Translate this page into:
Catalytic activity of bimetallic AuPd alloys supported MgO and MnO2 nanostructures and their role in selective aerobic oxidation of alcohols
⁎Corresponding author. h.alshammari@uoh.edu.sa (Hamed Alshammari)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The use of metal oxides as supports for gold and palladium (Au-Pd) nano alloys constitutes new horizons to improve catalysts materials for very important reactions. From the literatures, Pd-based bimetallic nanostructures have great properties and active catalytic performance. In this study, nanostructures of magnesium oxide (MgO) and manganese dioxide (MnO₂) were synthesised and utilized as supports for Au-Pd nanoparticle catalysts. Gold and palladium were deposited on these supports using sol-immobilisation method. The MgO and MnO2 supported Au-Pd catalysts were evaluated for the oxidation of benzyl alcohol and 1-octanol, respectively. These catalysts were found to be more selective, active and reusable than the corresponding monometallic Au and Pd catalysts. The effect of base supports on the disproportionation reaction during the oxidation process was investigated. The results show that MgO stopped the disproportionation reaction for both aromatic and aliphatic alcohols while MnO₂ stopped it in the case of benzyl alcohol only. The outcomes of this work shed light on the selective aerobic oxidation of alcohols using bimetallic Au-Pd nanoalloys and pave the way to a complete investigation of more basic metal oxides for various aliphatic alcohols.
Keywords
Aerobic oxidation
Nano gold catalysis
Metal oxide
Oxidation of alcohol
1 Introduction
Converting alcohols by selective oxidation to their corresponding carbonyl compounds plays an important role in the industrial productions (Sheldon and Kochi, 1981; Zhang et al., 2011; Mallat and Baiker, 2004; Mori et al., 2007). However, the traditional methods of the conversion process are usually conducted with costly and environmentally unfriendly stoichiometric reagents. Improving the conversion reaction by making a green process for the more selective aerobic oxidation of different types of alcohols to their corresponding aldehydes and ketones is therefore of paramount importance for both economic and environmental reasons. The attention has been turned to aerobic oxidation using recyclable metal catalysts which are green and low cost catalysts (Gamez et al., 2004; Qi et al., 2015; Yu et al., 2014). Furthermore, the discovery of catalytic properties of supported gold nanoparticles (Au NPs) and their uses in oxidation reactions under mild temperatures with air as an oxidant (Briñas et al., 2008; Wilson and Lee, 2012; Montero et al., 2009) has brought attention to heterogeneous gold catalysts improvement and their uses in various types of catalytic reactions. However, these catalysts suffer from instability due to the agglomeration of Au NPs during the reactions (Dhakshinamoorthy and Garcia, 2014).
Recently, it has been found that bimetallic Au-Pd catalyst has better activity and stability than the monometallic counterparts and that has been mainly attributed to the geometric and electronic effects (Villa et al., 2015). In addition, it has been noticed that the support can significantly influence the performance of Au through specific support—metal interaction (Villa et al., 2015). Supported Au nanoparticle catalysts have been employed as catalysts for the oxidation of alcohols to their aldehydes. One major drawback of the supported Au monometallic catalysts is their low activity towards alcohol oxidation relatively (Zhou et al., 2008). The addition of Pd to Au to form Au-Pd nano-alloys significantly enhances the catalytic activity towards alcohols oxidation (Zhou et al., 2008). However, it creates another issue, which is the higher selectivity to toluene by-product (Zhou et al., 2008). Metal oxides have been used as supports for gold and gold-palladium nano alloys catalysts for many oxidation reactions in particular oxidation of alcohols (Zhou et al., 2008). Various metal oxide such as TiO₂, Al₂O₃, CeO₂, Fe₂O₃, MnO₂ and ZrO₂ have been investigated as a support for Au-Pd catalysts for the oxidation of alcohols (Dimitratos et al., 2012).
Nobel metals (e.g. palladium and gold) can be reduced easily during the activation by hydrogen reduction or calcination and form large metal particles. That is mainly due to the unique physical and chemical properties of noble metals such as mobility and autocatalysis. However, that leads to poor metal dispersion and reduction in the activity and therefore preparing high performance heterogeneous metal catalyst with high dispersion of the active components becomes a major challenge. The catalysts performance of bimetallic Au-Pd supported catalysts has been reported to be very active compared to the monometallic Au supported catalysts and their activity relies strongly on their properties including their size, composition and their structure (Hutchings and Kiely, 2013). Many studies have been carried out to control the mentioned properties (Dimitratos et al., 2012; Hutchings and Kiely, 2013). Hutchings group illustrated that, in benzyl alcohol oxidation, using MgO or ZnO as supports for Au-Pd nano alloys switches off the production of toluene by- product and enhanced the selectivity towards benzaldehyde dramatically (Hutchings and Kiely, 2013; Sankar et al., 2011). They reported that these basic metal oxide supports could stop the disproportionation reaction and hence the selectivity towards toluene by-products and significantly improve the selectivity to benzaldehyde. However, this has been reported for the oxidation of aromatic alcohol and has inspired us to investigate the effect of these catalysts on aliphatic alcohols oxidation.
