and Alarfaj, 2020; AlSalhi et al., 2016).
Further, the presence of Ag-NPs is reported to produce ROS stress-causing microbial growth inhibition (Qing et al., 2018). It has been reported that smaller Ag-NPs can anchor to the bacterial cell wall and consequently infiltrate it by doing damages in the bacterial membrane, which can lead to cellular contents leakage and bacterial death (Abbaszadegan et al., 2015; Dhandapani et al., 2020). The antibacterial effect of Ag-NPs on Gram-negative bacteria was stronger than Gram-positive bacteria because of the difference in the cell wall thickness (Mandal et al., 2016). Ag-NPs also interact with ribosomes and lead to their denaturation, causing inhibition of translation and protein synthesis (Singh et al., 2014; Shaik et al., 2016). The present study concludes that the AgNPs synthesized using the plant product extract showed high antimicrobial activities because of the influence of phytochemical involvement in AgNPs synthesis. Microbiological study using varying concentrations of green and chemically synthesized NPs showed that green nanoparticles have a high biocidal activity against various pathogens than the chemically synthesized NPs (Shaik et al., 2016; Devanesan et al., 2020).
7 Conclusion
The extract of the inflorescence of T. procumbens is rich in flavonoids and polyphenols, and this has promoted a cap formation in AgNPs. The phytochemical coatings around the AgNPs triggered its functional mechanism. The AgNPs with the layers of T. procumbens bioactive compounds helped to target the cell membrane of the bacteria and made cellular damages both externally. AgNPs with a small size (20–40 nm) binds with bacterial surface easily in comparison to larger AgNPs. The smaller sized particles have a large contact area, and easy reachability to cellular organelles. The present study concludes that the AgNPs prepared using the extracts of the inflorescence of T. procumbens are potential antibiotic agents against the multidrug-resistant bacteria isolates. So further research can be carried out to develop for from urinary tract bacterial pathogens that escape several antibiotics.
Acknowledgment
The authors extend their appreciation to the Researchers Supporting Project number (RSP-2020/228), King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
We declare that we have no financial and personal relationships with other people or organizations that could inappropriately influence (bias) their work or state.
References
- The effect of charge at the surface of silver nanoparticles on antimicrobial activity against gram-positive and gram-negative bacteria: a preliminary study. J Nanomater.. 2015;16:53.
- [CrossRef] [Google Scholar]
- An evaluation of antibiotics prescribing patterns in the emergency department of a tertiary care hospital in Saudi Arabia. Infect Drug Resist.. 2019;12:3241-3247.
- [CrossRef] [Google Scholar]
- Green synthesis of silver nanoparticles from Neurada procumbens and its antibacterial activity against multi-drug resistant microbial pathogens. J. King Saud Univ. Sci.. 2020;32:1346-1352.
- [CrossRef] [Google Scholar]
- Eco-friendly green synthesis of silver nanoparticles from the sesame oil cake and its potential anticancer and antimicrobial activities. J. Photochem. Photobiol. B.. 2019;192:83-89.
- [CrossRef] [Google Scholar]
- Green synthesis of silver nanoparticles using Pimpinella anisum seeds: antimicrobial activity and cytotoxicity on human neonatal skin stromal cells and colon cancer cells. Int. J. Nanomed.. 2016;11:4439-4449.
- [CrossRef] [Google Scholar]
- Impact of antibiotic de-escalation on hospitalized patients with urinary tract infections: a retrospective cohort single center study. J Infect Public Health.. 2020;13:985-990.
- [CrossRef] [Google Scholar]
- Nano-strategies to fight multidrug resistant bacteria-“a battle of the titans. Front. Microbiol.. 2018;9:1441.
- [CrossRef] [Google Scholar]
- Beck, S., Mathison, H., Todorov, T., Calderón-Juárez, E.A., Kopp, O.R. A Review of Medicinal Uses and Pharmacological Activities of Tridax Procumbens (L.) . J. Med. Plants Stud., 7 (2018), pp.19-35. doi:10.5539/jps.v7n1p19.
- Christy, V.R., Athinarayanan, G., Mariselvam, R., Dhasarathan, P., Singh, A.J.A. Epidemiology of urinary tract infection in south India. Biomed Res Clin Prac., 4(2019), doi. 10.15761/BRCP.1000190
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for bacteria Isolated from Animals: Approved Standard – Third Edition. CLSI document M31-A3 (ISBN 1-56238-659-X). Clinical and Laboratory Standards Institute, 940 West Valley Road, Suite 1400, Wayne, Pennsylvania 19087-1898 USA, 2008.
- Cytotoxic and antimicrobial efficacy of silver nanoparticles synthesized using a traditional phytoproduct, asafoetida gum. Int. J. Nanomed.. 2020;15:4351-4362.
- [CrossRef] [Google Scholar]
- Antimicrobial and cytotoxicity effects of synthesized silver nanoparticles from Punica granatum Peel Extract. Nanoscale Res. Lett.. 2018;13:315.
- [CrossRef] [Google Scholar]
- Bio-approach synthesis of nanosilver impregnation on calcium hydroxyapatite by biological activated ammonia from urinary waste. Arab. J. Chem.. 2020;13:5878-5889.
