Translate this page into:
Cardioprotective molecular mechanism of syringic acid against isoproterenol induced post- myocardial toxicity in male albino wistar rats
⁎Corresponding author. salthaf@ksu.edu.sa (Althaf Hussain Shaik)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
Background
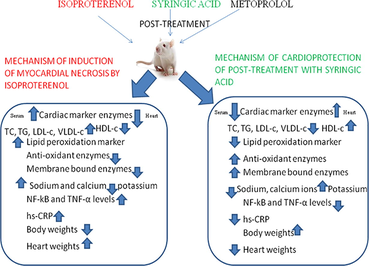
Syringic acid (SA) is a natural phenolic compound and act as anti-diabetic, anti-cancer, anti-inflammatory agents. In our current research, investigation was carried out on the effect of SA on post conditioning on isoproterenol (ISO) induced myocardial injury in wistar rats.
Methods
Male albino wistar rats were administered with ISO (30 mg/kg bw) to second, third and fourth group rats on 1st and 2nd days (with a 24 h interval) of experimental period. SA and metoprolol were orally given immediately after the second dose of ISO to second and fourth group of rats respectively. Myocardial damage was assessed by estimating the cardiac marker enzymes such as creatine kinase-MB (CK-MB), lactate dehydrogenase (LDH) and gamma glutamyl transferase (GGT), anti-oxidant enzymes such as superoxide dismutase (SOD) and catalase (CAT), inflammatory markers such as nuclear factor-kappa B (NF-kB), tumor necrosis factor-α (TNF-α) and high sensitivity-C reactive protein (hs-CRP) along with body and heart weights. Transmission electron microscopy (TEM) was utilized to find any ultra-structural alterations in the heart tissues.
Results
CK-MB, LDH and GGT levels and hs-CRP levels were found to be increased in serum while decreased in hearts of ISO group of rats. SOD and CAT were found to be decreased significantly while NF-kB and TNF-α expression levels were significantly increased in heart tissues of ISO administered rats. Body weights were decreased significantly and heart weights were increased significantly in ISO group of rats. TEM study showed alterations in ISO group hearts. Post-treatment with SA significantly recovered myocardial damage caused by ISO administration.
Conclusion
By counteracting with the above mentioned effects of ISO, our natural compound, SA exhibited recovery of myocardial damage caused by ISO administration in wistar rats. This is the first report revealing the cardioprotective activity of SA in post-myocardial infarction in rats.
Keywords
Syringic acid
Isoproterenol
NF-kB
TNF-α
Transmission electron microscopy
1 Introduction
Cardiovascular disease (CVD) stays the principal cause of loss of lives in both developed and developing countries. It is regarded as a silent infarct due to detection at advanced stages. When blood delivery to the heart is not in the equilibrium with cardiac myocyte requirement, it will result in prolonged ischemia to cardiomyocytes and eventually necrosis is seen in heart myocytes, a condition referred to as acute myocardial infarction (AMI) (Boarescu et al., 2019). Sweating, chest pain, weakness, vomiting, arrhythmia and cause loss of consciousness and even sudden loss of life are key characters of AMI. Several elements increasing the chance of heart attack encompass expanded degree of low density lipoprotein cholesterol (LDL-c), triglycerides (TG), reduces high density lipoprotein-cholesterol (HDL-c) levels, blood cholesterol and blood pressure. An elevated hazard of coronary heart disorder (CHD) is related with excessive levels of serum total cholesterol (TC) (Grundy, 1986) and low density lipoprotein-cholesterol (LDL-c) (Brown and Goldstein, 1986) and decreased levels of high density lipoprotein-cholesterol (HDL-c) (Castelli et al., 1986).
A non-specific β-agonist isoproterenol (ISO), which elevates systemic, pulmonary vasodilation, chronotropy and ionotropic effects (Procaccini et al., 2019). Heart failure, hypertrophy and myocardial ischemia will occur due to the excessive release of catecholamine. ISO in excess doses causes severe myocardial stress, resulting in sub endocardial myocardial ischemia, necrosis and hypoxia (Rona, 1985; Sahu et al., 2015). Number of researches stated that the morphological, pathophysiological and metabolic changes arise inside the hearts of experimental animals following ISO management resembled the ones produced by means of myocardial ischemia in humans (Sahu et al., 2015; Rona et al., 1959; Singal et al., 1982).