In this work, metal oxides such as MgO and MnO₂ were synthesised and used as supports for Au-Pd nano alloy catalysts for the oxidation of benzyl alcohol and 1-octanol to investigate their effect on the disproportionation reaction.
2 Experimental details
2.1 Preparation of MgO and MnO₂
The synthesis of MgO is described in an earlier paper (Ding et al., 2001). Briefly, 1 M of MgCl₂.6H₂O (Aldrich) solution was prepared in 100 ml of de-ionized water. Then 1 M of NH₄OH solution was added at 100 °C and followed by stirring at 25 °C for 2 h. After that, the solution was kept for ageing for 24 h. The resulting white precipitates were filtered and washed with de-ionized water and then ethanol (Aldrich) for several times to remove the by-products and/or impurities. The filtered cake was dried in air at 100 °C for 4 h. The as synthesized samples were calcined at 400 °C in static air for 3 h to obtain MgO nanoparticles.
Well-stable MnO₂ nanoparticles were prepared by directly stirring 5.68 g of hydrated manganese sulphate MnSO₄.H₂O (Aldrich) in 66 ml of water at room temperature until dissolved. Then, 7.67 g of ammonium persulfate (NH4)₂S₂O₈ (Aldrich) were added to the solution while stirring until a clear solution was formed. The solution was transferred to a sealed autoclave (Baskerville, 125 ml) and heated at 120 °C for 72 h. Following the reaction, the resulting black material was recovered by filtration then washed by water (0.5 L) and dried at 60 °C for 24 h.
2.2 Catalyst preparation
The aim of this study is to prepare and test a new Au-Pd catalyst immobilized onto MgO and MnO₂. To understand the motivation and progression of this work, highlights of the important preparation parameters of catalyst are mentioned here. Full details of catalyst preparation protocols can be found in literatures (Pritchard et al., 2010). 1% Au-Pd catalysts were synthesised using the sol-immobilisation method. Typically, a freshly prepared solution of PVA (1 wt%, Aldrich, Mw = 10,000, 80% hydrolysed) was added to an aqueous solutions of PdCl₂ (Johnson Matthey, 6 mg in 1 ml) mixed with HAuCl₄.3H₂O (Johnson Matthey, 12.25 g in 1000 ml) by stirring for 15 min (PVA/metal 0.65 w/w). 0.1 M of NaBH₄ [Aldrich, NaBH₄/(Au + Pd) (mol/mol = 5)] was prepared freshly and then added to form a dark brown solution. Then, it was stirred for 30 min and the pH value was adjusted to 1 by adding H₂SO₄. Then, 1.98 g of support (MgO or MnO₂) was added to the mixture and stirred for 1 h. The catalyst was then recovered by filtration, washed with distilled water (2 l) and dried at 110 °C for 24 h. Based on the calculation, the weight percentage of Au and Pd in the metal alloy are 0.65 and 0.35 respectively .
2.3 Catalyst testing
The Radleys carousel reactor (50 ml glass stirred reactor) was employed for the liquid phase benzyl alcohol and 1-octanoloxidation. The supported Au-Pd catalyst (200 mg) was suspended in the substrate (2 g) under solvent-free conditions. The experimental method used here is similar to the standard reaction protocols that are used for solvent-free oxidation of benzyl alcohol which was reported earlier (Morad et al., 2014). The reaction mixture was stirred at 1000 rpm and the oxygen pressure was kept at 1 bar. The pressure was maintained during the reaction by using pressure gauge to avoid any pressure fluctuations. After a specific reaction period (Morad et al., 2014), a sample was taken for analysis by using gas chromatography (GC - Varian star CP-3800) with CP-wax 52 column and a flame ionization detector (FID). The catalyst loading (mol%) has been calculated and found be 0.356% and 0.429% for benzyl alcohol and 1-octanol respectively.
