Translate this page into:
Butterfly pea flower (Clitoria ternatea L.) extract displayed antidiabetic effect through antioxidant, anti-inflammatory, lower hepatic GSK-3β, and pancreatic glycogen on Diabetes Mellitus and dyslipidemia rat
⁎Corresponding author. wahyu_w60@yahoo.com (Wahyu Widowati)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Diabetes Mellitus (DM) is hyperglycemic or elevated blood glucose level and deficiency of insulin level. DM treatment using synthetic drugs has several complexities, side effects. Reducing the side effects of synthetic drugs, the utilization of herbal medicines is increasingly in demand. Butterfly pea flower (Clitoria ternatea L.) extract (CTE) has pharmacological activities such as hepatoprotective, diuretic, antioxidant, antidiabetic, and anti-inflammatory activities. Objective: This research was conducted to evaluate antidiabetic potent of CTE in DM and dyslipidemia rats model. Methods: LC-MS/MS was used to analyze the CTE compounds. Rats were given high fat diet for 28 days followed by nicotinamide and streptozotocin for inducing DM rats model. DM and dyslipidemia rats model were given CTE at 200, 400, 800 mg/kg of BW, glibenclamide, and simvastatin for 28 days. The glucose and insulin levels on day 28 were measured after treatment of CTE. The CAT, SOD, MDA, IL-18 and protein of pancreas were measured. The glycogen gene expression in pancreas was measured using q-RTPCR method. The GSK-3β expression of liver, IL-6 expression of pancreas were analyzed using IHC method. The data were analyzed using ANOVA and then continued to be analyzed using Tukey’s HSD post-hoc test. Results: CTE increased level of pancreatic CAT, SOD and protein, reduced pancreatic MDA, IL-18 levels, glycogen gene expression of pancreas, GSK-3β protein expression of liver, and IL-6 protein expression of pancreas in DM and dyslipidemia rats. CTE improved liver histopathology, reduced serum glucose, and enhanced insulin levels. Conclusion: CTE has the potency for DM treatment, through antioxidant, and anti-inflammatory in DM and dyslipidemia rats.
Keywords
Antioxidant
Clitoria ternatea
Dyslipidemia
Diabetes mellitus
Glycogen
- BSA
-
Bovine Serum Albumin
- CAT
-
Catalase
- CTE
-
Clitoria ternatea L.extract
- DM
-
Diabetes Mellitus
- eIFs
-
eukaryotic Initiation Factors
- eEFs
-
eukaryotic Elongation Factors
- GSK-3β
-
Glycogen Synthase Kinase-3 β
- GMP
-
Good Manufacturing Practices
- HFD
-
High Fat Diet
- IL-6
-
Interleukin-6
- IHC
-
Immunohistochemistry
- IL-18
-
Interleukin-18
- q-RTPCR
-
quantitative- Reverse Transcriptase Polymerase Chain Reaction
- LC-MS/MS
-
Liquid Chromatography Mass Spectrometer
- MDA
-
Malondialdehyde
- OGTT
-
Oral Glucose Tolerance Test
- PTU
-
Propylthiouracil
- SOD
-
Superoxide Dismutase
- ROS
-
Reactive Oxygen Species
- STZ
-
Streptozotocin
Abbreviations
1 Introduction
Diabetes Mellitus (DM) is an illness that is characterized by high glucose level, glycosuria. Dyslipidemia with high plasma triglyceride, low High Density Lipoprotein (HDL) and high Low Density Lipoprotein (LDL) is one of the major risk factors for cardiovascular disease in DM. Lack insulin levels in the body caused by any mechanism such as insulin resistance by genetic, lipotoxicity, inflammation, negative regulation by hyperglycemia and mitochondrial dysfunction (Zamora and Villena, 2019).
Insulin is synthesized in β-pancreatic cells and has an important role in glucose homeostasis. This excess glucose is dealt with by glycogenesis in which the liver converts glucose into glycogen for storage (Sullivan and Forbes, 2019). Numerous metabolic diseases, including type-2 DM, have been connected to Glycogen Synthase Kinase-3 (GSK-3) signaling (Gupte et al., 2022). The increasing GSK-3β inhibits the activation of glycogen synthase which converting glucose to glycogen (Beurel et al, 2015). Patients with DM experience in oxidative stress conditions, decrease antioxidant activity such as Superoxide Dismutase (SOD), and increase Malondialdehyde (MDA) (Sunita et al., 2020). Patients with type-2 DM associated with inflammatory conditions, by increasing Interleukin-1β (IL-1β), IL-18, IL-6, and Tumor Necrosis Factor- α (TNF-α) (Zaharieva et al., 2018).
DM pharmacological therapy is administered such as sulfonylureas, metformin, and α-glucosidase inhibitors (acarbose). The side effects of pharmacological therapy include weight gain, hypoglycemia, and digestive disorders (Rosni et al., 2021). Therefore, the natural product therapy which fewer side effects and safer is needed for treating DM. Butterfly pea or telang flower (Clitoria ternatea L.) has the potential as antioxidant (Widowati et al., 2022a), anti-inflammatory, antidiabetic because it contains flavonoid compounds such as delphinidin, rutin, kaempferol, malvidin, and quercetin (Verma et al., 2013). This study was done to determine the antidiabetic, anti-inflammatory and antioxidant effects of C. ternatea L. extract (CTE) toward expression of liver GSK-3β, pancreatic IL-6, liver histopathology, glycogen gene expression of pancreas, CAT, SOD, MDA, IL-18 levels, blood glucose, insulin in DM and dyslipidemia rats model.
2 Materials and Methods
2.1 Preparation of C. ternatea extract, LC-MS/MS assay
The telang flower plant were collected from Kampung Herbal, Pasuruan, East Java, Indonesia. CTE was processed by PT FAST (Depok, Indonesia) based on Good Manufacturing Practices (GMP) with CoA No. Batch 00103211072. The butterfly flower peas were extracted using 70 % ethanol, then it added lactose (Widowati et al., 2021). The bioactive component was analyzed and identified qualitatively using a liquid chromatography mass spectrometer (LC-MS/MS). Hypersil Gold column with 150 mm × 2.1 mm × 1.9 mm was used for the analysis (Gondokesumo et al., 2019a; Widowati et al, 2022b).