A considerable phenolic compound found in spices, pumpkin, grapes, red wine, olives, dates and honey is syringic acid (SA), which has various beneficial properties by acting as anti-microbial, anti-oxidant, anti-inflammation, anti-diabetic, anti-cancer and protecting liver and brain (Srinivasulu et al., 2018). Recent findings suggested that hydrazones of SA acts as carbonyl and radical scavenger and involved in the prevention of carbonyl and oxidative stress in the pathophysiology of atherosclerosis (Belkheiri et al., 2010).
No previous studies were found on the post-treatment with SA against isoproterenol induced myocardial infarction in rats. So we selected the SA to check the therapeutic role in post-treatment to decrease MI. This is the first report revealing the cardioprotective activity of SA in a post-infarction heart failure in albino wistar rats.
2 Materials and methods
ISO, SA and metoprolol (MP) were purchased from Sigma Aldrich Company, USA. All other chemicals used were of analytical grade.
2.1 Animals
Male albino wistar rats were allowed to acclimatization for one week in animal house. 25 °C temperature, 12/12 light and dark cycles and ventilation were subjected to better management. Rats were provided with sufficient food and water. Sri Krishnadevaraya University, ananthapuramu, India got ethical clearance for conducting experiments on animals from Committee for the purpose of Control and Supervision of Experiments on Animals (CPCSEA) (Regd. 1889/GO/Re/S/16/CPCSEA), and this study was accepted by the Indian Animal Ethics Committee (IAEC) protocol No. SKU / Biochem / 08 / 2017.
2.2 Experimental protocols
Totally 24 rats were divided into 4 groups, each group with 6 rats.
Group I: Control group: rats received Dimethyl sulfoxide (DMSO) alone for 7 days
Group II: ISO + SA group: on 1st and 2nd days (with a gap of 24 h), rats received ISO (30 mg/kg bw) and dialy intake of SA (50 mg/kg bw) orally for 7 days
Group III: ISO group: on 1st and 2nd days (an interval of 24 h was maintained), rats received ISO (30 mg/kg bw)
Group IV: ISO + MP group: on 1st and 2nd days (with a 24 h interval), rats received ISO (30 mg/kg bw) and rats dialy intake with metaprolol (MP) (10 mg/kg bw) orally for 7 days.
DMSO was used to dissolveSA, water was used to soluble MP and ISO. Rats of group II and IV were subjected to oral post-treatment with SA and MP respectively through intragastric tube for 7 days. On 1st and 2nd days of experiment period (with a gap of 24 h), ISO was injected sub-cutaneously to rats of group II, III and IV and rats of group II and IV were treated with SA and MP the respectively, immediately after the second dose of ISO and continued post-treatment for upto 7 days.
2.3 Sample collection
All rats were sacrificed using cervical decapitation method following anaesthetization with sodium pentobarbital (35 mg/kg I.P.) at the end of study period. Serum and hearts were gathered right away and finally homogenate of heart tissue was prepared after washing with physiological saline (ice-cold) and subjected to centrifugation for the collection of supernatant to carry out different biochemical examinations. At −80 °C, serum and heart tissues were conserved for further analysis.
2.4 Biochemical measurements
All marker enzymes for heart injury such as creatine kinase-MB (CK-MB) and lactate dehydrogenase (LDH) were estimated by diagnostic kits except gamma glutamyl transferase (GGT) which was analysed by the method of Young and Stirling Donald, 1995. Anti-oxidant enzymes such as superoxide dismutase (SOD) and catalase (CAT) were measured by the methods of Marklund and Marklund, 1974; BEERS and SIZER, 1952 respectively. High sensitivity-C reactive protein (hs-CRP) was measured with diagnostic kit. Body weights and heart weights of rats were also measured.
2.5 Western blot
Left ventricles of myocardium were taken to separate proteins. Cardiac tissues were homogenized, lysed and 10% (w/v) Polyacrylamide gel and 0.1% (w/v) SDS were used to resolve tissue homogenate by the technique of electrophoresis. A film of nitrocellulose was utilized for the transfer of proteins electrophoretically. Incubation of nitrocellulose layer was done with primary and secondary antibodies that are linked with horse-radish peroxidase enzyme. The formation of antigen-antibody complexes were recognized with the enhanced chemiluminiscence detection reagent kit (Biorad). 1:1000, 1:10,000 dilutions were used for anti-Nuclear factor-kappa B (NF-kB) and anti-Tumor necrosis factor-α (TNF-α) antibodies and secondary antibodies respectively.