2.4 Catalyst characterization
Powder X-ray diffraction was conducted using a PANalyticalX’Pert Pro with a CuKα X-ray source operated at 40 kV and 40 mA fitted with an X’Celerator detector. The XRD patterns of MgO and MnO₂ are shown in Fig. 1. BET surface area was measured using nitrogen adsorption and a micrometrics surface area analyser. Samples were prepared for Transmission electron microscopy (TEM) examination by dispersing the catalyst powder. TEM was performed using a Jeol 2100 with a filament operating at 200 kV. The TEM images of the recovered catalyst are reported in Fig. 2. The morphology of the catalysts was observed by scanning electron microscopy (SEM) using a Carl Zeiss EVO 40 SEM fitted with a BSD, an Everhart-Thornley detector, and a variable pressure chamber with a tungsten source. SEM images of the catalyst are presented in Fig. 3.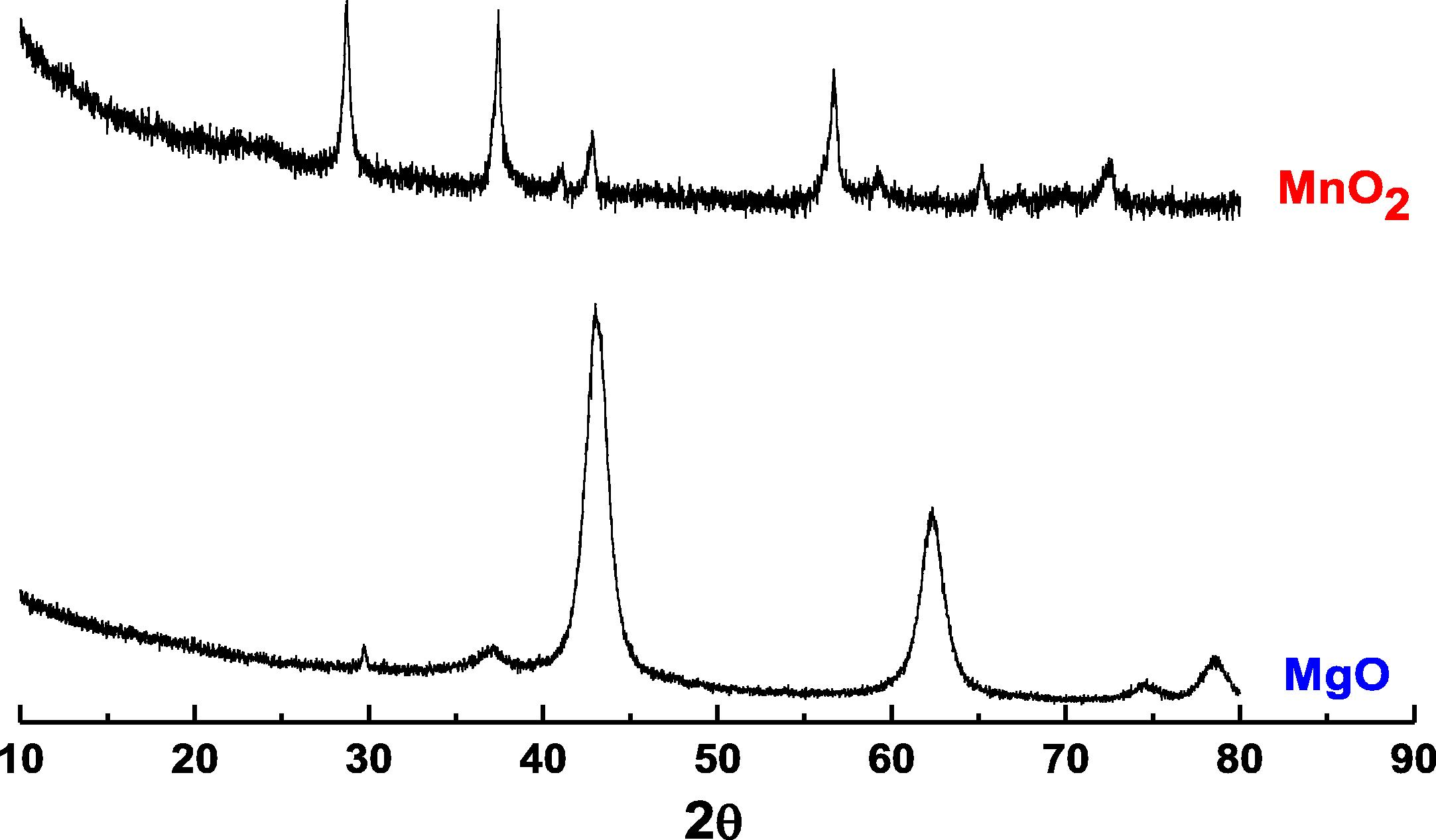
The XRD pattern of MgO and MnO2.
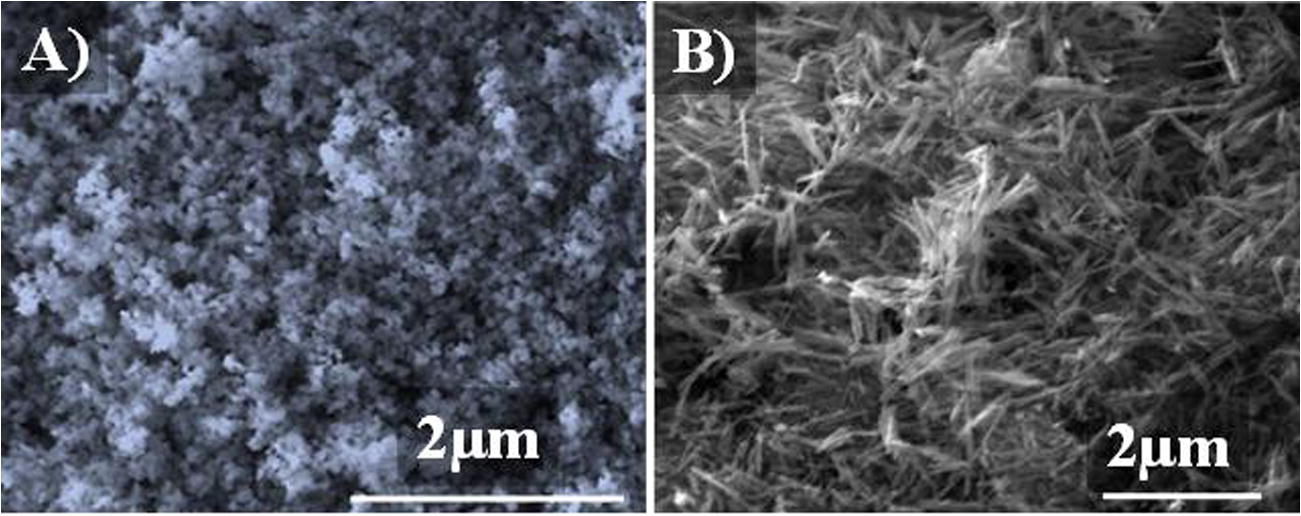
SEM image of A) MgO and B) MnO2.