2.2 Induction of Diabetes Mellitus and dyslipidemia in rats
The DM and dyslipidemia research protocols have been approved by Maranatha Christian University Ethical Committee, Bandung, Indonesia (147/KEP/VI/2021). Male rats of Sprague Dawley species aged 6 weeks and weighed 120–140 g were obtained from IratCo Laboratory, Bogor, Indonesia. The rats were kept in individual cages in 25 °C room temperature and a 12-h dark/12-h light cycle. The rats were given standard diet and water ad libitum for 7 days (Widowati et al, 2022b). After acclimatization, the rats were administered High Fat Diet (HFD) with 5.5 % crude fiber, 18 % crude protein, 50 % crude fat (PT Indoofeed) and 0.01 % Propylthiouracil (PTU, Dexa Medica) in drunk water (Kurniati et al., 2021), while standard diet had 7.37 % crude fat (Widowati et al., 2013). The induction of dyslipidemia to rats was done by giving HFD and PTU for 28 days. Dyslipidemia of rats was confirmed by serum cholesterol level using Cholesterol Kit (Elabscsi, E-BC-K109-M) ≥ 200 mg/dL. Single intraperitoneal injection of streptozotocin (STZ) for inducing DM (Sigma Aldrich SO130) 60 mg/kg BW 60 min after intraperitoneal (ip) administration of nicotinamide (NA, Sigma Aldrich-N3376) 120 mg/kg of BW. After five days, 12 h Fasting Blood Glucose (FBG) was evaluated using Autocheck blood glucose, DM rats have glucose level > 250 mg/dL (Elamin et al., 2018). After the rats were confirmed to have DM and dyslipidemia, the rats were treated with CTE (200, 400, 800 mg/kg BW), glibenclamide 0.45 mg/kg of BW (Generic, GKL9520905004A2), simvastatin 0.9 mg/kg BW (Generic, GKL131670271A), combination of glibenclamide and simvastatin while distilled water for negative control was given for 28 days (Florence et al., 2014).
2.3 Oral glucose tolerance test
Oral Glucose Tolerance Test (OGTT) was managed as a modification of the tests conducted by Anusooriya et al. (2014), Elamin et al. (2018). The fasted rats were treated for 12 h with unrestricted access to water and glucose given orally 2 g/kg BW, and then their glucose levels were tested in the coccygeal vein blood at 0, 2, 4, and 6 h after glucose loading using Glucose Kit (Elabsci, E-BC-K234-M) (Elamin et al., 2018).
2.4 Serum Collection, rats termination, liver collection
On day 28 after treating CTE, blood samples were taken from plexus retro-orbitalis in 12 h-fasted rats before anesthesia. The blood samples were kept at 4 °C for 2 h and centrifuged at 3,500 g for 10 min to obtain the serum (Widowati et al., 2022b). Rats were administered 100 mg/kg BW ketamine HCl (Ikapharmindo Putramas), xyla at 10 mg/kg BW (Interchemie, 361453), and then liver and pancreas were collected (Widowati et al., 2022b). The liver and pancreas were preserved at −80 °C and the remaining liver and pancreas were fixed in formalin (10 %) for immunohistochemistry (IHC), histopathological assay (Florence et al., 2014; Gondokesumo et al., 2019b; Widowati et al., 2022b).
2.5 Serum glucose, insulin assay
The glucose, insulin levels of the serum were measured using Glucose Colorimetric Kit. Rat Insulin Elisa Kit (Elabsci, E-EL-R3034), according to the manufacturer protocol (Elamin et al., 2018).
2.6 CAT, SOD, MDA, IL-18 assay
The CAT, SOD, MDA, IL-18 levels of pancreas were assessed using Kit respectively CAT Kit (Elabsci, E-BC-K031-S), SOD Kit (Elabsci, E-BC-K020), MDA Kit (Elabsci, E-EL-0060), Rat IL-18 Elisa Kit (Elabsci, E-EL-R0567) at 405, 450, 450, 450 nm respectively and Microplate reader (MultiskanTM Spectrophotometer, Thermo Scientific) was utilized to measure absorbance respectively of CAT, SOD, MDA, IL-18 (Widowati et al., 2019; Widowati et al., 2022b).
2.7 Glycogen gene expression assay
Total RNA of rat pancreas was isolated and purified using Direct-zol RNA Miniprep Plus Kit (Zymo, R2073). Complementary DNA synthesis was carried out using the iScript Reverse Transcription Supermix for RT-PCR (Bio-Rad, 170–8841). Quantitative gene expression was performed using the AriaMx 3000 Real-Time PCR System (Agilent). Evagreen master mix was performed as the q-RTPCR reaction mixture (Bio-Rad, 1725200) (Afifah et al., 2019). Table 1 shows the Glycogen and GAPDH sequences primer.
Gene
Primer Sequence (5′ to 3′)
Annealing (°C)
Product length (bp)
Reference
Glycogen
AGAGTTCGTCCGTGGCTGTC
GTTCGTGGAGATGCTCGGGA57
109
https://www.ncbi.nlm.nih.gov NM_001161587.2
GAPDH
TCAAGATGGTGAAGCAG
ATGTAGGCCATGAGGTCCAC57
217
https://www.ncbi.nlm.nih.gov NM_001289726
2.8 Protein assay
The 20 µL of standard solutions of Bovine Serum Albumin (BSA) stocks (Sigma Aldrich, A9576) in 1000 µL ddH2O, samples (serum, pancreas homogenate), each well plate was filled with Quick Start Dye Reagent 1X (Biorad, 5000205) 200 µL at room temperature, incubated for five minutes. After the incubation, Microplate reader was used for measure the sample with absorbance at 595 nm (Widowati et al., 2019).
2.9 Histopathological assay
Liver specimens were fixed in formalin (10 %) and examined by Haematoxylin Eosin (HE). Graded alcohols were used to dry the liver, which was then cleaned with xylene before being embedded in paraffin wax. The microtome was used to cut four or five micrometer (μM) thickness slices, which were subsequently stained with HE and coated with a cover glass. A light microscope was used to observe the samples and ImageJ was used to do quantitative data analysis. The quantitative data of the liver included necrosis, inflammation, and lipide degeneration (Elamin et al., 2018; Gondokesumo et al., 2019b; Rosni et al., 2021).
2.10 Immunohistochemistry assay
Cut paraffin blocks 4–5 μM (pancreas, liver), antigen (Abcam, ab208572) in pH 6.0, 121 °C for 10 min, 3 % of H2O2 in methanol for 15 min, 37 °C for blocking endogenous peroxidase. Then, 5 % BSA for pre-incubating the samples for 10 min. The primary antibody reaction using GSK-3β Polyclonal (Elabsci, E-AB-70043) for liver, IL-6 Polyclonal (Elabsci, E-AB-65801) for pancreas were done overnight at 37 °C. The HRP/DAB (ABC) rabbit specific detection IHC kit was used to visualize the protein target (Abcam, ab64261). Two-step plus Poly-HRP Anti Mouse/Rabbit IgG Detection System (with DAB solution) (Elabsci, E-IR-R217) was used for visualizing the protein target (Gondokesumo et al., 2019b; Rosni et al., 2021; Widowati et al., 2022b).
2.11 Statistical analysis
The data were examined using One Way ANOVA, and a post-hoc analysis was performed using the Tukey's HSD test. Significant at P 0.05.
