Translate this page into:
Calliphorids as forensic indicator to facilitate PMI estimation: A case study from Chhattisgarh, India
⁎Corresponding author. madhubaladhakane@gmail.com (Madhu Bala)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objectives
Calliphorids especially blowflies are act as important forensic indicators as they are foremost visitors of the corpse and act as silent witness after the accomplishment of crime.
Methods
In present case study immature stages of blowflies utilized for PMI estimation. Postmortem Interval (PMI) of a highly putrefied male corpse in advanced stage of decomposition was estimated. Corpse was found under the culvert of Bijapur – Bhopalpatnam road, Village Modakpal, District Bijapur, State Chhattisgarh, India. PMI was determined with the help of Accumulated Degree Hours (ADH) method based on developmental period of third instar larvae of Blowfly Chrysomya megacephala (Fabricius). Tentative PMI of corpse is 5.5 days. Apart from Chrysomya megacephala immature stages as well as adults of Chrysomya rufifaces (Macquart) were also collected from deceased body. Entomological samples were sent for identification to the department of Zoology and Environmental Sciences, Punjabi University, Patiala, India.
Results and conclusions
Larval stages were identified based on anterior and posterior spiracles and cephalophrangeal apparatus. Adults were identified based on their morphological characters. For accurate PMI estimation correct insect identification and meteorological data of crime scene are key parameters.
Keywords
Blowfly Chrysomya megacephala (Fabricius)
Maggots
Postmortem Interval (PMI) ADH method
1 Introduction
Forensic entomology is a study grounded by the insects arrived on the decaying carrion and plays an important role in the estimation of minimum postmortem interval. In the carrion depletion insects plays an important contribution and consequently are of great forensic interest. Blow flies commonly acts as forensic indicator as their larvae feeds on decomposing carcass. Odor of decaying corpse can attract blow flies over several kilometers (Braack, 1981). The developmental times of an immature are species specific and dependent upon the various environmental conditions to which the immature have been grown (Donovan et al., 2006). The carrion undergoes various changes because of various factors including the environmental conditions, physiochemical variables, decomposition stages of the corpse and occupation of scavenger animals (Marshall, 1989). During PMI estimation forensic pathologists are able to estimate time since death within 72 h after death (Prieto et al., 2004). The estimation of PMI commonly based on the physical changes occurs after a person’s death, including algor mortis, livor mortis, rigor mortis etc but these changes do not remain active after 72 hrs of postmortem (Guo et al., 2016). After that time interval, forensic experts face many obstacles regarding the estimation of postmortem interval (Prieto et al., 2004). Insects remain on corpse for a longer period varies from several days to months. Flies laid their eggs and development of immature stages stared on corpse, which helps in PMI estimation. Development data and succession study of necrophagous insects on corpse can be used for the estimation of postmortem interval (Amendt et al., 2007). Therefore, a sound knowledge about the habitat, geographic distributions, morphology, and developmental statistic of each species of necrophagous insects required for accurate PMI estimation (Catts and Goff, 1992).
The blow flies are the first arrivers on the corpse and utilize it as a substrate for egg laying. The oriental latrine fly Chryosomya megacephala is the most dominant necrophagous species on the corpse due to its large population size (Shiao and Yeh, 2008; Sukontason et al., 2008). The distribution of C. megacephala has increased gradually and now this species available all over the world (Villet and Williams, 2006). The various studies have already shown the forensic important of C. megacephala as it is one of the first fly appears on the corpse (Heo et al., 2008; Wang et al., 2008; Shi et al., 2009; Silahuddin et al., 2015; Wang et al., 2017). Under favorable condition, within an hour females of C. megacephala can arrive on the carcass and lays about a mass of 220–325 eggs on it (Badenhorst and Villet, 2018). In present case study, PMI of a skeletonized body of about 45 years old male from Bijapur (Chhattisgarh, India) was estimated by utilizing ADH method based on the development of larvae of blow fly C. megacephala.