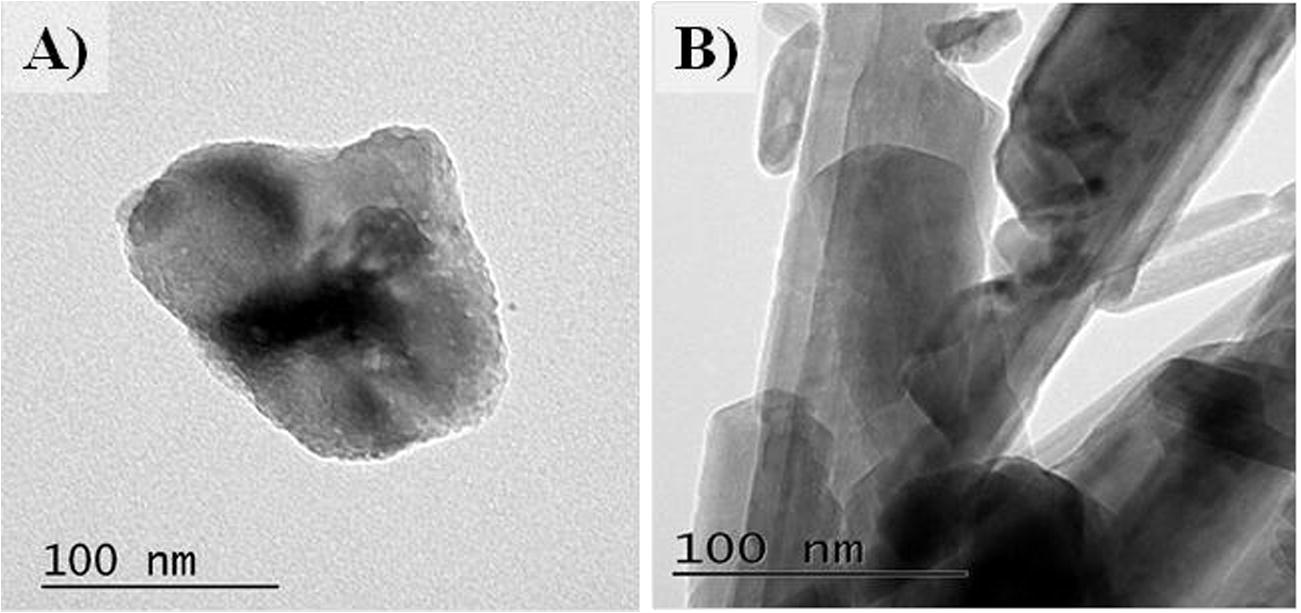
TEM image of A) MgO and B) MnO2.
3 Results and discussion
3.1 Synthesis of MgO and MnO₂
X-ray diffraction patterns of the synthesised MgO and MnO₂ materials are illustrated in Fig. 1. For MgO sample, the figure shows that the sample essentially consists of MgO phase. The peaks at 2θ = 36.86°, 42.82° and 62.09° are related to (1 1 1), (2 0 0) and (2 2 2) planes of MgO, respectively (Boo et al., 1999). For MnO₂ sample, the presence of the diffraction peaks at 28.88°, 37.32°, 42.62°, 56.65°, 59.10°, 64.96°, 67.15° and 72.30° confirmed the formation of β-MnO₂ phase (Liang et al., 2008). It was noticed after the electrochemical experiments that the β-MnO₂ nanomaterials showed different specific capacitance (Cs) values which allows the β-MnO₂ to exhibit an excellent long-term stability (Ede et al., 2014).
The morphology of the prepared MnO2 and MgO samples is shown in Fig. 2. The SEM images of MgO in Fig. 2A show that the sample contains agglomerated nanoparticles with the particle size of about 100 nm. The obtained MnO2 sample contains nanowires with an average length of 1 µm and average diameter of 50 nm as shown in Fig. 2B. The TEM images in Fig. 3 illustrate the size of each material and it is obvious from the figure that both synthesised materials are in nano scale.