Quantification of western blot was done with image J software (version 1.49) and expressed as percentage of control group.
2.6 Ultra structural studies of heart tissues by transmission electron microscopy (TEM)
Tiny pieces of heart tissues were rinsed by 0.1 M phosphate buffer (pH 7.2). After the cutting of 1mm thick pieces of heart tissues, fixation was done into 2.5% ice chilled glutaraldehyde in 0.1 M phosphate buffer (pH 7.2) and maintained for 12 h at 4 °C, finally post fixed with the help of osmium tetroxide (buffered). TEM examination was performed by the heart tissue processing. Staining of grids containing sections was done with the help of uranyl acetate (2%) and lead acetate (0.2%). TEM (4000×) was utilized for analyzing these segments of heart.
2.7 Statistical study
To perform the statistical study, results were subjected to Duncan’s Multiple Range (DMR) test by considering p value (p < 0.05). Data was expressed as means ± SD.
3 Results
3.1 Effect of SA on cardiac marker enzymes
SA effect on Cardiac marker enzymes such as CK-MB, LDH and GGT in heart tissue and serum are shown in table 1. A significant (p < 0.05) change of CK-MB, LDH and GGT levels in heart and serum were found to be decreased and augmented respectively in ISO managed rats, compared to control rats. When ISO managed rats were post-treated with SA and MP, these marker enzyme levels were significantly (p < 0.05) brought up and down in heart and serum respectively, compared to ISO alone operated rats. Results are mean ± SD values with superscript letters (a, b, c and d) significantly differ from one another [p<0.05, Duncan’s multiple range test (DMRT)].
parameters
Control
ISO + SA
ISO
ISO + MP
Serum
Heart
Serum
Heart
Serum
Heart
Serum
Heart
CK-MB
313 ± 3.5a
82 ± 4.8a
411.66 ± 43.0b
64.33 ± 1.9b
611 ± 0.8c
52.5 ± 2.6c
355.16 ± 4.53d
72.16 ± 0.9d
LDH
713 ± 7.8a
373 ± 5.7a
891.5 ± 29.9b
254 ± 2.6b
1593.83 ± 5.2c
233.33 ± 10.7c
799.16 ± 22.9d
321.33 ± 6.2d
GGT
7.58 ± 0.1a
5.71 ± 0.4a
9.56 ± 0.31b
3.1 ± 0.2b
12.41 ± 0.09c
2.04 ± 0.1c
7.89 ± 0.06d
4.12 ± 0.1d
3.2 Effect of SA on anti-oxidant enzymes
SOD and CAT are affected by SA post-treatment in heart tissues (Table 2). A significant (p < 0.05) reduced activities of SOD and CAT were detected by ISO administration in rats, while comparing with control rats. SA and MP dramatically increased the activities of these enzymes significantly (p < 0.05) after post-treatment, compared to ISO injected rats. Results are mean ± SD values with superscript letters (a, b, c and d) significantly differ from one another [p<0.05, Duncan’s multiple range test (DMRT)].
Parameters
Control
ISO + SA
ISO
ISO + MP
SOD
39.21 ± 1.22a
27.79 ± 0.39b
23.85 ± 0.99c
32.66 ± 0.81d
CAT
26.47 ± 0.38a
18.45 ± 2.58b
12.50 ± 0.38c
23.45 ± 0.26d
3.3 Effect SA on expression levels of NF-kB and TNF-α
Fig. 1 demonstrate the effect of SA on the expressed protein levels of NF-kB and TNF-α in heart tissues. There was a significant (p < 0.05) increase in protein expression of NF-kB and TNF- α in ISO treated rats, when compared with control rats. Decreased levels of NF-kB and TNF- α were found significantly (p < 0.05) in SA and MP post-treated rats, compared to ISO administered rats.![Effect of post-treatment of SA on the protein levels of NF-kB and TNF-α levels in heart tissues of control and isoproterenol (ISO) treated rats. Results are mean ± SD values with superscript letters (a, b, c and d) significantly differ from one another [p<0.05, Duncan’s multiple range test (DMRT)].](/content/185/2020/32/2/img/10.1016_j.jksus.2019.11.030-fig2.png)
Effect of post-treatment of SA on the protein levels of NF-kB and TNF-α levels in heart tissues of control and isoproterenol (ISO) treated rats. Results are mean ± SD values with superscript letters (a, b, c and d) significantly differ from one another [p<0.05, Duncan’s multiple range test (DMRT)].