2 Material and methods
Numerous fly maggots were collected from the skeletonized corpse with the help of forceps from the scene of occurrence i.e., village Modakpal, Chhattisgarh, India, by the first author and placed into 20 ml clean plastic vials. Some maggots were killed and preserved in spirit solution, whereas some were reared to get adult flies by the investigating officer of the case in the police station Modakpal under the supervision of the first author. Both the preserved and reared samples were sent to the Department of Zoology and Environmental Sciences, Punjabi University, Patiala, Punjab, India for identification. The measurement of adults and immature stages was done with the help of measuring scale. The third instars larvae of C. megacephala, was identified by analyzing the anterior spiracles, posterior spiracles, Cephalopharyngeal apparatus and arrangements of spines. The slides were prepared to analyze the anterior spiracle, posterior spiracle and Cephaloskelton. Adult flies were identified by running keys based on their morphological characters.
For PMI estimation, Accumulated Degree Hours (ADH) method was utilized. ADH was calculated based on developmental time taken by Chrysomya megacephala to reach up to third instar larval stage at 28 ± 1 ℃. Meteorological data of crime scene was obtained from the weather forecast station of Bijapur, Chhattisgarh. Total ADH was calculated by taking average temperature from 9th June to 15th June 2019. The ADH method was applied by using formula, ADH = Time (hrs) × (average temperature − minimum development threshold temperature).
3 Results
On 15/06/2018, an unidentified skeletonized body of about 45 years old male was found on the Bijapur-Bhopalpatnam road (Chhattisgarh), under the culvert (Fig. 1A&B). The body was brought to the mortuary of Late BRKM Govt. Medical College for postmortem. The corpse was wrapped in black plastic sheet. There was no evidence of clothes or any personal belongings on corpse for personnel identification. The body was in the advanced stage of decomposition. Face was skeletonized with few skin and muscle tissues remain adherent at places. Postmortem lividity and rigor mortis were also absent. Body was blackish brown in color and maggots were present all over the face, chest, upper limbs, abdominal cavity, and perineum at places. Strong foul odor of decomposition was present. The skull seemed with mandible and included raised supra-orbital ridges, acute fronto-nasal junction, sloping forehead, prominent styliod processes both sides, low set square shaped orbits, prominent zygomatic arches, prominent occipital protuberance. Metopic suture was present, not fused completely. Moreover Sagittal, coronal, temporal, lambdoid suture was all partially fused. Both upper and lower jaw had 14 teeth with space for third molar on both sides. Anatomical features of all bones were male type. All the ossification center of long bones was fused. Approximate height was estimate as about 173 cm. Maggots were collected from the body and preserved in the spirit during postmortem examination (Fig. 1C–F).
A & B. Skeletonized remain of male corpse. C. Third instar larvae of C. megacephala. D. Third instar larvae of C. rufifacies E. Pupa of C. megacephala F. adult of C. megacephala collected from corpse.
Since there was no information regarding when the deceased was last seen, when he was alive or any missing report of person. Therefore, tentative PMI was estimated, by analyzing the development period of Chrysomya megacephala and by using ADH method. In ADH method, Accumulated Degree Hours (ADH) taken by Chrysomya megacephala to reach the pupal stage at 28 ± 1 °C was calculated by using the data mentioned in literature (Bharti et al., 2007) (Table 2). The average temperature was calculated from 9th June to 15th June by using the temperature data obtained from the weather forecast station of Bijapur, Chhattisgarh (Fig. 2). Average temperature of each day was subtracted from threshold temperature which is 10 °C in case of insects to obtain accumulated growth degree day values. Accumulated degree days then multiplied with 24 to obtain Accumulated Degree Hours (Table 1). Total ADH was calculated by adding the Accumulated Degree Hours from 9th June to 15th June (Table 2).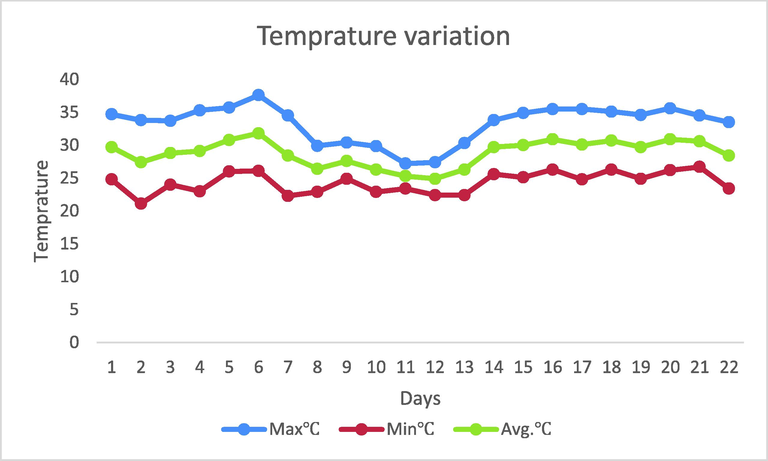
Temperature variation from 1 June 2018 to 22 June 2018.