The surface areas of the synthesised MgO and MnO2 samples and their average pore diameters are shown in Table 1. MgO nanoparticles sample exhibits higher surface area than MnO2 nanowire sample. The BET surface area and pore diameter distribution are shown in Fig. 4. It can be observed from the figure that MnO2 sample has narrower pore diameter distribution than MnO2.
Area (m2/g)
Average pore radius (Å)
Average pore volume (cc/g)
MgO
80
40
0.45
MnO2
16
50
0.16
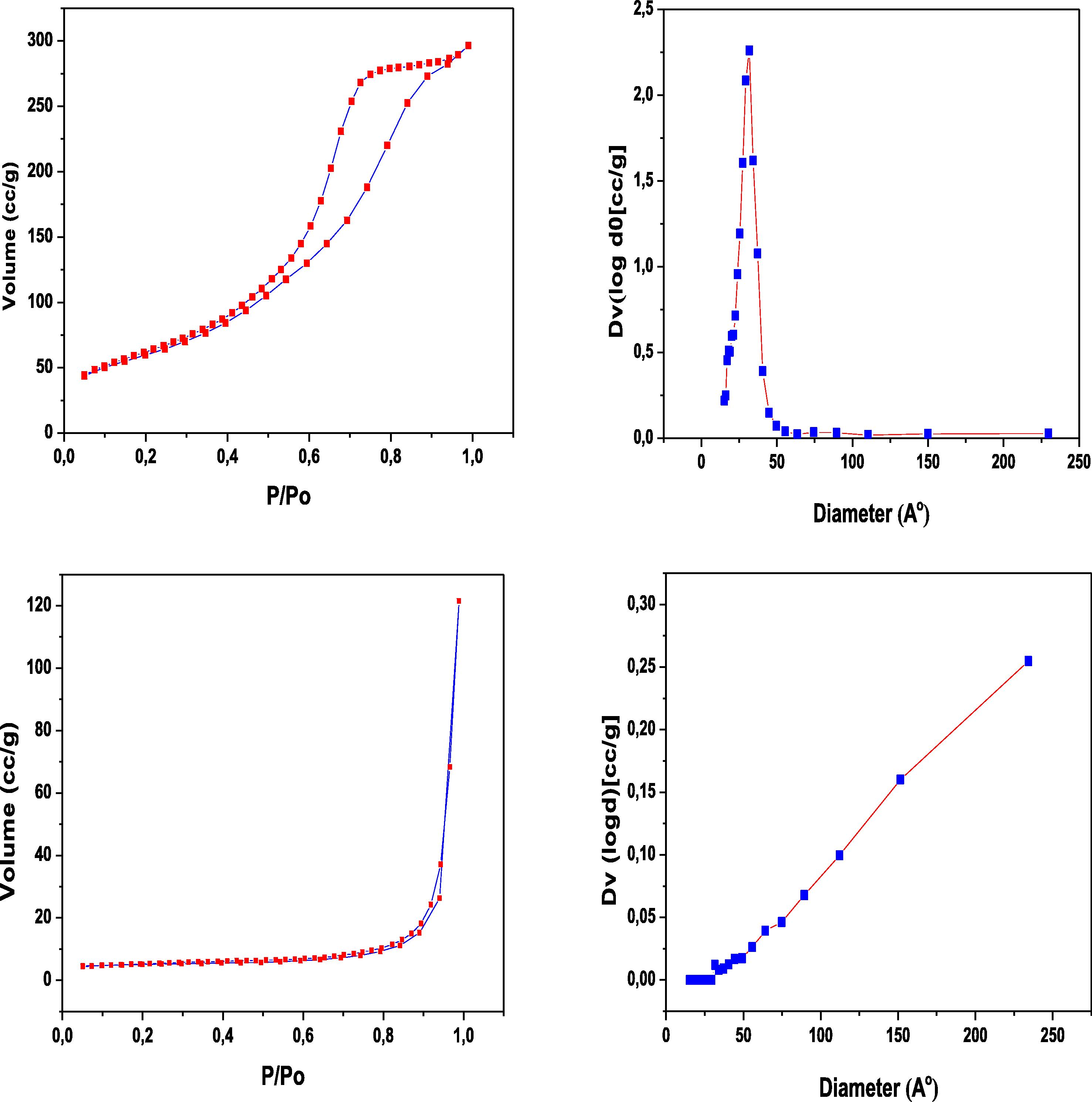
BET surface area and pore diameter plots. Upper panel for MgO. Lower panel for MnO2.