3 Results
3.1 Compound analysis in CTE by LC-MS/MS
The various compounds were described by the chromatogram peak with various molecular weights. In our investigation, the results of LC-MS/MS analysis were compared to the standard 2-hydroxycinnamic acid which had a molecular weight of 164.16 g/mol, inositol 180.16 g/mol, (+) catechin 7-O-β-glucoside 160.2 g/mol, (+)-catechin 7-O-β-glucoside 452.13 g/mol, and delphinidin-3-O-(6-O-p-coumaroyl) glucoside-pyruvic acid 679.12 g/mol. This result indicated that CTE contained: 1). 2-hydroxycinnamic acid, 2). inositol, 3). (+) catechin 7-O-β-glucoside, 4). delphinidin-3-O-(6-O-p-coumaroyl) glucoside-pyruvic acid (Fig. 1).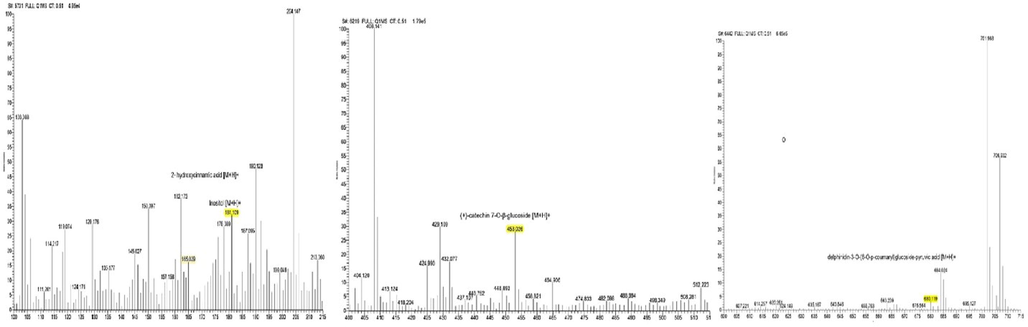
LC-MS/MS Spectrum of CTE. Mass spectra 100–200 m/z (165.029): 2-hydroxycinnamic acid with molecular weight of 164.16 g/mol. Mass spectra 100–200 m/z (181.109): inositol with molecular weight of 180.16 g/mol. Mass spectra 400–500 m/z (181.109): (+)-catechin 7-O-β-glucoside with molecular weight of 452.13 g/mol. Mass spectra 400–500 m/z (181.109): delphinidin-3-O-(6-O-p-coumaroyl) glucoside-pyruvic acid with molecular weight of 680.119 g/mol.
3.2 Effect of CTE toward OGTT, serum Glucose, insulin levels
The effect of CTE toward serum OGTT in DM rats can be seen in Fig. 2A. Diabetic rats model showed an increase glucose levels at 0–4 h, then decreased at 6 h. The CTE treatment lowered blood glucose in DM rats. The blood glucose level at 0 h after CTE treatment did not show a decrease. Treatment CTE 800 mg/kg BW was the most effective to decrease OGTT.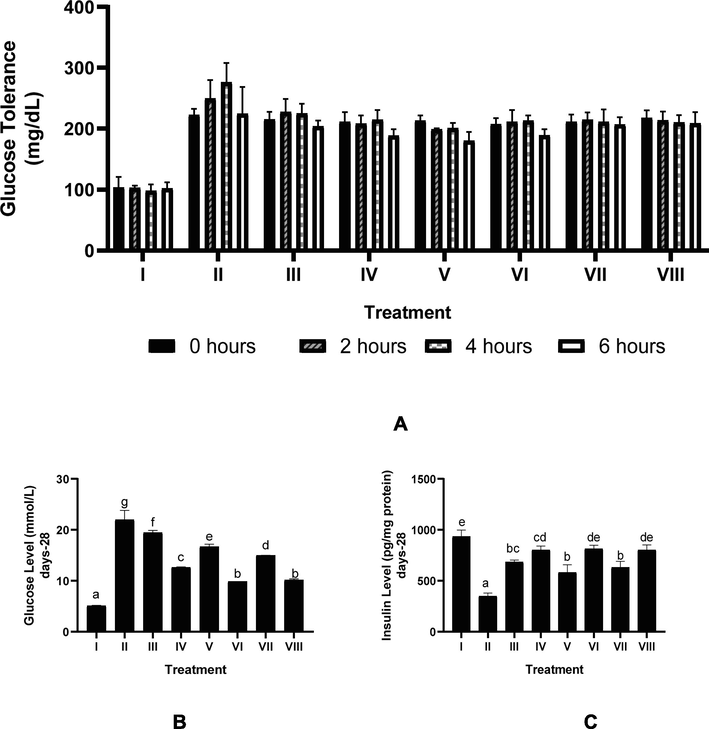
Effect of CTE toward OGTT, Serum Glucose, and Serum Insulin in DM Rats Model. *Data provided as mean and standard deviation. I: negative control; II: positive control (DM rats); III: DM rats + CTE 200 mg/kg BW; IV: DM rats + CTE 400 mg/kg BW; V: DM rats CTE + 800 mg/kg BW; VI: DM rats + Glibenclamide 0.45 mg/kg BW; VII: DM rats + Simvastatin 0.9 mg/kg BW; VII: DM rats + Glibenclamide 0.45 mg/kg BW and Simvastatin 0.9 mg/kg BW *A. Effect CTE toward OGTT. *B. Various letters (a, b, c, d, e, f, g) differences between treatments toward glucose level (Tukey’s HSD test, p < 0.05). *C. Various letters (a, b, bc, cd, de, e) significantly differences among treatments toward insulin level (Tukey’s HSD test, p < 0.05).
The effect of CTE toward serum glucose, insulin levels in DM rats on day 28th can be seen in Fig. 2B and C. The CTE decreased glucose level (p < 0.05), increased insulin level of DM rats (p < 0.05). The effective CTE to decrease glucose level, increase insulin level was CTE 400 mg/kg BW.