Determination of Accumulated Degree Hours (ADH)
Date
Temperature
Threshold temp. ℃
Accumulated Degree Day Value (DD)
Accumulated Degree Hours
Max ℃
Min ℃
Avg. ℃
DD × 24 h
1/06/2018
34.7
24.8
29.7
10
19.7
472.8
02/06/2018
33.8
21.1
27.4
10
17.4
417.6
03/06/2018
33.7
24
28.8
10
18.8
451.2
04/06/2018
35.3
23
29.1
10
19.1
458.4
05/06/2018
35.7
26
30.8
10
20.8
499.2
06/06/2018
37.6
26.1
31.8
10
21.8
523.2
07/06/2018
34.5
22.3
28.4
10
18.4
441.6
08/06/2018
29.9
22.9
26.4
10
16.4
393.6
09/06/2018
30.4
24.9
27.6
10
17.6
422.4
10/06/2018
29.85
22.9
6.3
10
16.3
391.2
11/06/2018
27.2
3.4
25.3
10
15.3
367.2
12/06/2018
27.4
22.4
24.9
10
14.9
357.6
13/06/2018
30.3
22.4
6.3
10
16.3
391.2
14/06/2018
33.8
25.6
29.7
10
19.7
472.8
5/06/2018
34.9
25.1
30
10
20
480
16/06/2018
35.5
26.3
30.9
10
20.9
501.6
17/06/2018
35.5
4.8
30.1
10
20.1
482.4
18/062018
35.1
26.3
30.7
10
496.8
19/06/2018
34.6
24.9
29.7
10
19.7
472.8
20/06/2018
35.6
26.2
30.9
10
20.9
501.6
21/06/2018
34.5
26.7
30.6
10
20.6
494.4
22/06/2018
33.5
23.4
28.4
10
18.4
441.6
Accumulated Degree Hours Calculation
Collection of maggots
15 June
Accumulated Degree Hours (ADH) taken by Chrysomya megacephala to reach the third instar larval stage at 28 ± 1 °C
(15 + 14 + 21 + 25 + 34 + 97) × 24 = 4944
Total ADH from 9th June to 15th June
(422.4 + 391.2 + 367.2 + 357.6 + 391.2 + 472.8 + 480) = 2882.4 ADH
Difference between Accumulated Degree Hours (ADH) taken by Chrysomya megacephala to reach the pupal stage at 28 ± 1 °C Total ADH from 9 to 15 June
4944–2882.4 = 2061.6 ADH
Determination of PMI
422.4 × Y = 2061.6, Y = 2061.6/422.4 = 4.8 Days
Chrysomya megacephala laid eggs on corpse on 10 June 2019
The Total ADH from 9 to 15 June was subtracted from Accumulated Degree Hours (ADH) taken by Chrysomya megacephala to reach pupal stage at 28 ± 1 ℃ which was about 2061.6 ADH (Table 2). For the determination of PMI, 2061.6 ADH was divided by 422.4 ADH of the 9th June (Table 2). The estimated PMI of male corpse was about 5.5 days.
Both adults and third instars larvae were identified by using keys based on morphological characters under stereo zoom binocular microscope (RI-90-02). The larvae were identified by analyzing their morphological characters i.e., anterior spiracles, posterior spiracles, Cephaloskeleton. Both the Larvae and adult specimens were identified as blowfly species Chrysomya megacephala and Chrysomya rufifacies.