3.2 Oxidation of benzyl alcohol using 1% Au-Pd/MgO and 1%Au-Pd/MnO₂
Synthesised sol-immobilised Au-Pd/MgO and Au-Pd/MnO₂ catalysts were evaluated for the oxidation of benzyl alcohol (Table 2). Both catalysts exhibited good catalytic activity towards benzyl alcohol oxidation. Under the optimised reaction conditions, there was a stable increase in the conversion with reaction time for both catalysts. However, Au-Pd/MnO₂ catalyst showed better activity than Au-Pd/MgO catalyst. This can be attributed to the Au-Pd particle size distribution. Fig. 5 demonstrates that although Au-Pd/MgO catalyst has smaller mean particle size (mean = 4.43 nm), Au-Pd/MnO₂ catalyst has narrower particle size distribution (the majority of particle size was between 2 and 5 nm) unlike the Au-Pd/MgO catalyst. The size of the gold particles is the major factor that determines the catalyst performance in addition to other factors such as oxidation state of gold, synthesis method and the choice of catalyst support. These factors are, in the case of supported gold catalysts, strongly influenced by the morphology of the support and as a result the catalyst activity is influenced by the support properties as well (Alhumaimess et al., 2014). The main observation was that both MgO and MnO₂ supports almost switched off the disproportionation reaction and hence the toluene production. It is important to note that it is well known that the disproportionation of benzyl alcohol has been identified as the source of toluene formation in the solvent-free oxidation of benzyl alcohol using supported gold palladium catalysts. There is a slight increase in the disproportionation reaction, and hence the toluene selectivity, when this reaction is performed in a continuous mode (Cao et al., 2013).This is in agreement with Hutchings' group work (Enache et al., 2006; Sankar et al., 2012; Bartley et al., 2012) which illustrated that basic supports completely switch-off this disproportionation reaction, whereas acidic supports like activated C, Nb₂O₅ and Al₂O₃ promote this disproportionation reaction. The effect of the reaction time on selectivity is shown in Table 2. It can be seen from the Table 2 that the reaction with both catalysts resulted in >98% selectivity for benzaldehyde with increasing conversion. It should be mentioned that Hutching's group did not investigate MnO₂ support for benzyl alcohol oxidation in their work (Hutchings and Kiely, 2013; Sankar et al., 2011, 2012; Enache et al., 2006; Bartley et al., 2012). Reaction conditions: 0.02 g catalyst, 1 bar of O2, 2 g benzyl alcohol, 120 °C and stirring at 1000 rpm.
Time (h)
Au-Pd/MgO
Au-Pd/MnO2
Conversion%
Selectivity %
TOF (h−1)
Conversion%
Selectivity%
TOF (h−1)
0.5
7.6
98.3
2136
8.2
98.5
2304
1
11.4
98.5
1612
13.3
97.4
1874
1.5
16.7
98
1564
18.2
97.4
1706
2
21.4
96.3
1501
24.7
96.4
1733
2.5
29.4
97.8
1650
31.6
96.5
1774
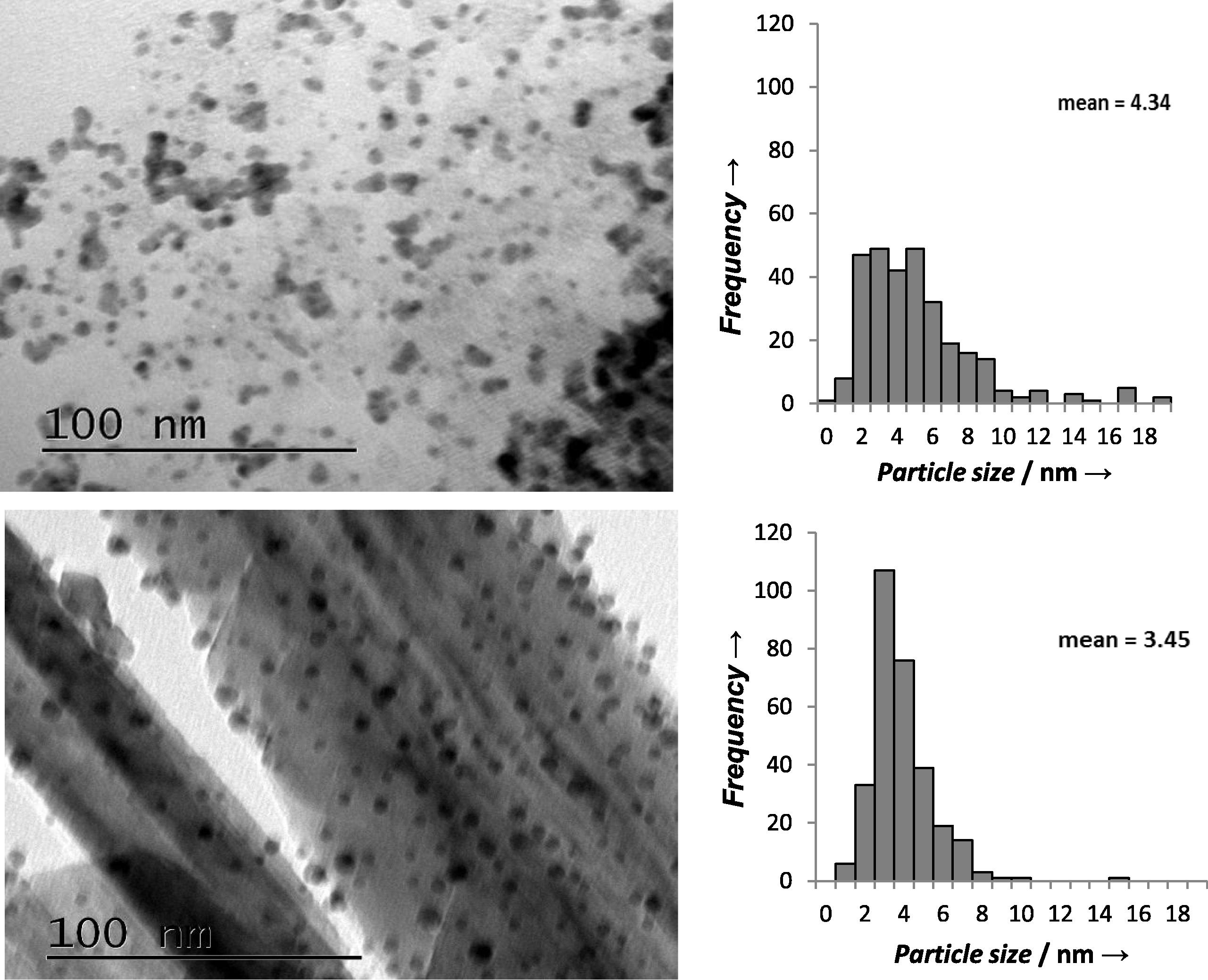
Particle size distributions and TEM images of A) Au-Pd/MgO and B)Au-Pd/MnO2 prepared by sol-immobilisation method.