3.4 Effect of SA on hs-CRP
SA effect on inflammatory markers is exhibited in Fig. 2. A significant (p < 0.05) increase in the serum hs-CRP levels were detected in ISO injected rats in contrasting with control group of rats. Levels of hs-CRP in the serum revealed a significant (p < 0.05) decrease in SA and MP post-treated rats, when compared to ISO administered rats.![Effect of post-treatment of SA on high sensitivity c - reactive protein (hs-CRP) levels in serum samples of control and isoproterenol (ISO) treated rats. Results are mean ± SD values with superscript letters (a, b, c and d) significantly differ from one another [p<0.05, Duncan’s multiple range test (DMRT)].](/content/185/2020/32/2/img/10.1016_j.jksus.2019.11.030-fig3.png)
Effect of post-treatment of SA on high sensitivity c - reactive protein (hs-CRP) levels in serum samples of control and isoproterenol (ISO) treated rats. Results are mean ± SD values with superscript letters (a, b, c and d) significantly differ from one another [p<0.05, Duncan’s multiple range test (DMRT)].
3.5 Effect of SA on body weights and heart weights
Impact of SA on weights of body and heart are expressed in Figs. 3A and B. After the completion of experimental period, in ISO group we found that body weights were decreased significantly (p < 0.05) and heart weights were increased significantly (p < 0.05). Interestingly we observed that a significant (p < 0.05) increase in body weights and a significant (p < 0.05) decrease in heart weights in rats, which were post-treated with SA and MP for 7 days.![Effect of post-treatment of SA on body weights of control and isoproterenol (ISO) treated rats. Results are mean ± SD values with superscript letters (a, b and c) significantly differ from one another [p<0.05, Duncan’s multiple range test (DMRT)].](/content/185/2020/32/2/img/10.1016_j.jksus.2019.11.030-fig4.png)
Effect of post-treatment of SA on body weights of control and isoproterenol (ISO) treated rats. Results are mean ± SD values with superscript letters (a, b and c) significantly differ from one another [p<0.05, Duncan’s multiple range test (DMRT)].
![Effect of post-treatment of SA on heart weights of control and isoproterenol (ISO) treated rats. Results are mean ± SD values with superscript letters (a, b and c) significantly differ from one another [p<0.05, Duncan’s multiple range test (DMRT)]. * Group is significantly not different with ISO+SA group.](/content/185/2020/32/2/img/10.1016_j.jksus.2019.11.030-fig5.png)
Effect of post-treatment of SA on heart weights of control and isoproterenol (ISO) treated rats. Results are mean ± SD values with superscript letters (a, b and c) significantly differ from one another [p<0.05, Duncan’s multiple range test (DMRT)]. * Group is significantly not different with ISO+SA group.
3.6 Effect of SA on ultra structural changes of myocardial tissues
Post-treatment effect of SA on heart tissues in control and ISO treated groups are shown in Fig. 4. Irregular ‘z’ lines, swollen mitochondria and fragmented myofibrils were found in the ISO administered heart tissues, when compared to control rats. SA post-treated heart tissues exhibited ‘z’ lines with regular arrangement, decreased content of fragmented myofibrils and decrease in the swelling of mitochondria.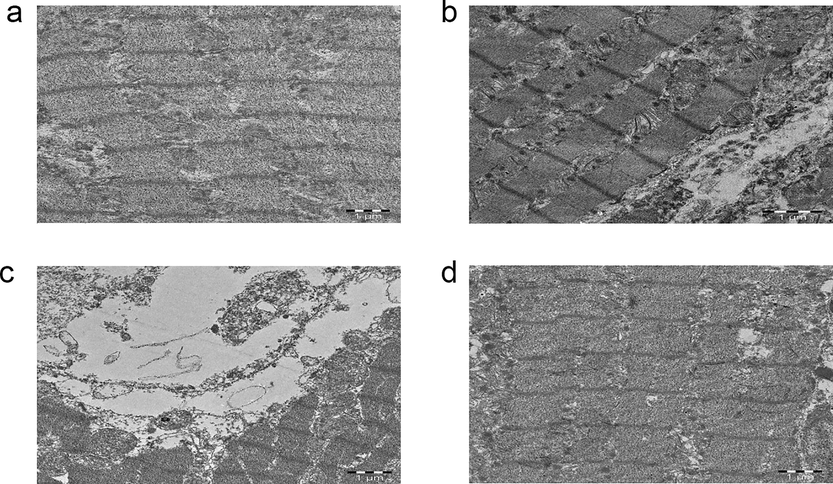
Electron photomicrographs (TEM 4000×) of the heart tissues of normal, treated and isoproterenol (ISO) treated experimental rats.