3.1 Description of Chrysomya megacephala adults
In the adults of Chrysomya megacephala anterior thoracic spiracle may be white or brown. Thorax of adults is blueish green in color with two short longitudinal stripes present on the dorsal position. Anterior spiracle in female appeared white (Fig. 2A).
In the eyes of female of C. megacephala the facets are comparably smaller than male and uniformly arranged without any demarcation (Fig. 3B). In female the Parafrontalia slightly narrower than the width of frons (Fig. 3B). It is covered with golden tomentum and appeared black toward vertex (Fig. 3A, B). Gena or jowl is golden (Fig. 3A, B). The frons of female is nearly parallel and dark to reddish in color (Fig. 3B). Rows of small hairs are present on the upper part with dark fronto-orbital plate (Fig. 3B). Parafacialia, face and epistome are orange in color (Fig. 3B). The antennae are orange in color with black and orange antennal arista (Fig. 3B). The upper enlarge facets in the eyes of the male of C. megacephala is joined and demarcated separately from lower small facets (Fig. 3D). In male Paraforontalia is much shortens than the female and appeared like a fine line (Fig. 3D). Gena is golden and covered with golden tomentum (Fig. 3C, D). Frons in male is almost eradicated throughout its length (Fig. 3D). Anterior spiracle in male is appeared dark brown (Fig. 3C).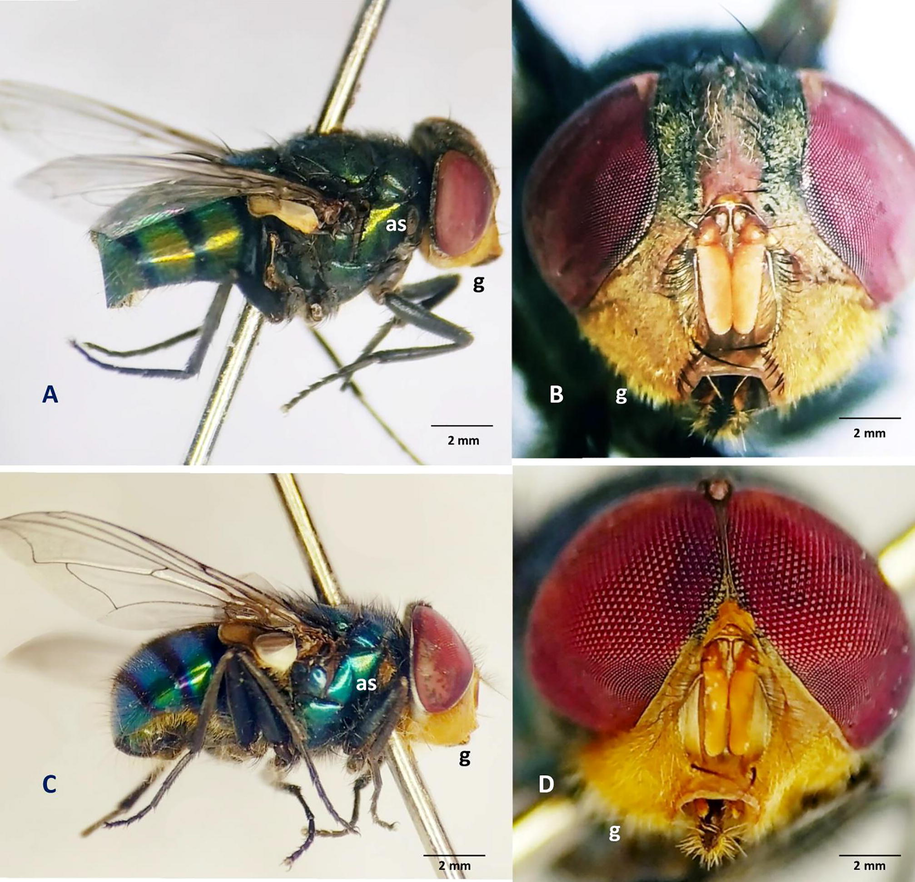
A. Female of C. megacephala lateral view, B. Head of C. megacephala female, C. Male of C. megacephala lateral view, D. Head of C. megacephala male. Abbreviations: as- anterior spiracle, g- gena.