3.3 Effect of CTE toward CAT, SOD, MDA, IL-18 levels of pancreas
The effect CTE toward levels of serum CAT, SOD, MDA, IL-18 in DM rats on day 28th can be seen in Fig. 3. The results show that CTE increased CAT, SOD levels and lowered MDA, IL-18 levels (p < 0.05). The effective CTE to increase CAT, SOD levels, to lower MDA, IL-18 levels was CTE 400 mg/kg BW.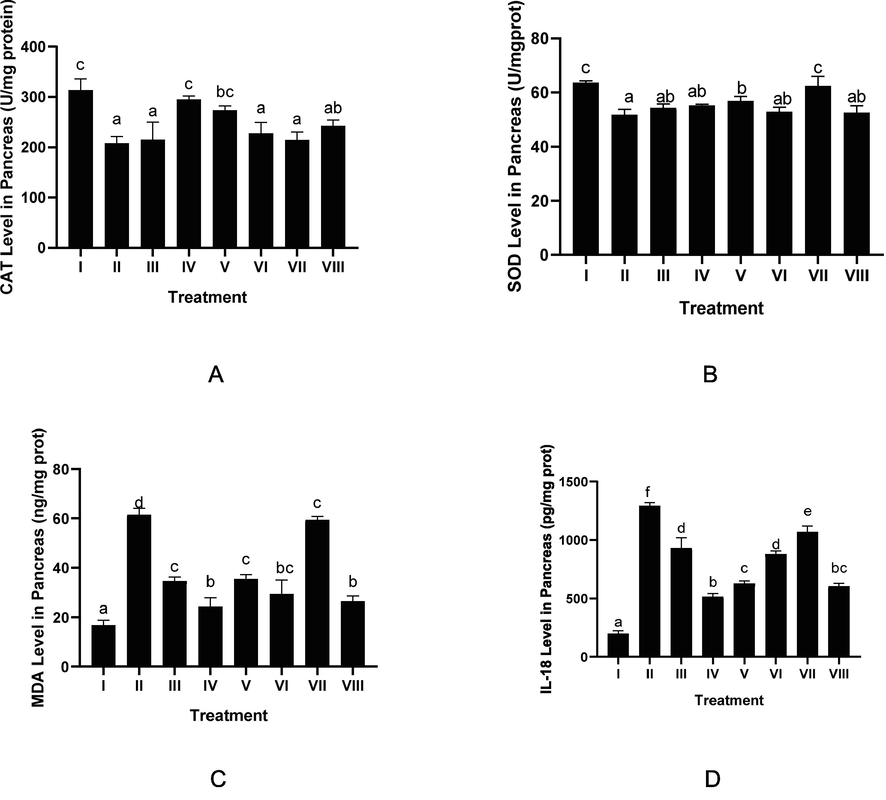
Effect of CTE toward CAT, SOD, MDA, IL-18 Level in DM Rats Model. *Data provided as a mean and standard deviation, I: negative control; II: DM rats; III: DM rats + CTE 200 mg/kg BW; IV: DM rats + CTE 400 mg/kg BW; V: DM rats CTE + 800 mg/kg BW; VI: DM rats + Glibenclamide 0.45 mg/kg BW; VII: DM rats + Simvastatin 0.9 mg/kg BW; VII: DM rats + Glibenclamide 0.45 mg/kg BW and Simvastatin 0.9 mg/kg BW. *A. Various letters (a, ab, bc, c) significantly differences between treatments toward CAT level (Tukey’s HSD test, p < 0.05). *B. Various letters (a, ab, b, c) significantly differences among treatments toward SOD level (Tukey’s HSD test, p < 0.05). *C. Various letters (a, b, bc, c, d) significantly differences among treatments toward MDA level (Tukey’s HSD test, p < 0.05). *D. Various letters (a, b, bc, c, d, e, f) significantly differences between treatments toward IL-18 level (Tukey’s HSD test, p< 0.05).
3.4 Effect of CTE toward protein level of pancreas
The effect of CTE toward pancreatic protein level in DM rats on day 28th can be seen in Fig. 4. The STZ induction decreased protein level (p < 0.05), CTE treatment increased protein level (p < 0.05). The most effective CTE to increase pancreatic protein was CTE 400 mg/kg BW.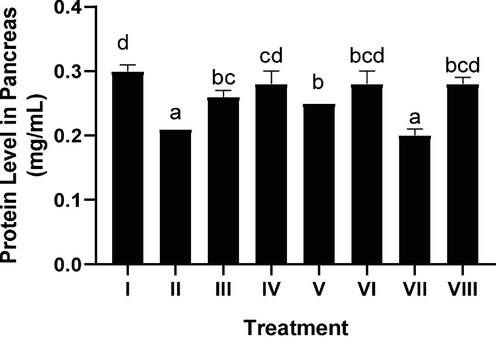
Effect of CTE toward Protein Level on DM Rats Model. *Data provided as a mean and standard deviation I: negative control; II: positive control; III: CTE 200 mg/kg of BW + positive control; IV: CTE 400 mg/kg of BW + positive control; V: CTE 800 mg/kg of BW + positive control; VI: positive control + Glibenclamide 0.45 mg/kg of BW; VII: Simvastatin 0.9 mg/kg of BW + positive control; VIII: Glibenclamide 0.45 mg/kg of BW and Simvastatin 0.9 mg/kg of BW + positive control. Various letters (a, b, bc, bcd, cd, d) reveal substantially differences between treatments toward protein level (Tukey’s HSD test, p < 0.05).
3.5 Effect of CTE toward IL-6 protein expression of pancreas
The effect of CTE toward IL-6 protein expression in DM rats on day 28th can be seen in Fig. 5. The CTE treatment decreased IL-6 expression (p < 0.05), the effective CTE to lower IL-16 expression were CTE 400, 800 mg/kg BW.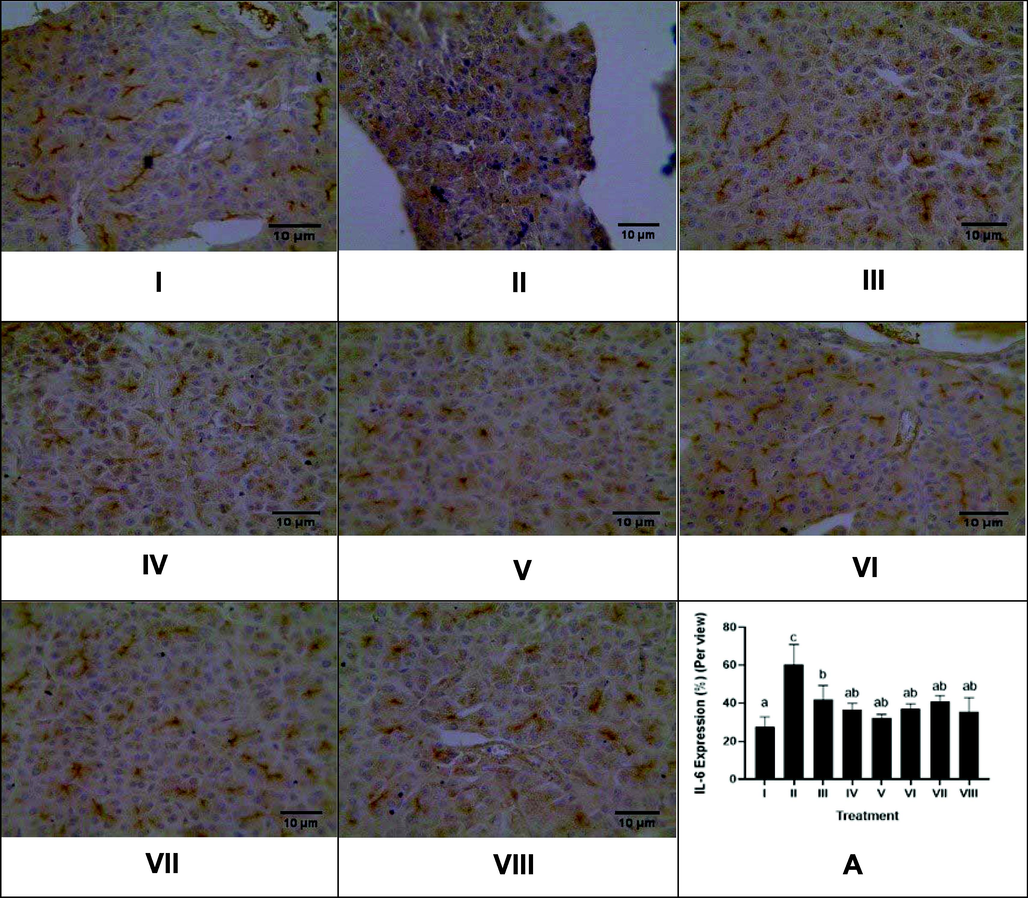
Effect of CTE toward IL-6 Expression of Pancreas in DM Rats Model. Hystopathology of pancreas tissues, IL-6 positive cells are distinguishable by their brown cytoplasmic staining and hemotoxylin eosin counterstain (magnification x40). I: negative control; II: DM rats; III: DM rats + CTE 200 mg/kg BW; IV: DM rats + CTE 400 mg/kg BW; V: DM rats CTE + 800 mg/kg BW; VI: DM rats + Glibenclamide 0.45 mg/kg BW; VII: DM rats + Simvastatin 0.9 mg/kg BW; VII: DM rats + Glibenclamide 0.45 mg/kg BW and Simvastatin 0.9 mg/kg BW. *A. Various letters (a, ab, b, c) significantly differences between treatments toward IL-6 expression (Tukey’s HSD test, p < 0.05).