4 Discussion
4.1 Effect of SA on cardiac marker enzymes
Cardiac marker enzymes such as CK-MB levels demonstrated that a marked increment in serum and reduction in heart tissue levels were detected in rats, which were induced by ISO. The reason may reflect that ISO administration leads to the necrosis of heart tissue, cellular damage with loss of permeability and functional integrity of the cell membrane (IR et al., 2008; Alam et al., 2018). Further increment in serum LDH and GGT, while decrement in heart LDH and GGT supported the cardiomyocyte membrane damage in ISO administered rats. SA post-administration exhibited a marked reduction in ISO induced elevated serum marker enzymes. Serum marker enzyme levels are reduced due to action of SA on maintaining integrity of the membrane, thereby preventing these enzymes leakage. Myocardial injury, which is caused by ISO administration, is mediated through the β1-adrenergic receptor.
Acute stimulation of β-adrenergic receptor not only produces reactive oxygen species (ROS) but also depresses total cellular anti-oxidant capability, down regulates the catalytic nature of copper-zinc SOD enzyme, m-RNA and protein and level of glutathione, leads to the damage to the integrity of the membrane and reducing the contractile capability of the heart and toxicity of myocytes, finally leads to the myocardial necrosis (Rathore et al., 1998; Srivastava et al., 2007). In our current approach, we detected that SA conserved the myocardium from ISO-caused myocardial functional and structural damage via decreasing the levels of serum diagnostic marker enzymes. Our results are in agreement with the previous results (Jia et al., 2017; Mnafgui et al., 2016; Ulla et al., 2017).
4.2 Effect of SA on anti-oxidant enzymes
SOD and CAT which are endogenous anti-oxidant enzymes are the first-line defense system against oxidative stress, destroying oxygen (O2) and hydrogen peroxide (H2O2) before they interact to produce highly reactive hydroxyl radical. For the effective removal of oxygen stress in intracellular organelles, an effective equilibrium must be established between these anti-oxidant enzymes. Free radical induced cell injury is prevented efficiently with the help of SOD and CAT. SOD is the only enzyme, which uses a free radical as a substrate. Enhancement in the activity of SOD is a chief requirement to scavenge free radicals. CAT and/or GSH are in inactivated state even though SOD is in activated state; this situation is detrimental because SOD forms more cytotoxic product, hydrogen peroxide, which is not removed by the CAT and/or GSH when they are in inactivated state. So increase in SOD must sync with the increase in the activities of CAT and GSH for an overall beneficial effect (Syeda Nishat Fathima, 2019).
In ISO treated rats, we found a drastic decrease in SOD and CAT content in heart tissues. This may be due to ISO causes an imbalance between anti-oxidative defense system and production of ROS leads to the occurrence of MI (Zhou et al., 2008) through the generation of adrenochromes such as quinines via ISO auto-oxidation, finally leads to the synthesis of highly toxic O2 free radicals such as superoxide (O2−) and H2O2. Moreover Ca2+ overload and their consequences are crucial in ISO-caused injury and synthesis of ROS (Díaz-Muñoz et al., 2006). Cell membrane phospholipids and fatty acids are the key targets for the attack by ROS forming lipid peroxides, which survive for longer periods than ROS and can begin the chain reactions that enrich the oxidative damage to cells (Sharma et al., 2012). However the depletion of the anti-oxidant systems such as SOD occurs when the oxidative pressure increases. Reduction in the activity of SOD in ISO induced rats might be due to the enhanced formation of ROS, such as O2− and H2O2, which could reduce the activity of SOD. Post-treatment with SA to ISO administered rats increased these anti-oxidants due to possessing free radical scavenging, anti-oxidant activities and by increasing the availability of GSH levels. Earlier report supports the results of our research (Ashokkumar et al., 2018).