3.2 Description of adults of C. rufifacies
Adults of C. rufifacies are greenish blue in color which reflects purple color under lights (Fig. 4B). In the eyes of adults of C. rufifacies the facets are small and constant (Fig. 4B). In females the Parafrontalia are of equal width to frons which shades black on upper half and the lower half is covered with the silver tomentum (Fig. 3A, B). Gena or jowl is white (Fig. 4A, B). The Parafacialia, palpi and epistome are orange in color (Fig. 4B). The antennae are reddish-brown in color (Fig. 4B). Anterior spiracle in female appeared white (Fig. 4A).
A. Female of C. rufifacies lateral view, B. Head of C. rufifacies female. Abbreviations: as- anterior spiracle, g- gena.
3.3 Description of Chrysomya megacephala larvae
The fully developed 3rd instars of C. megacephala was vermiform and muscoid shaped. The 3rd instarslarvae were about 16 mm long. The integumental structure of 3rd instars is very smooth with small spines scattered at each segments. On the last segments an incomplete spinules band is present. The cephalic region has a cluster of cuticular spines with one to three pointed tips. The spines are large fleshy with dark serrated tips.
3.4 Cephaloskeleton
The cephaloskeleton shows curved mouth hook part (mh) (Fig. 5A). The Dorsal cornu (dc) is longer than the ventral cornu (vc) (Fig. 5A). Parastomal bar (pb) is well developed along the length of dorsal sclerite (ds) (Fig. 5A). Parastomal bar (pb) is almost equals to the dorsal sclerite (ds) and curved upward at the apex (Fig. 5A). Length of ventral cornu (lvc) is smaller than the Width of ventral plate (wvp) (Fig. 5A). Dorsal cornu has an open window at apex (Fig. 5A). The ventral cornu has a well-developed close window (Fig. 5A).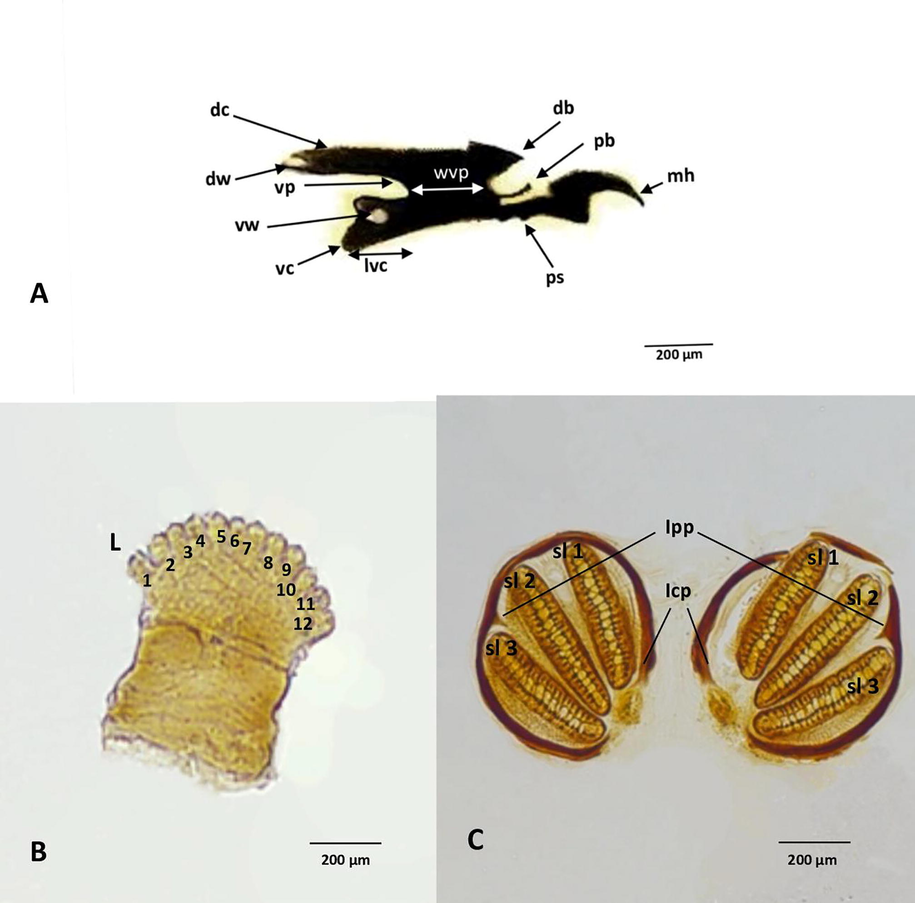
Third instars morphology of C. megacephala A. Cephaloskeleton, B. Anterior spiracle, C. Posterior spiracle. Abbreviations: db- dorsal bridge, dc- dorsal cornua, ps- pharyngeal sclerite, dw- dorsal window, length of ventral cornu (lvc), mh- mouthhook, pb- parastomal bar, vc- ventral cornua, vp- ventical plate, vw- ventral window, wvp- width of ventical plate, l- spiracular lobes, Icp- incomplete peritreme, Ipp- internal peritremal projections, sl-spiracular slit.