3.6 Effect of CTE toward glycogen gene expression of pancreas
The effect of CTE toward pancreatic glycogen gene expression in DM rats on day 28th can be seen in Fig. 6. The CTE treatment decreased glycogen gene expression (p < 0.05), the most effective of CTE to decrease glycogen gene expression was CTE 400, 800 mg/kg BW.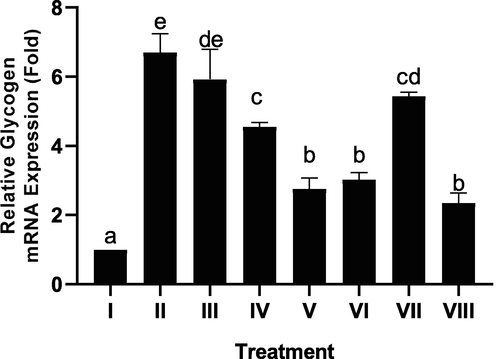
Effect of CTE toward Glycogen Gene Expression of Pancreas in DM Rat Model. *Data provided as a mean and standard deviation. I: negative control; II: DM rats; III: DM rats + CTE 200 mg/kg BW; IV: DM rats + CTE 400 mg/kg BW; V: DM rats CTE + 800 mg/kg BW; VI: DM rats + Glibenclamide 0.45 mg/kg BW; VII: DM rats + Simvastatin 0.9 mg/kg BW; VII: DM rats + Glibenclamide 0.45 mg/kg BW and Simvastatin 0.9 mg/kg BW. *Various letters (a, b, c, cd, de, e) significantly differences between treatments toward glycogen gene expression of pancreas (Tukey’s HSD test, p < 0.05).
3.7 Effect of CTE toward GSK-3β protein expression of liver
The effect of CTE toward liver GSK-3β protein expression in DM rats on day 28th can be seen in Fig. 7. Based on the result, CTE was able to lower GSK-3β expression in DM rats, the effective CTE to lower GSK-3β expression were CTE 400, 800 mg/kg BW.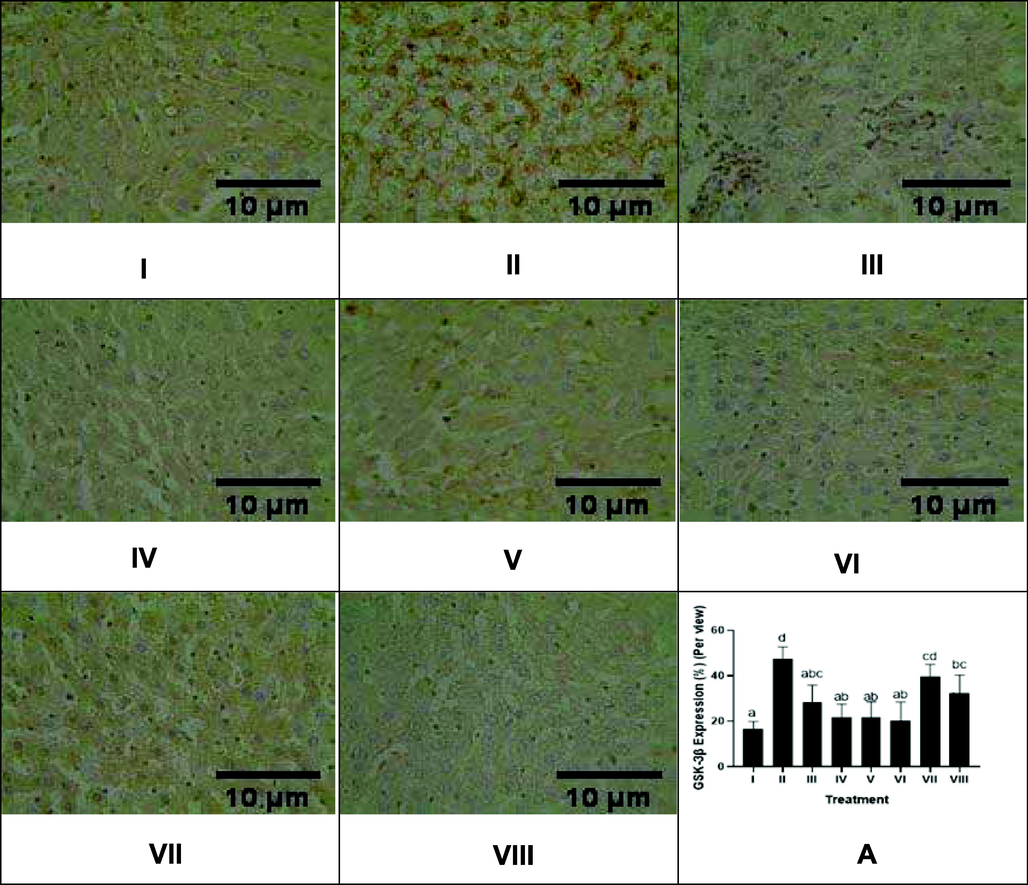
Effect of CTE toward GSK-3β Expression of Liver in DM Rats Model. Hystopathology of liver tissues, GSK-3β cells are distinguishable by their brown cytoplasmic staining and hemotoxylin eosin counterstain (magnification x40) I: negative control; II: DM rats; III: DM rats + CTE 200 mg/kg BW; IV: DM rats + CTE 400 mg/kg BW; V: DM rats CTE + 800 mg/kg BW; VI: DM rats + Glibenclamide 0.45 mg/kg BW; VII: DM rats + Simvastatin 0.9 mg/kg BW; VII: DM rats + Glibenclamide 0.45 mg/kg BW and Simvastatin 0.9 mg/kg BW. *A Various letters (a, ab, abc, bc, cd, d) significantly differences between treatments toward GSK-3β expression (Tukey’s HSD test, p < 0.05).