4.3 Effect SA on expression levels of NF-kB and TNF-α
Regulation of oxidative pressure, expression of inducible nitric oxide synthase, apoptosis and NF-kB activation will be carried out by the binding of TNF-α with TNF- α receptor (TNFR1) and TNFR2 (Ozsoy et al., 2008; Varfolomeev and Vucic, 2008). Nuclear factor-kappa B (NF-kB) is a complex of proteins, which participates in the transcription of DNA, production of cytokines and survival of cells. NF-kB is actually activated by the central receptor namely receptor activator of NF-kB (RANK), a type of TNFR. In normal conditions, NF-kB receptor is under inactivation by exhibiting interaction with Inhibitory kappa-B (Ik-B) in the cytoplasm. Whenever NF-kB is to be stimulated, Ik-B will get phospharylated and degraded, which results in the release of NF-kB from Ik-B and enters the nucleus, where we can observe binding with target gene promoter. An inflammatory response was shown generally in CVD. In myocardial infarctions, studies have found increased levels of pro-inflammatory cytokines such as TNF-α and NF-kB, which depend on the NF-kB activation (Karin and Ben-Neriah, 2000). In our research, we detected enhanced levels of NF-kB and TNF-α expression in the heart tissues of ISO operated rats, when compared with control rats, which might be due to ISO induced inflammation. Upon post-treating the rats with SA, we got a result that showing the drastic decrease in the NF-kB and TNF-α expression in the heart tissues, when compared to ISO rats. This result was due to the anti-inflammatory nature of SA, which may be brought by preventing the Ik-B degradation or by directly blocking the RANK. Our results show agreement with the past studies (Dai et al., 2019; Jia et al., 2017; Guo et al., 2011).
4.4 Effect of SA on hs-CRP
A major sensitive marker of inflammation, tissue damage and infection is indirectly participate in the coagulation process is a classic acute-phase protein, called hs-CRP. The lifespan of the hs-CRP is nearly 19 h in the plasma. There was a best correlation between total infarct size in AMI and circulating hs-CRP levels in clinical studies. Thus we can conclude that hs-CRP is a reliable diagnostic indicator for underlying coronary inflammation along with extent of myocardial necrosis. However previous research clearly exhibited the correlation between hs-CRP and AMI in human beings (Badiger et al., 2014; Carrero et al., 2019). Co-deposition of CRP was seen with activated complement in AMI (Lagrand et al., 1997) and research outcomes have clearly demonstrated that the response of CRP not only reflect the damage to tissues in this context but also show a marked role in the development of myocardial injury (Griselli et al., 1999). A recognizable role of CRP was noticed in the pathogenesis of CVD and as a predictor of CVD contain many effects of CVD such as clotting, oxygen radical synthesis, enhancing the adhesion molecule expression and plasminogen activator inhibitor1 and destabilization of plaque, which could lead to CVD.
A drastic surge in the hs-CRP levels was detected in the rats of ISO group, when compared to the normal group of rats. This may be occurred due to necrosis of myocardial cells, caused by ISO. CRP attaches to the phosphocholine, which is expressed on the surface of dead and dying cell. This results in the complement system activation, enhancing phagocytosis by macrophages (Bray et al., 2016). Levels of hs-CRP were decreased significantly in the serum of SA post-treated rats, upon comparing with control rats. This may be due to anti-oxidant and anti-inflammatory activity of SA.
4.5 Effect of SA on body weights and heart weights
In the present study, ISO injection was associated with decrease in body weights and marked increase in heart weights. Decrease in the body weights were linked to consumption of low diet by ISO administration (Patel et al., 2010). However post-treatment with SA exhibited a marked increase in body weights, which could be attributed to the capacity of SA to increase the diet intake in the rats of SA group. Increase in heart weight is might be due to increase in water content, increase in protein content and edematous intravascular space (Patel et al., 2010). When post-treated with SA, a recognizable decrement in heart weights was noticed. This effect will come due to SA post-treatment showed a marked decrease in the water content and protein content in heart tissues. Previous research studies are in good support with our research output (Wang et al., 2019).