3.5 Anterior spiracular
The anterior spiracles consist of 12 marginal spiracular lobes (Fig. 5B). The spiracular lobes are arranged in a single symmetrical row (Fig. 5B).
3.6 Posterior spiracles
At the caudal segment, a pair of the posterior spiracles arranged without a spiracular cavity at the end (Fig. 5C). The posterior spiracles are arranged close to each other (Fig. 5C). The peritreme was heavily sclerotized and incomplete structure (Fig. 5C). The peritreme encloses the three spiracular slits and present without bottom (Fig. 5C). The spiraculaer slits are relatively straight, oval, and elongated in structure (Fig. 5C). A triangular shape inner peretremal projection appears only between the second and third slit (Fig. 5C).
3.7 Pupa
The pupa and 3rd instars larva carry the same identifying character as the pupa formation takes places from the mature larvae. The pupa of C. megacephala is dark brown in color with yellowish-brown anterior spiracles (Fig. 1E).
4 Discussion
Present work is related to a case study from Bijapur, Chhattisgarh, India, where forensic entomology is utilized for estimation of PMI. A dead body infested with insect specimen was brought to the mortuary of Late BRKM Govt. Medical College from Maharani Hospital for investigation. The body was wrapped in plastic bag. The personal data of person i.e., sex, age, cause of death, time since death etc. was not available. Postmortem lividity was absent. Rigor mortis was also absent. Body was blackish brown in color all over. There were maggots present over Face, Chest, Upper limbs, abdominal cavity, Perineum at places. Photographs of the corpses with insect’s maggots were taken. The larvae collected from corpse were belonged to blowfly species Chrysomya megacephala and Chrysomya rufifacies. These blow flies are mostly found in all the seasons of the year and their life cycle are temperature dependent (Siddiki and Zambare, 2017). Calliphoridae has the ability to smell the carcass from about 16 km and usually are the first insects to colonize it (Greenberg, 1991). Since there was no information regarding when the deceased was last seen, when he was alive or any missing report of person. Therefore, tentative PMI was estimated, by analyzing the development period of Chrysomya megacephala and by using ADH method. This blow fly species has been reported frequently from the various parts of South-West Asia and regarded as an early invader of remains (Bharti and Kurahashi, 2009).
Moreover, at high temperature of summers carcasses decays much faster rate than the low temperature of winter (Siddiki and Zambare, 2017). The development of insects is temperature dependent (Anderson, 2000). Warmer temperature increases the succession rate by rapidly increasing the development rate of immature stages of insects (Siddiki and Zambare, 2017). The fly larval activity is enhances by the higher temperatures, while their activity inhibited by the cold weather conditions (Smith, 1986). The rate of development increases faster with increased temperature due to the increase in metabolic rate (Anderson, 2000). Zhang et al. (2018) observed that the duration of development of Chrysomya megacephala at seven constant temperatures 16 ℃, 19 ℃, 22 ℃, 25 ℃, 28 ℃, 31 ℃ and 34 ℃ which provides useful information to use C. megacephala in forensic death investigations.