3.8 Effect of CTE toward histopathology of liver
The effect of CTE toward liver histopathology in DM rats on day 28th can be seen in Fig. 8. Liver histopathology was performed using HE staining, the scoring of the inflammation, necrotic cell, and lipid accumulation of liver. The result shows that STZ induction triggered liver inflammation, necrotic cell, and lipid degeneration. The CTE treatment lowered the inflammation, necrotic cell, and lipid degeneration in the liver of DM rats. The CTE 800 mg/kg BW was the most effective to reduce the inflammation, necrotic cell, and lipid accumulation in liver rats.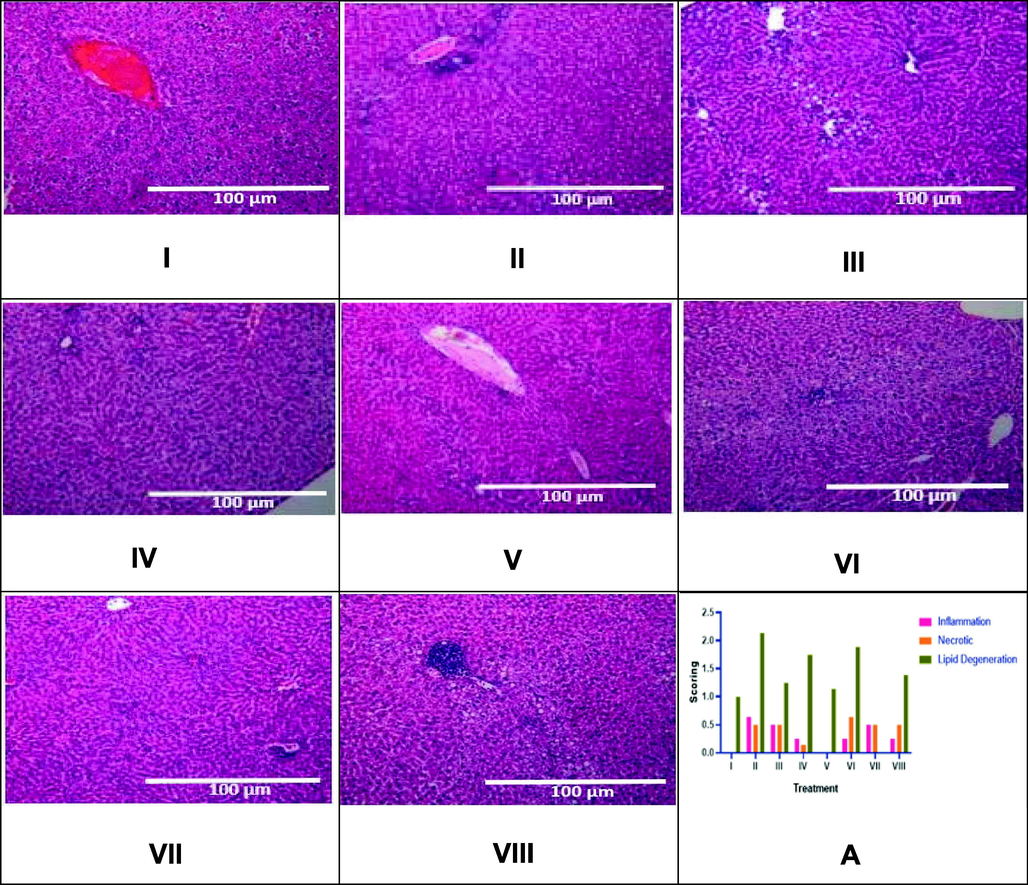
Effect of CTE toward Liver Histopathology in DM Rats Model. Positive control over lipid degradation, necrotic cells, and inflammation. The inflammatory infiltration was initially noticed near the portal triad, where there was more severe inflammation that could be observed in the central vein (x10 magnification). I: negative control; II: DM rats; III: DM rats + CTE 200 mg/kg BW; IV: DM rats + CTE 400 mg/kg BW; V: DM rats CTE + 800 mg/kg BW; VI: DM rats + Glibenclamide 0.45 mg/kg BW; VII: DM rats + Simvastatin 0.9 mg/kg BW; VII: DM rats + Glibenclamide 0.45 mg/kg BW and Simvastatin 0.9 mg/kg BW. A* The data is provided as a mean and the scores of inflammation, necrotic cell, lipid degeneration of liver are based on category 0: normal (no change), 1: mild, 2: moderate, 3: severe.
4 Discussion
Based on LC-MS/MS result, CTE contains flavonoids and phenols which have antioxidant activity (Jaafar et al., 2020). Previous researches exhibited that catechin and inositol were effective for treating diabetic patients (Pawar and Karthiyekan, 2020). Anthocyanins are blue, red, or purple pigments found in butterfly flower pea (Syafa’atullah et al., 2020), anthocyanins was potent antioxidant including based on electron donors, the polyphenol stabilize and delocalize free radicals as well as chelating the transition of metal ions (Devi et al., 2012). Thus, CTE can scavenge the free radicals and inhibit ROS production in DM rats (Zhang et al., 2021).
Glucose tolerance is the body's ability to absorb glucose in the blood. Diabetics patients absorb the glucose slowly due to lack of insulin level (Mukhtar et al., 2020). Based on our result, CTE reduced glucose tolerance in the DM rat model. This result was confirmed by testing serum glucose and insulin levels. Previous studies showing quercetin decreased insulin levels (Dokumacioglu et al., 2018), glucose level (Talpate et al., 2013). CTE contains flavonoids and have antioxidant potent and free radicals scavenging activity in the body (Jeyaraj et al., 2021; Widowati et al., 2022a), reduced the damage of pancreatic β-cell and normalized insulin production. Previous researches exhibited that CTE had the antidiabetic ability (Daisy, 2009), by reducing apoptotic cell caused by ROS (Zhang et al., 2021).
The damage of pancreatic β-cells in diabetics is triggered by oxidative stress. The lack of antioxidant enzyme activity causes uncontrolled free radical reactions to lead apoptotic cells. A previous study has shown that STZ induction in rats enhanced the production of free radicals and decreased CAT level (Ghosh et al., 2015). Patients with type 2-DM shows the decreasing antioxidant level and activity such as SOD, CAT and increasing MDA level (Kumawat et al., 2013; Verma et al., 2013).
The IL-6 protein expression is shown in brown colorization inside the cells cytoplasm, the negative control was weak cytoplasmic brown colorization, the pancreatic IL-6 expression positively stained with darker brown color. The administration of CTE, control drug (Glibenclamide and Simvastatin) decreased IL-6 expression. This data was validated with previous research that inflammatory cytokines IL-6 had higher level in DM patients, IL-6 are strong predictors of type-2 DM (Kreiner et al., 2022). CTE decreased pancreatic IL-6 expression, These results were consistent with earlier studies that flavonoid decreased IL-6, MDA levels in STZ-induced DM rats (Dokumacioglu et al., 2018).
Hyperglycemia, obesity, or insulin resistance could induce the expression inflammatory cytokines including IL-1α, IL-1β, IL-6, IL-18 (Farzaei et al., 2019). The CTE decreased pancreatic IL-18 level in DM rats, this data was validated with prior research that flavonoid as anti-inflammatory (Farzaei et al., 2019), antidiabetic effect (Dhanya, 2022).