4.6 Effect of SA on ultrastructural studies of myocardial tissues-TEM
To evaluate the cardioprotection of SA in post-myocardial infarction, TEM study was conducted to support the biochemical and molecular studies. Our results confirmed the cardioprotection of SA by exhibiting regeneration of heart tissues, which was previously damaged by ISO. Thus in our research, we found that all biochemical, molecular studies were further supported by TEM observations. Due to the generation of ISO induced more number of free radicals, mitochondrial damage was observed along with the dysfunction of mitochondria, which are the main characteristics of heart diseases. Heart tissues of ISO administered rats showed fragmentation of myofibrils as well as detectable alterations in size, shape and swelling of mitochondria. Deposition of products of lipid peroxidation occurs due to the GSH depletion, which is the major cause for the mitochondrial swelling. A significant regular arrangement of ‘Z’ lines, myofibrils as well as reduced swelling of mitochondria was detected in ISO administered rats, which were post-treated with SA. This outcome could be due to the free radical scavenging property and anti-oxidant activity of SA. Therefore mitochondrial swelling reduces by decreasing accumulation of lipid peroxidation products in mitochondria by increasing the GSH synthesis.
5 Conclusion
Oral post-treatment with SA reduced cardiotoxicity via minimizing alterations in cardiac marker enzymes, anti-oxidant enzymes, NF-kB and TNF-α. Thus our examination reveals cardioprotective potency of SA in ISO induced oxidative stress in rats.
Acknowledgements
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding this work through research group number (RG-1438-058).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Astaxanthin prevented oxidative stress in heart and kidneys of isoproterenol-administered aged rats. J. Diet. Suppl.. 2018;15:42-54.
- [CrossRef] [Google Scholar]
- Vitexin protects isoproterenol induced post myocardial injury by modulating hipposignaling and ER stress responses. Biochem. Biophys. Res. Commun.. 2018;496:731-737.
- [CrossRef] [Google Scholar]
- hs-C-reactive protein as an indicator for prognosis in acute myocardial infarction. J. Sci. Soc.. 2014;41:118.
- [CrossRef] [Google Scholar]
- A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J. Biol. Chem.. 1952;195:133-140.
- [Google Scholar]
- Synthesis and antioxidant activity evaluation of a syringic hydrazones family. Eur. J. Med. Chem.. 2010;45:3019-3026.
- [CrossRef] [Google Scholar]
- Effects of curcumin nanoparticles in isoproterenol-induced myocardial infarction. Oxid. Med. Cell. Longev. 2019
- [CrossRef] [Google Scholar]
- Erythrocyte sedimentation rate and C-reactive protein measurements and their relevance in clinical medicine. Wis. Med. J.. 2016;115:317-321.
- [Google Scholar]
- hsCRP Level and the risk of death or recurrent cardiovascular events in patients with myocardial infarction: a healthcare-based study. J. Am. Heart Assoc.. 2019;8:e012638
- [CrossRef] [Google Scholar]
- Incidence of coronary heart disease and lipoprotein cholesterol levels. The Framingham Study. JAMA. 1986;256:2835-2838.
- [Google Scholar]
- Phosphocreatine attenuates isoproterenol-induced cardiac fibrosis and cardiomyocyte apoptosis. Biomed. Res. Int.. 2019;2019
- [CrossRef] [Google Scholar]
- Correlation between oxidative stress and alteration of intracellular calcium handling in isoproterenol-induced myocardial infarction. Mol. Cell. Biochem.. 2006;289:125-136.
- [CrossRef] [Google Scholar]
- C-reactive protein and complement are important mediators of tissue damage in acute myocardial infarction. J. Exp. Med.. 1999;190:1733-1740.
- [CrossRef] [Google Scholar]
- Telmisartan attenuates isoproterenol-induced cardiac remodeling in rats via regulation of cardiac adiponectin expression. Acta Pharmacol. Sin.. 2011;32:449-455.
- [CrossRef] [Google Scholar]
- IR, M., Arya DS, G.S., 2008. Dietary Curcuma longa protects myocardium against isoproterenol induced hemodynamic, biochemical and histopathological alternations in rats. Int. J. Appl. Res. Nat. Prod. 1, 19–28.