In other countries, the forensic entomologists are considered as the expert witnesses and employ as special agents in crime investigations but in India forensic entomology has remained abandoned due to the lack of information about necrophagous insects (Singh et al., 1999). In India, Forensic entomology is a new developing approach in the field of forensic investigation in which insects found to be an important crime indicator. Therefore, various scientists all over India are contributing to the field of forensic entomology and providing valuable information about the relationship between insects and decomposing carcass. Bharti et al. (2007) reared the C. megacephala larvae in the laboratory conditions at four different temperatures 22 ℃, 25 ℃, 28 ℃ and 30 ℃ and measured the duration of developmental stages at different temperatures. Suri Babu et al. (2013) determined the PMI of a 9-month-old male fetus corpse found in Chhattisgarh (India) by using the development rate of the larvae of Chrysomya rufifacies (Macquart). Sharma and Bala (2016) calculated PMI of mummified body of a female from Punjab, India by utilizing insects as evidence with the help of ADH (Accumulated Degree Hours) method. Siddiki and Zambare (2017) also studied the life cycle of two blow fly species C. megacephala and C. rufifacies at different temperature in three different seasons (i.e. rainy, winter and summer seasons) in order to increase the accuracy of PMI estimation. Suri Babu et al. (2017) demonstrated that at low temperature Sarcophaga dux (Sarcophagidae) delayed its larval growth, whereas at high temperature accelerated larval growth if proper food is available. Sharma et al. (2018) collected necrophagous insects colonizing the human corpses from Northern India and provided information about their role in PMI estimation. Kaur and Bala (2018) studied the insect Succession during four decomposition stages of pork i.e., Fresh, Bloated, Decay and Dry stages in which the Diptera, Coleoptera and Hymenoptera were the dominating fauna found on the pork. Singh and Bala (2019) also study the succession of forensically important Coleopteran species on goat carrion from Himachal Pradesh, India, during which they observed Histeridae and Silphidae were the dominant Coleopteran families throughout the decomposition. These types of study provide useful data for PMI estimation as very little is known about the role of insects in postmortem colonization in our country. In present study the estimated PMI of male corpse was about 5.5 days. ADH was calculated by analyzing the developmental rate of Chrysomya megacephala from the time when it first deposit eggs on the corpse.
Acknowledgements
The authors are deeply indebted to Mr. Pradeep Gupta, IPS, Director, State Forensic Science Laboratory, Raipur (C.G.) for encouragement and to Mr. Kamalochan Kasyap, IPS, Supdt. of Police, District Bijapur (C.G.) for providing necessary facilities and encouragement throughout the course of this study and for permission to publish this case study. The authors are also grateful to the Head Department of Zoology and environmental sciences, Punjabi University, Patiala, India for providing necessary facilities for the study. This work was funded by Taif University Researches Supporting Project number (TURSP-2020/139), Taif University, Taif, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Best practice in forensic entomology - standards and guidelines. Int. J. Legal Med.. 2007;121(2):90-104.
- [Google Scholar]
- Minimum and maximum development rates of some forensically important Calliphoridae (Diptera) J. Foren. Sci. 2000;45(4):14778J.
- [CrossRef] [Google Scholar]
- The uses of Chrysomya megacephala (Fabricius, 1794) (Diptera: Calliphoridae) in forensic entomology. Foren. Sci. Res.. 2018;3(1):2-15.
- [CrossRef] [Google Scholar]
- Finding of feral derived form (fdf) of Chrysomya megacephala (Fabricius) from India with an evolutionary novelity (Diptera, Calliphoridae) Jpn. Soc. Syst. Entomol.. 2009;15(2):411-413.
- [Google Scholar]
- Effect of temperature on the development of forensically important blowfly, Chrysomya megacephala (Fabricius) (Diptera: Calliphoridae) Entomon. 2007;32:149-151.
- [Google Scholar]
- Visitation patterns of principal species of the insect complex at carcasses in the Kruger National Park. Koedoe. 1981;24(1):33-49.
- [Google Scholar]
- Forensic Entomology in criminal investigations. Ann. Rev. Entomol.. 1992;37(1):253-272.
- [Google Scholar]
- Larval growth rates of the blowfly, Calliphora vicina, over a range of temperatures. Med. Vet. Entomol.. 2006;20(1):106-114.