3.3 Oxidation of 1-octanol using 1% Au-Pd/MgO and 1% Au-Pd/MnO₂
Having evaluated the catalysts performance for the oxidation of aromatic alcohol, the obtained catalysts were then evaluated for the oxidation of the aliphatic alcohol for 1-octanol oxidation (Table 3). Under the optimised conditions showed in Table 2 both catalysts illustrated a steady increase in conversion as the reaction time increased. Moreover, the catalyst prepared by using MnO₂ as support showed enhanced activity for 1-octanol oxidation compared to MgO catalyst, which could be due to the narrower gold and palladium nanoparticle distribution shown in Fig. 5 as explained above in the case of benzyl alcohol oxidation. Reaction conditions: 0.02 g catalyst, 1 bar of O2, 2 g 1-octanol, 120 °C and stirring at 1000 rpm.
Time (h)
Au-pd/MgO
Au-pd/MnO2
Conversion %
Selectivity (%)
TOF (h−1)
Conversion %
Selectivity (%)
TOF (h−1)
1-Octanal
1-Octanal
1-Octanoic acid
0
0
0
0
0
0
0
0
2
1.36
100
78.9
1.77
84.9
15
109.1
4
2
100
58.1
2.3
73.6
26.3
68.3
6
2.3
100
43.6
2.95
67.1
32.8
57.5
8
2.6
100
39.7
3.2
63.2
36.7
47.6
Table 3 also shows the reaction time effect on the selectivity for 1-octanol oxidation using Au-Pd/MnO₂ and Au-Pd/MgO catalysts. The Au-Pd/MgO achieved the highest selectivity toward 1-octanal (100% selectivity to 1-octanal) whereas Au-Pd/MnO₂ catalyst achieved significant selectivity toward 1-octanoic (15%) acid after 2 h of the reaction time. Furthermore, the selectivity toward 1-octanal remained constant with the reaction time (100%) when using Au-Pd/MgO catalyst unlike the Au-Pd/MnO₂ catalyst, which showed a significant decrease in selectivity toward 1-octanal and hence increase the production of 1-octanoic acid. For the Au-Pd/MnO₂ catalyst, at short reaction times (2 h) the selectivity towards the 1-Octanal is around 80%, however, the selectivity towards 1-Octanal decreases dramatically as the reaction proceeded suggesting that this may oxidised to 1-octanoic acid. The selectivity of 1-octanoic acid increased with reaction time, which is likely to be a result of the oxidation of 1-Octanal, which remains quite constant throughout the reaction time, suggesting that it is oxidised at a similar rate to which it is formed.
4 Conclusion
Two main metal oxide support materials (MgO and MnO₂) and their correspondent bimetallic catalyst Au-Pd nanoalloys were successfully prepared and characterized. MgO and MnO₂ nanomaterials were synthesised hydrothermally and were used as supports for the Au-Pd catalysts. The Au-Pd supported on MgO and MnO₂ materials were prepared via sol-immobilisation method and used for solvent free oxidation of benzyl alcohol and 1-octanol. Under the optimized reaction conditions, Au-Pd/MnO₂ catalyst exhibited better catalytic activity compared with Au-Pd/MgO for both benzyl alcohol and 1-octanol oxidation reactions. This was attributed to the narrower gold and palladium particle size distribution for Au-Pd/MnO₂ catalysts. Both MgO and MnO₂ materials switched off the disproportionation for benzyl alcohol oxidation when used as supports while for 1-octanol oxidation only MgO material switched it off. These findings show the key role of these nanostructured metal oxides in the oxidation of aliphatic alcohol and therefore the importance of extending the present work further to study more nanostructured metal oxides for various aliphatic alcohols oxidation reactions.