- Protective effect of diethylcarbamazine inhibits NF-κB activation in isoproterenol-induced acute myocardial infarction rat model through the PARP pathway. Mol. Med. Rep.. 2017;16:1596-1602.
- [CrossRef] [Google Scholar]
- Phosphorylation meets ubiquitination: the control of NF-κB activity. Annu. Rev. Immunol.. 2000;18:621-663.
- [CrossRef] [Google Scholar]
- C-reactive protein colocalizes with complement in human hearts during acute myocardial infarction. Circulation. 1997;95:97-103.
- [CrossRef] [Google Scholar]
- Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem.. 1974;47:469-474.
- [CrossRef] [Google Scholar]
- Anti-inflammatory, antithrombotic and cardiac remodeling preventive effects of eugenol in isoproterenol-induced myocardial infarction in wistar rat. Cardiovasc. Toxicol.. 2016;16:336-344.
- [CrossRef] [Google Scholar]
- Oxidative stress promotes ligand-independent and enhanced ligand-dependent tumor necrosis factor receptor signaling. J. Biol. Chem.. 2008;283:23419-23428.
- [CrossRef] [Google Scholar]
- Cardioprotective effect of melatonin against isoproterenol induced myocardial infarction in rats: a biochemical, electrocardiographic and histoarchitectural evaluation. Eur. J. Pharmacol.. 2010;644:160-168.
- [CrossRef] [Google Scholar]
- Pharmacology of cardiovascular drugs. In: Critical Heart Disease in Infants and Children (third ed). Elsevier Inc.; 2019.
- [Google Scholar]
- Lipid peroxidation and antioxidant enzymes in isoproterenol induced oxidative stress in rat tissues. Pharmacol. Res.. 1998;38:297-303.
- [CrossRef] [Google Scholar]
- An infarct-like myocardial lesion and other toxic manifestations produced by isoproterenol in the rat. AMA. Arch Pathol. 1959
- [Google Scholar]
- Lagerstroemia speciosa L. attenuates apoptosis in isoproterenol-induced cardiotoxic mice by inhibiting oxidative stress: possible role of Nrf2/HO-1. Cardiovasc. Toxicol.. 2015;15:10-22.
- [CrossRef] [Google Scholar]
- Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot.. 2012;2012:1-26.
- [CrossRef] [Google Scholar]
- Role of free radicals in catecholamine-induced cardiomyopathy. Can. J. Physiol. Pharmacol.. 1982;60:1390-1397.
- [CrossRef] [Google Scholar]
- Syringic acid (SA) - a review of its occurrence, Biosynthesis, Pharmacological and Industrial Importance. Biomed. Pharmacother.. 2018;108:547-557.
- [CrossRef] [Google Scholar]
- Downregulation of CuZn-superoxide dismutase contributes to beta-adrenergic receptor-mediated oxidative stress in the heart. Cardiovasc. Res.. 2007;74:445-455.
- [CrossRef] [Google Scholar]
- Syeda Nishat Fathima, S.V.M., 2019. Cardioprotective Effects to Chronic Administration of Rosa Damascena Petals in Isoproterenol Induced Myocardial Infarction: Biochemical, Histopathological and Ultrastructural Studies. Biomed. Pharmacol. J. 12.
- Coenzyme Q10 prevents oxidative stress and fibrosis in isoprenaline induced cardiac remodeling in aged rats. BMC Pharmacol. Toxicol.. 2017;18
- [CrossRef] [Google Scholar]
- (Un)expected roles of c-IAPs in apoptotic and NFkappaB signaling pathways. Cell Cycle. 2008;7:1511-1521.
- [CrossRef] [Google Scholar]
- Stevioside attenuates isoproterenol-induced mouse myocardial fibrosis through inhibition of the myocardial NF-κB/TGF-β1/Smad signaling pathway. Food Funct.. 2019;10:1179-1190.
- [CrossRef] [Google Scholar]
- Effects of Drugs on Clinical Laboratory Tests (fourth ed.). Washington D.C: AACC Press; 1995.
- Cardioprotective effect of fluvastatin on isoproterenol-induced myocardial infarction in rat. Eur. J. Pharmacol.. 2008;586:244-250.
- [CrossRef] [Google Scholar]