- [Google Scholar]
- The potential use of bacterial community succession for estimating post-mortem interval as revealed by high-throughput sequencing. Sci. Rep.. 2016;6:24197.
- [Google Scholar]
- Insect succession on a decomposing piglet carcass placed in a man-made freshwater pond in Malaysia. Trop. Biomed.. 2008;25:23-29.
- [Google Scholar]
- Insect faunal succession studies on pork carrion in Punjab, India. J. Entomol. Res.. 2018;42(2):287-294.
- [Google Scholar]
- Bone modification and “the laws of burial”. In: Bonnichsen R., Sorg M., eds. Bone modification. Orono: Center for the Study of the First Americans. University of Maine; 1989. p. :724.
- [Google Scholar]
- Interpretation of postmortem change in cadavers in Spain. J. Forensic Sci.. 2004;49(5):1-6.
- [Google Scholar]
- Postmortem Interval Estimation of Mummified Body Using Accumulated Degree Hours (ADH) Method: A Case Study from Punjab (India) J. Foren. Sci. Crim. Invest.. 2016;1(1):552.
- [Google Scholar]
- Five Case Studies Associated with Forensically Important Entomofauna Recovered from Human Corpses from Punjab, India. J. Foren. Sci. Crim. Invest.. 2018;7(5):721.
- [Google Scholar]
- Shi, Y., Liu, X., Wang, H., et al., 2009. Seasonality of insect succession on exposed rabbit carrion in Guangzhou, China. Insect Scencei,16:425–439.
- Larval competition of Chrysomya megacephala and Chrysomya rufifacies (Diptera: Calliphoridae): behavior and ecological studies of two blow fly species of forensic significance. J. Med. Entomol.. 2008;45(4):785-799.
- [Google Scholar]
- Studies on time duration of life stages of Chrysomya megacephala and Chrysomya rufifacies (Diptera: Calliphoridae) during different seasons. J. Foren. Res.. 2017;8(3):379.
- [Google Scholar]
- Silahuddin, S.A, Latif, B., Kurahashi, H., et al., 2015. The importance of habitat in the ecology of decomposition on rabbit carcasses in Malaysia: implications in forensic entomology. J. Med. Entomol., 52:9–23.
- Forensic Entomology in the Indian perspective. J. Punjab Acad. Sci.. 1999;1:217-220.
- [Google Scholar]
- Succession study on forensically important Coleoptera from India: a preliminary study and its forensic implications. Egypt. J. Foren. Sci.. 2019;9:66.
- [Google Scholar]
- A Manual of Forensic Entomology. Ithaca, NY: Cornell University Press; 1986.
- Morphology and developmental rate of blowflies Chrysomya megacephala and Chrysomya rufifacies in Thailand: application in forensic entomology. Parasitol. Res.. 2008;102(6):1207-1216.
- [Google Scholar]
- Studies on the larval growth of forensically important flesh fly Sarcophaga dux Thompson 1869 (Diptera: Sarcophagidae) under outdoor ambient temperatures from Central India. Int. J. Appl. Res.. 2017;3(7):366-370.
- [Google Scholar]
- Suri Babu, B., Sharma, H., Bharti M., 2013. Estimation of postmortem interval by rearing Chrysomya rufifacies (Macquart, 1842) (Diptera: Calliphoridae). A case study from central India. Anil Aggrawal’s Internet J. Forensic Med. Toxicol. 14(2): 12.
- A new and earlier record of Chrysomya megacephala in South Africa, with notes on another exotic species, Calliphora vicina (Diptera: Calliphoridae) Afr. Invertebr.. 2006;47:347-350.
- [Google Scholar]
- The succession and development of insects on pig carcasses and their significances in estimating PMI in south China. Foren. Sci. Int.. 2008;179(1):11-18.
- [Google Scholar]
- Insect succession on remains of human and animals in Shenzhen, China. Foren. Sci. Int.. 2017;271:75-86.
- [Google Scholar]
- Zhang, Y., Wang, Y., Yang, L., Tao, L., Wang, J., 2018. Development of Chrysomya megacephala at constant temperatures within its colony range in Yangtze River Delta region of China. 3(1): 74–82.