The research shows that STZ induction causes inflammation as well as necrotic and lipid degeneration of the liver, pancreas. Glycogen was accumulated in pancreas β-cells of DM patients (Ashcroft et al., 2017). Another study also shows that impairment in β-cells causes an increase pancreatic glycogen level (Brereton et al., 2016). In the normal body, glycogen stored in muscle and liver as well as the impaired β-cells causes pancreas store the glycogen (Beurel et al., 2015; Brereton et al., 2016). The CTE reduced pancreatic glycogen expression, the data were supported by previous studies that Gnetum gnemon L. seed extract which contains flavonoids has ability to decrease glucose, increase insulin levels as well as Langerhans cells density, and decrease β-cell death (Rosni et al., 2021), while flavonoids inhibit oxidative stress-related pancreatic apoptosis and reduce pancreatic glycogen level (Lee et al., 2020).
The total protein level in DM rats were lower compared than normal rats because of the impaired of β-cells, disorder of insulin production, and it affects the protein production. Insulin rapidly activates protein synthesis by activating the translational machinery of eukaryotic elongation factors (eEFs) and eukaryotic initiation factors (eIFs) (Proud, 2006).
Previous studies show that DM causes oxidative stress in many organs especially liver (Mohamed et al., 2016). Hyperglycemia generates free radicals, which cause inflammation, cellular necrosis, and fatty liver disease (Mohamed et al., 2016). CTE reduced the liver inflammation, this result was in line with earlier research that flavonoid quercetin reduced liver inflammation and fibrosis in DM mice (Li et al., 2016). The CTE lowered hepatic necrosis, this result was supported by previous study that flavonoid from Malus doumeri leaf inhibited liver injury and reduced stem cell necrosis caused by liver injury (Zhu et al., 2019). The CTE lowered lipid degeneration, this data was consistent with earlier research that flavonoids which had antioxidant activity improved lipid metabolism, decreased lipid peroxidation and other oxidative stress (Wier et al., 2017).
The inhibition of GSK-3β can be used for DM treatment. Based on the result, STZ induction increased pancreatic GSK-3β expression, the CTE treatment reduced liver GSK-3β expression. This research was in line with previous research that flavonoid quercetin reduced GSK-3β enzymatic activity (Beurel et al., 2015; Jung et al., 2017).
5 Conclusion
The butterfly pea flower extract contains 2-hydroxycinnamic acid, inositol, (+) catechin 7-O-β-glucoside, delphinidin-3-O-(6-O-p-coumaroyl) glucoside-pyruvic acid. The butterfly pea flower extract decreases glucose, increases serum insulin levels of diabetic rats. The butterfly pea flower extract increases serum CAT, SOD, decreases MDA levels. The expressions of glycogen and GSK-3β are also reduced by the butterfly pea extract. The butterfly pea extract also reduces IL-6 and IL-18 cytokines of pancreas, decreases necrotic cells, lipid degeneration in diabetic rats’ liver. In conclusion, butterfly pea extract has the potency as a diabetes mellitus treatment through antioxidant, anti-inflammatory, improve glycogen and GSK-3β expression.
6 Disclosure of funding
The financial support from Maranatha Christian University, Bandung, Indonesia.
CRediT authorship contribution statement
Wahyu Widowati: Conceptualization, Supervision, Validation, Writing – review & editing. Lusiana Darsono: Conceptualization. Johan Lucianus: Conceptualization. Edwin Setiabudi: Data curation. Selonan Susang Obeng: Methodology. Shiela Stefani: Validation. Roro Wahyudianingsih: Validation. Kaleb Reynaldo Tandibua: Validation. Richard Gunawan: Data curation. Cahyaning Riski Wijayanti: Data curation, Writing – original draft. Agung Novianto: Methodology, Writing – original draft. Hanna Sari Widya Kusuma: Methodology. Rizal Rizal: Validation.
Acknowledgments
We are gratefully acknowledging Maranatha Christian University, Bandung, Indonesia for their financial support (Proposal Penelitian Pendanaan Skema B Internal 2021) (034/SK/ADD/UKM/VI/2021). Aretha Medika Utama, Biomolecular and Biomedical Research Center, Bandung, West Java, Indonesia, also sponsored, facilitated, and supported this research. We also appreciate PT FAST, Depok, Indonesia, for preparing the C. ternatea extract
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Induction of matrix metalloproteinases in chondrocytes by interleukin IL-1β as an osteoarthritis model. J. Math. Fund. Sci.. 2019;51(2):103-111.
- [CrossRef] [Google Scholar]
- Antioxidant and antidiabetic effect of aqueous fruit extract of Passiflora ligularis Juss. on streptozotocin induced diabetic rats. Int. Sch. Res. Notices. 2014
- [CrossRef] [Google Scholar]
- Is type 2 diabetes a glycogen storage disease of pancreatic β cells? Cell. Metab.. 2017;26(1):17-23.
- [CrossRef] [Google Scholar]
- Glycogen synthase kinase-3 (GSK3): regulation, actions, and diseases. Pharmacol. Ther.. 2015;148:114-131.
- [CrossRef] [Google Scholar]
- Hyperglycaemia induces metabolic dysfunction and glycogen accumulation in pancreatic β-cells. Nat. Commun.. 2016;7(1):1-15.
- [CrossRef] [Google Scholar]
- Hypoglycemic effects of Clitoria ternatea Linn. (Fabaceae) in alloxan-induced diabetes in rats. Trop. J. Pharm. Res.. 2009;8(5):393-398.
- [Google Scholar]
- DNA damage protecting activity and free radical scavenging activity of anthocyanins from red Sorghum (Sorghum bicolor) Bran. Biotechnol. Res. Int.. 2012;2012 (258787):1-9.
- [CrossRef] [Google Scholar]
- Quercetin for managing type 2 diabetes and its complications, an insight into multitarget therapy. Biomed. Pharmacother.. 2022;146:112560.
- [CrossRef] [Google Scholar]
- The effects of hesperidin and quercetin on serum tumor necrosis factor-alpha and interleukin-6 levels in streptozotocin-induced diabetes model. Pharmacogn. Mag.. 2018;14(54):167.
- [CrossRef] [Google Scholar]
- Histopathological alteration in STZ-nicotinamide diabetic rats, a complication of diabetes or a toxicity of STZ? Int. J. Diabetes. Res.. 2018;5(3):1-9.
- [CrossRef] [Google Scholar]
- Targeting inflammation by flavonoids: novel therapeutic strategy for metabolic disorders. Int. J. Mol. Sci.. 2019;20(19):4957.
- [CrossRef] [Google Scholar]
- Antidiabetic and antioxidant effects of Annona muricata (Annonaceae), aqueous extract on streptozotocin-induced diabetic rats. J. Ethnopharmacol.. 2014;151(2):784-790.
- [CrossRef] [Google Scholar]
- Curcumin protects rat liver from streptozotocin-induced diabetic pathophysiology by counteracting reactive oxygen species and inhibiting the activation of p53 and MAPKs mediated stress response pathways. Toxicol. Rep.. 2015;2:365-376.