- [CrossRef] [Google Scholar]
- Dos Santos, K.C., daSilva, M.F., Pereira-Filho, E.R., Fernandes, J.B., Polikarpov, I., Forim, M.R. Polymeric nanoparticles loaded with the 3,5,3’tri iodo thyro acetic acid (Triac), a thyroid hormone: Factorial design, characterization, and release kinetics. Nanotechnol Sci Appl., 5(2012), pp.7-48.doi.org/10.2147 /NSA. S32837.
- Durai, P., Chinnasamy, A., Gajendran, B., Ramar, M., Pappu, S., Kasivelu, G., Thirunavukkarasu, A. Synthesis and characterization of silver nanoparticles using crystal compound of sodium para-hydroxybenzoate tetrahydrate isolated from Vitex negundo . L leaves and its apoptotic effect on human colon cancer cell lines. Eur. J. Med. Chem, 84(2014), pp. 90–99. doi.org/10.1016/j.ejmech.2014.07.012
- Potential management of resistant microbial infections with a novel non-antibiotic: the anti-inflammatory drug diclofenac sodium. Int. J. Antimicrob. Agents. 2007;30:242-249.
- [CrossRef] [Google Scholar]
- Anti-inflammatory, cyclooxygenase inhibitory and antioxidant activities of standardized extracts of Tridax procumbens L. Fitoterapia.. 2017;82:173-177.
- [CrossRef] [Google Scholar]
- Miaomiao, L., El-Hossary, E.M., Oelschlaeger, T.A., Donia, M.S., Quinn, R.J. Abdelmohsen, U.R. Potential of marine natural products against drug-resistant bacterial infections. THE LANCET., 19(2019),pp.237-245.https://doi.org/10.1016/S1473-3099(18)30711-4.
- Mariselvam, R., Ranjitsingh, A.J.A., Usha Raja, N., Kalirajan, K., Padmalatha, C., Selvakumar, P. Green synthesis of silver nanoparticles from the extract inflorescence of Cocos nucifera (Family: Arecaceae) for enhanced antibacterial activity. Spectrochim Acta A Mol Biomol Spectrosc., 29(2014), pp.537-41. doi: 10.1016/j.saa.2014.03.066.
- Secondary metabolites in the green synthesis of metallic nanoparticles. Materials. 2018;11:940.
- [CrossRef] [Google Scholar]
- Bio-fabricated silver nanoparticles preferentially targets Gram positive depending on cell surface charge. Biomed. Pharmacother.. 2016;83:548-558.
- [CrossRef] [Google Scholar]
- Epidemiology of urological infections: a global burden. World J Urol. 2020
- [CrossRef] [Google Scholar]
- Antibacterial potential of silver nanoparticles synthesized using Madhuca longifolia flower extract as a green resource. Microb Pathog.. 2018;121:184-189.
- [CrossRef] [Google Scholar]
- Green synthesis of silver nanoparticles using Tridax procumbens: their characterization, antioxidant and antibacterial activity against MDR and reference bacterial strains. Chem. Pap.. 2020;74:1817-1830.
- [CrossRef] [Google Scholar]
- Regí, M.V., González, B., izquierdo-barba, I. Nanomaterials as Promising Alternative in the Infection Treatment . Int J Mol Sci., 20(2019), pp.3806. doi: 10.3390/ijms20153806.
- Current trends in cancer nanotheranostics: metallic, polymeric, and lipid-based systems. Pharmaceutics. 2019;11:22.
- [CrossRef] [Google Scholar]
- The role of nanotechnology in combating multi-drug resistant bacteria. J. Nanosci. Nanotechnol.. 2014;14:4745-4756.
- [CrossRef] [Google Scholar]
- Antimicrobial activity mechanism of inhibition of silver nanoparticles against extreme halophilic archaea. Front. Microbiol.. 2016;7:e1424
- [CrossRef] [Google Scholar]
- Alternative therapeutics against antimicrobial-resistant pathogens. Front. Microbiol.. 2019;19:2173.
- [CrossRef] [Google Scholar]
- Determination of the total charge in the cell walls of Gram-positive bacteria. Colloids Surf. B Biointerfaces. 1997;9:81-100.
- [Google Scholar]
- Valsalam, S., Agastian, P., Arasu, M.V., Al-Dhabi, N.A., Ghilan, A.M., Kaviyarasu, K., Ravindran, B., Chang, S.W., Arokiyaraj, S. Rapid biosynthesis and characterization of silver nanoparticles from the leaf extract of Tropaeolum majus L. and its enhanced in-vitro antibacterial, antifungal, antioxidant and anticancer properties. J Photochem Photobiol B. 191 (2019),65-74. doi:10.1016/j.jphotobiol.2018.12.010.
- Potential antibacterial mechanism of silver nanoparticles and the optimization of orthopaedic implants by advanced modification technologies. Int. J. Nanomed.. 2018;13:3311-3327.
- [CrossRef] [Google Scholar]
- Breathing new life into old antibiotics: overcoming antibacterial resistance by antibiotic-loaded nano gel carriers with cationic surface functional. Nanoscale. 2019;11:10472-10485.
- [CrossRef] [Google Scholar]
- Prevalence of urinary tract infection and antibiotic resistance pattern in Saudi Arabia population. GJBAHS. 2015;4:206-214.
- [Google Scholar]