Acknowledgment
This project was funded by the University of Hail (Deanship of Scientific Research), under Grant No. 0150411.
References
- Oxidation of benzyl alcohol and carbon monoxide using gold nanoparticles supported on MnO2 nanowire microspheres. Chemistry. 2014;20:1701-1710.
- [Google Scholar]
- Simple method to synthesize high surface area magnesium oxide and its use as a heterogeneous base catalyst. Appl. Catal. B. 2012;128:31-38.
- [Google Scholar]
- Growth of magnesium oxide thin films using single molecular precursors by metal–organic chemical vapor deposition. Thin Solid Films. 1999;341:63-67.
- [Google Scholar]
- Gold nanoparticle size controlled by polymeric Au (I) thiolate precursor size. J. Am. Chem. Soc.. 2008;130:975-982.
- [Google Scholar]
- Selective suppression of disproportionation reaction in solvent-less benzyl alcohol oxidation catalysed by supported Au–Pd nanoparticles. Catal. Today. 2013;203:146-152.
- [Google Scholar]
- Metal–organic frameworks as solid catalysts for the synthesis of nitrogen-containing heterocycles. Chem. Soc. Rev.. 2014;43:5750-5765.
- [Google Scholar]
- Selective liquid phase oxidation with supported metal nanoparticles. Chem. Sci.. 2012;3:20-44.
- [Google Scholar]
- Nanoscale magnesium hydroxide and magnesium oxide powders: control over size, shape, and structure via hydrothermal synthesis. Chem. Mater.. 2001;13:435-440.
- [Google Scholar]
- Enhanced catalytic and supercapacitor activities of DNA encapsulated β-MnO2 nanomaterials. Phys. Chem. Chem. Phys.. 2014;16:21846-21859.
- [Google Scholar]
- Solvent-free oxidation of primary alcohols to aldehydes using Au-Pd/TiO2 catalysts. Science. 2006;311:362-365.
- [Google Scholar]
- Room temperature aerobic copper-catalysed selective oxidation of primary alcohols to aldehydes. Adv. Synth. Catal.. 2004;346:805-811.
- [Google Scholar]
- Strategies for the synthesis of supported gold palladium nanoparticles with controlled morphology and composition. Acc. Chem. Res.. 2013;46:1759-1772.
- [Google Scholar]
- Effect of phase structure of MnO2 nanorod catalyst on the activity for CO oxidation. J. Phys. Chem. C. 2008;112:5307-5315.
- [Google Scholar]
- Oxidation of alcohols with molecular oxygen on solid catalysts. Chem. Rev.. 2004;104:3037-3058.
- [Google Scholar]
- Structure-sensitive biodiesel synthesis over MgO nanocrystals. Green Chem.. 2009;11:265-268.
- [Google Scholar]
- Solvent-free aerobic oxidation of alcohols using supported gold palladium nanoalloys prepared by a modified impregnation method. Catal. Sci. Technol.. 2014;4:3120-3128.
- [Google Scholar]
- Development of ruthenium-hydroxyapatite-encapsulated superparamagnetic γ-Fe2O3 nanocrystallites as an efficient oxidation catalyst by molecular oxygen. Chem. Mater.. 2007;19:1249-1256.
- [Google Scholar]
- The effect of catalyst preparation method on the performance of supported Au–Pd catalysts for the direct synthesis of hydrogen peroxide. Green Chem.. 2010;12:915-921.
- [Google Scholar]
- Nanoscaled copper metal-organic framework (MOF) based on carboxylate ligands as an efficient heterogeneous catalyst for aerobic epoxidation of olefins and oxidation of benzylic and allylic alcohols. Chemistry. 2015;21:1589-1597.
- [Google Scholar]
- Controlling the duality of the mechanism in liquid-phase oxidation of benzyl alcohol catalysed by supported Au–Pd nanoparticles. Chemistry. 2011;17:6524-6532.
- [Google Scholar]
- Synthesis of stable ligand-free gold–palladium nanoparticles using a simple excess anion method. ACS Nano. 2012;6:6600-6613.
- [Google Scholar]
- Metal-catalyzed Oxidations of Organic Compounds. New York: Academic Press; 1981.
- New challenges in gold catalysis: bimetallic systems. Catal. Sci. Technol.. 2015;5:55-68.
- [Google Scholar]
- Rational design of heterogeneous catalysts for biodiesel synthesis. Catal. Sci. Technol.. 2012;2:884-897.
- [Google Scholar]
- Hierarchical PS/PANI nanostructure supported Cu (II) complexes: facile synthesis and study of catalytic applications in aerobic oxidation. RSC Adv.. 2014;4:55028-55035.
- [Google Scholar]
- Chemoselective catalytic conversion of glycerol as a biorenewable source to valuable commodity chemicals. Chem. Soc. Rev.. 2008;37:527-549.
- [Google Scholar]