- [CrossRef] [Google Scholar]
- Xanthones analysis and antioxidant activity analysis (Applying ESR) of six different maturity levels of mangosteen rind extract (Garcinia mangostana Linn.) Pharmacogn. J.. 2019;11(2):369-373.
- [CrossRef] [Google Scholar]
- Garcinia mangostana extract enhances skin epithelialization in rat induced burn injury. Pak. Vet. J.. 2019;39(3):365-370.
- [CrossRef] [Google Scholar]
- Isoform-specific role of GSK-3 in high fat diet induced obesity and glucose intolerance. Cells.. 2022;11(3):559.
- [CrossRef] [Google Scholar]
- Optimum extraction condition of Clitorea ternatea flower on antioxidant activities, total phenolic, total flavonoid and total anthocyanin contents. Trop. Life Sci. Res.. 2020;31(2):1-17.
- [CrossRef] [Google Scholar]
- Extraction methods of butterfly pea (Clitoria ternatea) flower and biological activities of its phytochemicals. J. Food Sci. Technol.. 2021;58(6):2054-2067.
- [CrossRef] [Google Scholar]
- Flavones with inhibitory effects on glycogen synthase kinase 3β. Appl. Biol. Chem.. 2017;60(3):227-232.
- [CrossRef] [Google Scholar]
- Interleukin 6 in diabetes, chronic kidney disease, and cardiovascular disease: mechanisms and therapeutic perspectives. Expert Rev. Clin. Immunol.. 2022;18(4):377-389.
- [CrossRef] [Google Scholar]
- Antioxidant enzymes and lipid peroxidation in type 2 diabetes mellitus patients with and without nephropathy. N. Am. J. Med. Sci.. 2013;5(3):213.
- [CrossRef] [Google Scholar]
- The effect of ethanol extract of Punica granatum Linn. leaves on lipid profiles of dyslipidemic rat. Pharm. Sci. Res.. 2021;8(2):4.
- [Google Scholar]
- Flavonoids identification and pancreatic beta-cell protective effect of Lotus Seedpod. Antioxidants. 2020;9(8):658.
- [CrossRef] [Google Scholar]
- Quercetin protects mice from ConA-induced hepatitis by inhibiting HMGB1-TLR expression and down-regulating the nuclear factor kappa B pathway. Inflammation. 2016;39(1):96-106.
- [CrossRef] [Google Scholar]
- Mechanisms of diabetes-induced liver damage: the role of oxidative stress and inflammation. Sultan Qaboos Univ. Med. J.. 2016;16(2):e132.
- [Google Scholar]
- A modern overview on diabetes mellitus: a chronic endocrine disorder. Eur. J. Biol.. 2020;5(2):1-14.
- [CrossRef] [Google Scholar]
- Role of catechins in diabetes mellitus. Eur. J. Mol. Clin. Med.. 2020;7(11):2515-2826.
- [Google Scholar]
- Regulation of protein synthesis by insulin. Biochem. Soc. Trans.. 2006;34(2):213-216.
- [CrossRef] [Google Scholar]
- Potential of Gnetum gnemon L. seed extract against insulin levels and PDX-1 expression in diabetes mellitus rats model. 2021 IEEE Int. Conf. Health. Instrumentation & Measurement, and Natural Sciences (InHeNce) 2021:1-5.
- [CrossRef] [Google Scholar]
- Glucose and glycogen in the diabetic kidney: Heroes or villains? EBioMedicine.. 2019;47:590-597.
- [CrossRef] [Google Scholar]
- Evaluation of malondialdehyde in type 2 diabetes mellitus patients as oxidative stress markers in Bengkulu population. BSM : J. Biomed. Transl. Res.. 2020;4(3):45-54.
- [CrossRef] [Google Scholar]
- Syafa’atullah, A.Q., Amira, A., Hidayati, S., Mahfud, M., 2020. Anthocyanin from butterfly pea flowers (Clitoria ternatea) by ultrasonic-assisted extraction. In: AIP Conf. In: Proc, 2237 (1). AIP Publishing LLC, p. 020069. https://doi.org/10.1063/5.0005289.
- Antihyperglycemic and antioxidant activity of Clitorea ternatea Linn. on streptozotocin-induced diabetic rats. Ayu.. 2013;34(4):433-439.
- [CrossRef] [Google Scholar]
- The potential of flavonoids in the treatment of non-alcoholic fatty liver disease. Crit. Rev. Food. Sci. Nutr.. 2017;57(4):834-855.
- [CrossRef] [Google Scholar]
- Evaluation of antidiabetic antihyperlipidemic and pancreatic regeneration, potential of aerial parts of Clitoria ternatea. Rev. Bras. Farmacogn.. 2013;23(5):819-829.
- [CrossRef] [Google Scholar]
- Widowati, W., Prahastuti, S., Ekayanti, N.L.W., Munshy, U.Z., Kusuma, H.S.W., Wibowo, S.H.B., et al., 2019. Anti-inflammation assay of black soybean extract and its compounds on lipopolysaccharide-induced RAW 264.7 cell. In: J. Phys. Conf., 1374 (1). IOP Publishing, 012052. https://doi.org/10.1088/1742-6596/1374/1/012052.
- Widowati, W., Priyandoko, D., Wahyuni, C. D., Marthania, M., Kusuma, H. S. W., Handayani, T., 2021. Antioxidant properties of soybean (Glycine max L.) extract and isoflavone. In: 2021 IEEE Int. In: Conf. Health, Instrumentation & Measurement, Nat. Sci. (InHeNce). IEEE, pp. 1–6. https://doi.org/10.1109/InHeNce52833.2021.9537282.
- Hypolipidemic and antioxidant effects of black tea extract and quercetin in atherosclerotic rats. Int. J. Med. Sci. Eng.. 2013;7(10):64-71.
- [Google Scholar]
- Antioxidant activity of TEMON (Clitoria ternatea and Citrus sp.) as an infused herbal tea. Trad. Med. J.. 2022;27(1):32-39.
- [CrossRef] [Google Scholar]
- Detam 1 black soybean against cisplatin-induced acute ren failure on rat model via antioxidant, antiinflammatory and antiapoptosis potential. J. Tradit. Complement. Med.. 2022;12(4):426-435.
- [CrossRef] [Google Scholar]
- Interleukin-18 serum level is elevated in type 2 diabetes and latent autoimmune diabetes. Endocr. Connect.. 2018;7(1):179-185.
- [CrossRef] [Google Scholar]
- Contribution of impaired insulin signaling to the pathogenesis of diabetic cardiomyopathy. Int. J. Mol. Sci.. 2019;20(11):2833.
- [CrossRef] [Google Scholar]
- A review of phytochemistry and pharmacology perspectives of Clitoria ternatea L. Asian J. Tradit. Med.. 2021;16(3):153-160.
- [Google Scholar]
- Preventive effect of flavonoids from Wushan Shencha (Malus doumeri leaves) on CCl4-induced liver injury. Food Sci. Nutr.. 2019;7(11):3808-3818.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2023.102579.
Appendix A
Supplementary material
The following are the Supplementary data to this article:







