Translate this page into:
BTEX induces histopathological alterations, oxidative stress response and DNA damage in the testis of the freshwater leech Erpobdella johanssoni (Johansson, 1927)
⁎Corresponding author at: Department of Zoology, College of Science, King Saud University, 11451 Riyadh, Saudi Arabia. hharrath@ksu.edu.sa (Abdel Halim Harrath)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The widespread application of the hydrocarbons benzene, toluene, ethylbenzene and xylene (BTEX) has led to their consideration as major aquatic environmental pollutants, and exposure to these hydrocarbons has become a serious concern given their detrimental health effects on aquatic species. We investigated in the present study the impact of chronic exposure to BTEX on the testis of the freshwater leech Erpobdella johanssoni. The results revealed that BTEX induced severe histological alterations revealed by vacuolar degeneration in the spermatogenic cysts, and a complete loss of normal cysts was observed. The cytophore was grossly destroyed, and extensive necrosis and detached germ cells from cytophore were observed. Interestingly, BTEX exposure induced a significant increase in malondialdehyde (MDA) levels by 85.71% in the testicular tissue of treated leeches compared to the control (P < 0.00005), whereas the activity levels of SOD, GPx and CAT antioxidant biomarkers were significantly decreased by 60%, 81.81% and 47.5%, respectively. Additionally, using the alkaline comet assay, we observed that BTEX exposure induced significant DNA fragmentation in the testis cells of treated leeches compared to controls (P < 0.00005). Overall, this study unravels the histopathologic, oxidative stress and DNA damage induced by BTEX on the testis of the freshwater leech E. johanssoni.
Keywords
BTEX
Aquatic toxicity
Testis
Oxidative stress
DNA damage
Leech
1 Introduction
Benzene, toluene, ethylbenzene and xylene (BTEX) are found naturally in petroleum products, such as crude oil, diesel fuel and gasoline (Heibati et al., 2017). These hydrocarbons are widely used as industrial solvents and provide starting materials for the production of polymers, solvents, agrochemicals and pharmaceuticals (Singh et al., 2011). Derivatives of BTEX are among of the most important pollutants of soil and groundwater (Khodaei et al., 2017). Recommended BTEX groundwater concentrations ranging from 0.1 to 1.8 µg L−1, 1–100 µg L−1, 0.1–1.1 µg L−1 and 0.1–0.5 µgL-1, respectively. BTEX levels in drinking water should not exceed 0.1–1 µg L−1, 1–27 µg L−1, 1–10 µg L−1 and 0.1–12 µg L−1, respectively (ATSDR, 2000; ATSDR, 2007a,b; Organization, 2008; ATSDR, 2010). According to a great deal of research, BTEX toxicity has gained increasing attention and has become a serious concern with respect to human and animal health worldwide (Xiong et al., 2016; Doherty et al., 2019). Exposure to BTEX induces harmful health effects, such as cancer, damage to the liver and kidney, paralyzing the central nervous system, and even resulting in death at low concentrations (Li et al., 2013; Baghani et al., 2019). The alteration of reproductive function and gonadal tissue is one of the most devastating consequences of BTEX exposure (Khaled et al., 2016; Khaled et al., 2017; Cassidy-Bushrow et al., 2021). BTEX can exert negative effects on various female reproductive sites due to its potential to destroy chromosomes; affect cell metabolism, including the accumulation of free radicals; and lead to disturbances in the secretion of hormonal regulator of reproductive system and intracellular protein kinases (Sirotkin and Harrath, 2017). Recently, it has been described that toluene and ethylbenzene can disrupt ovarian function by causing histopathological alterations and inducing both apoptotic and autophagic mechanisms (Alrezaki et al., 2021; Harrath et al., 2022).
In human males, exposure to BTEX is associated with reduced sperm vitality and activity, male reproductive dysfunction, carcinogenicity and denaturation of sperm nuclear DNA (XIAO et al., 2001; Nakai et al., 2003; Mandani et al., 2013). Despite the excellent studies on BTEX toxicity, the toxic effect of these hydrocarbons on aquatic invertebrates deserves further investigation because most studies have focused the effects on mammals (Zhang et al., 2010; Singh et al., 2011; Saxena and Ghosh, 2012; Kumar et al., 2014; PT Manfo et al., 2014). Given their morphology, physiology, and behavior leeches are considered as a suitable biological model for aquatic toxicity assessment (Mihaljević et al., 2009). Leeches have been used as a typical bioindicator of toxic pollutants in aquatic environment studies (Petrauskien, 2003; Petrauskienė, 2004). In our previous studies, we revealed that acute exposure to BTEX (1.4 and 2.8 mg L−1) induced morphological disruptions and adverse effects on spermatogenesis and oogenesis of the leech Limnatis nilotica (Khaled et al., 2016). In Erpobdella johanssoni, the same treatment induced alterations and ultrastructural abnormalities, notably in previtellogenic and vitellogenic oocytes, and a significant increase in DNA fragmentation (Khaled et al., 2016; Khaled et al., 2017).
Biological indicators have been developed at various levels of biological organization to evaluate the impact of environmental stress on aquatic organisms. A cascade of stress-related responses, such as biochemical, molecular, cellular, physiological or behavioral changes, are now increasingly being used as biomarkers of environmental stress because they provide a definite biological endpoint of historical exposure (Eseigbe et al., 2013). As such, histopathological alterations, DNA damage and oxidative stress represent core tools and sensitive indicators in environmental toxicology to evaluate the effect of pollution in biomonitoring assessments (Li et al., 2015). Herein, we hypothesize that exposure to an environmentally realistic concentration of BTEX may lead to histopathological and molecular changes in the testis of the leech Erpobdella johanssoni since it is considered as a suitable model for the ecological monitoring and aquatic toxicity assessment of invertebrate species. Thus, we evaluated the effects of chronic exposure to BTEX (25 µg L−1) on a) testis structure, b) the level of oxidative damage in the testis by measuring lipid peroxidation and antioxidant defense enzyme activity, and c) DNA damage using the comet assay.
2 Materials and methods
2.1 Sample collection
Specimens of Erpobdella johanssoni tested in this work were collected throughout the breeding season of May and June 2019 from the Tamerza waterfall (mountain oasis situated in south west of Tunisia) (Fig. 1A, B). Leeches were maintained in laboratory conditions in aerated glass at room temperature (20 °C) and fed weekly with crushed chicken liver.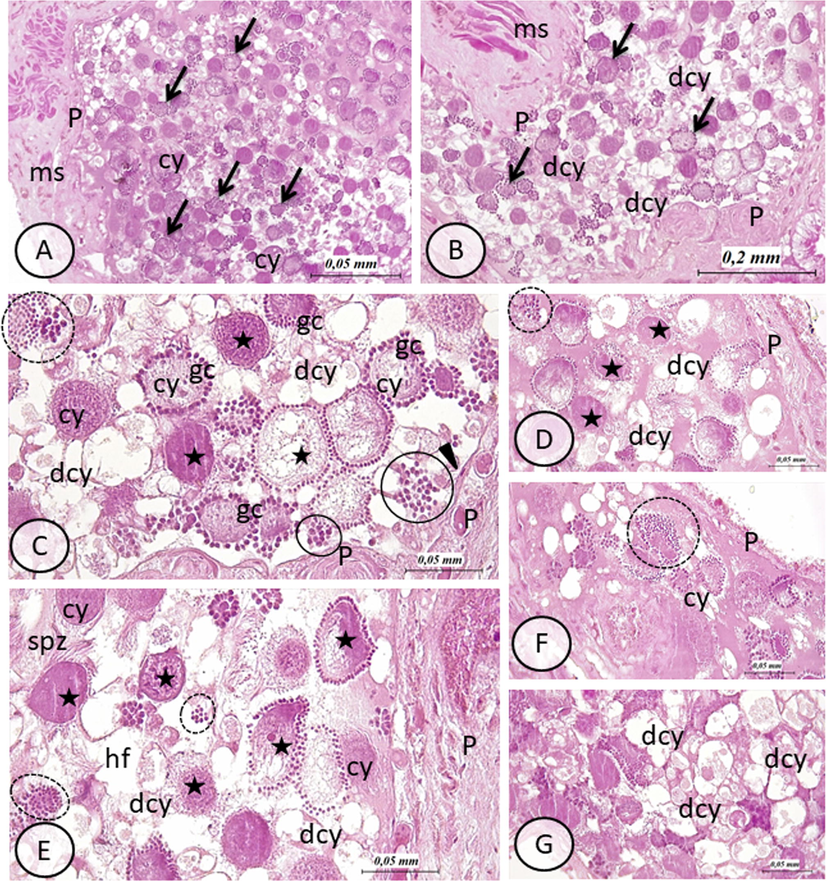
Testis of E. johanssoni: hematoxylin and eosin (H&E) staining of paraffin sections. (A) General view of testis in control group. Note the normal appearance of germ cells (gc) in a cystic organization (arrows). During spermatogenesis, germ cells are shown connected to a common cytoplasmic mass, the cytophore (cy). Testes wall (P) and muscle cells (ms) are visible (B) General view of testis in treated group with BTEX.. Arrows indicate cysts of germ cells. Note the increased number of disorganized cysts marked especially by degenerative cytophores (dcy). The wall of the testes (P) (ms) are also visibles (H&E, 100×). (C)–(G): details of testis in treated group with BTEX showing different types of alterations: stars indicate vacuolated and or damaged cytophore with cytoplasmic protrusions, circles show disorganized cysts expressed by dispersed germ cells (gc) (not connected to cytophore), degenerating cytophore (dcy) are also visible hf: hemocoelomic fluid. In (C) note parietal ciliated cells (arrow head) lying on the inner side of the testis wall (P) (H&E, 400×). (H&E, 400×).
2.2 Chronic exposure of E. johanssoni to BTEX
We purchased benzene, toluene, ethylbenzene and xylene with greater than 99% purity from Labo Chimie PVT. Ltd. 107. For BTEX chronic exposure, adult and alive leeches were chosen and divided randomly into two groups: untreated group contained 15 leeches and served as a control group. In the second group, leeches were treated with a concentration of 25 µg L−1 of BTEX prepared with equal amount of each component. Treatments were conducted in triplicate with 15 leeches per replicate. Chronic exposure was carried out for 26 days with respect to acclimation condition. Aeration was removed to avoid BTEX volatilization. Upon treatment, leeches were fed weekly and the entire exposure solution was modified daily so as to conserve the required concentration of BTEX. The selection of this concentration was based mostly to our preliminary laboratory tests and available literature data that focused on BTEX chronic exposure in other aquatic invertebrates and fish (Fan et al., 2009; Li et al., 2013).
2.3 Light microscopy
For histopathological assessment, five specimens of E. johanssoni from each group were dissected. Briefly, removed testes were fixed in 4% neutral buffered formalin (NBF), dehydrated in graded ethanol series and embedded in paraffin wax. Then, sections of 6-µm thick were stained with eosin and hematoxylin and examined under a light microscope.
2.4 Evaluation of oxidative stress biomarkers
Ten specimens of E. johanssoni from each group were dissected, and testes weighing 1 g were removed and immersed in 2 ml ice-cold Tris buffered saline and centrifuged for 15 min at 5000 G and 4 °C. Supernatants were collected and used for further analysis.
2.4.1 Lipid peroxidation assay
Lipid peroxidation was determined indirectly by measuring the level of MDA as an index of lipid peroxidation according to a previous study (Buege and Aust, 1978). Briefly, a total of 125 µL of supernatant and 125 µL of 20% trichloroacetic acid containing 1% butyl-hydroxy-toluene were mixed and centrifuged (1000g for 10 min at 4 °C). Two hundred microliters of the sample was added to HCl (6 M) and 80 µL Tris–thiobarbituric acid (TBA), and the content was mixed at 80 °C for 10 min. The MDA level (nmol mg−1 of proteins) was estimated from the absorbance at 530 nm using the molar extinction coefficient of MDA (156 mM−1 cm−1).
2.4.2 Determination of superoxide-dismutase (SOD) activity
SOD activity was evaluated by measuring its ability to inhibit the oxidation of nitro blue tetrazolium (NBT) using a method described previously (Beyer Jr and Fridovich, 1987). One unit of SOD activity represents the amount of enzymes that causes 50% inhibition of the reduction of NBT at 25 °C.
2.4.3 Determination of catalase (CAT) activity
The activity of catalase (CAT) was assayed following the method of Aebi (Aebi, 1984). 20 µL of the sample was added to H2O2 solution in phosphate buffer (pH 7). The activity of CAT was determined by measuring H2O2 decomposition at 240 nm and calculated using the molar extinction coefficient (0.043 mM−1 cm−1).
2.4.4 Determination of glutathione peroxidase (GPx) activity
Glutathione peroxidase (GPx) activity was measured by the subsequent oxidation of NADPH at 340 nm using the method described previously (Flohé and Günzlervz, 1984).
2.5 Comet assay
For DNA damage assessment, five specimens from each treatment were dissected. Briefly, extracted testes were immersed in 100 µL of phosphate buffered saline (PBS). Cell suspension was obtained after mincing samples using very fine scissors. 60 µL of obtained suspension was mixed with 60 µL of 1.0% low melt point agarose at 37 °C. Then, cell suspension was spread on slides predicted with a layer of 1% (w/v) normal melting point agarose prepared in PBS. Thereafter, slides were solidified for 5–10 min at 4 °C and were immersed in lysis solution at 4 °C overnight. Slides were then placed into a horizontal electrophoresis tank filled with 0.3 M NaOH and 1 mM ethylenediamine tetraacetic acid for 20 min. Electrophoresis was performed for 20 min at 25 V and 300 mA at ambient temperature. After electrophoresis, slides were then neutralized using 0.4 M Tris, pH 7.5, and immersed in absolute ethanol for 10 min. After staining with 40 µL ethidium bromide, slides were examined under a Nikon Eclipse TE300 fluorescence microscope (Nikon, Tokyo, Japan). The experiment was repeated thrice for each sample. The evolution of DNA damage is carried out with the visual scoring method, a total of 100 comets on each slide were visually scored according to the relative intensity of fluorescence in the tail and classified into one of five categories. According to (Collins et al., 1996), comet classes were given values (0, 1, 2, 3 or 4; from undamaged, 0, to maximally damaged, 4). The total score was estimated by the subsequent formula: (percentage of cells in class 0 × 0) + (percentage of cells in class 1 × 1) + (percentage of cells in class 2 × 2) + (percentage of cells in class 3 × 3) + (percentage of cells in class 4 × 4). Accordingly, the total score varied from 0 to 400.
2.6 Statistical analysis
Statistical analysis was carried out using GraphPad Prism version 9. Data are represented as the mean ± standard deviation (SD). Significant differences between groups were analyzed using the Mann–Whitney U test. p < 0.05 was considered to be statistically significant.
3 Results
3.1 Histopathological evaluation
Testis of untreated leeches showed normal structure, as evidenced by the well-organized distribution of developing germline clones organized in spermatogenic cysts (Fig. 1A). In fact, germ cells are interconnected to a central, cytoplasmic mass called cytophore to form syncytial groups (cysts) (Fig. 1A). However, testes of leeches that were exposed to 25 µg L−1 BTEX, when compared to control group, showed many apparent histological changes. These later, resulted in extensive alterations that appeared in spermatogenic cysts, leading to a complete loss of normal cysts (Fig. 1B-G). Interestingly, the normal morphology of cytophores was affected (Fig. 1B-G). In fact, degenerative, vacuolated and finally cytophores with less compacted cytoplasm was observed (Fig. 1B-G). Moreover, extensive necrosis and disseminated germ cells which appeared floating freely in the testis hemocoelomic fluid, as consequence of the loss of connexion to cytophore, was noted (Fig. 1C-F).
3.2 Oxidative stress and antioxidant enzyme biomarkers
3.2.1 Lipid peroxidation assay
Lipid peroxidase status was evaluated by the measure of MDA content in the testes of the leech E. johanssoni and the results are shown in Fig. 2. Exposure to BTEX exhibited a significant elevation in MDA content. In fact, after 26 days of treatment with 25 µg L−1 of BTEX, the levels of MDA in the testes of experimental leeches were significantly increased (p < 0.05) from a range of 85.71% as compared to the untreated group (p < 0.05).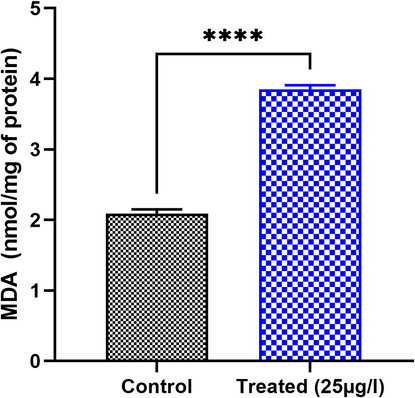
Effect of BTEX on MDA levels in the testis of E. johanssoni from the treated group compared to the control. Values are expressed as means ± SD. *P < 0.05 indicates a significant difference from the control ****P < 0.00001.
3.2.2 Superoxide dismutase (SOD)
Our results showed that the activity of the enzyme SOD was inhibited in the leeches exposed to BTEX (Fig. 3). Measurements of SOD activity in the testes of leeches E. johanssoni exposed to 25 µg L−1 for 26 days have a decreasing tendency as compared to the control group (p < 0.05) by about 60%.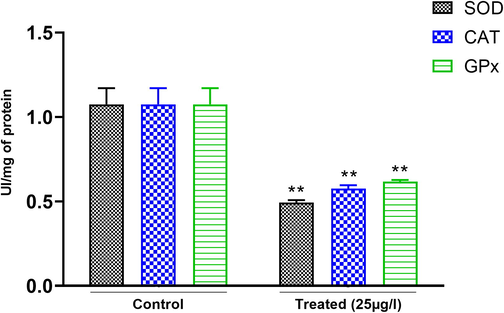
Effects of BTEX on SOD, CAT, and GPx activity in the testes of E. johanssoni from treated leeches compared to the control. All these biomarkers were significantly decreased after exposure to 25 µg L−1 BTEX. Values are expressed as means ± SD. (*) P < 0.05 and (**) P < 0.001 indicate a significant difference compared with the control.
3.2.3 Catalase (CAT)
Our results showed that exposure to BTEX exhibited a significant decrease in catalase activity in the testes of the leeches E. johanssoni exposed to 25 µg L−1 over 26 days (Fig. 3) by about 81.81% as compared to the control group (p < 0.05).
3.2.4 Glutathione peroxidase (GPx)
As shown in (Fig. 3), GPx activity was found to be significantly decreased in the testes of leeches E. johanssoni exposed to 25 µg L−1 for 26 days. The level of GPx activity decreased from a range of 47.5% when compared to control group (p < 0.05).
3.3 DNA damage level
To address the potential genotoxic effects of BTEX in the testis of the leech E. johanssoni, we used the comet assay, which can detect primary DNA damage and single and double DNA strand breaks. Comet assay findings showed a significant increase in DNA damage in exposed leeches compared to leches of the untreated group (Fig. 4). Testis of exposed leeches have approximatively five-fold higher levels of DNA damage.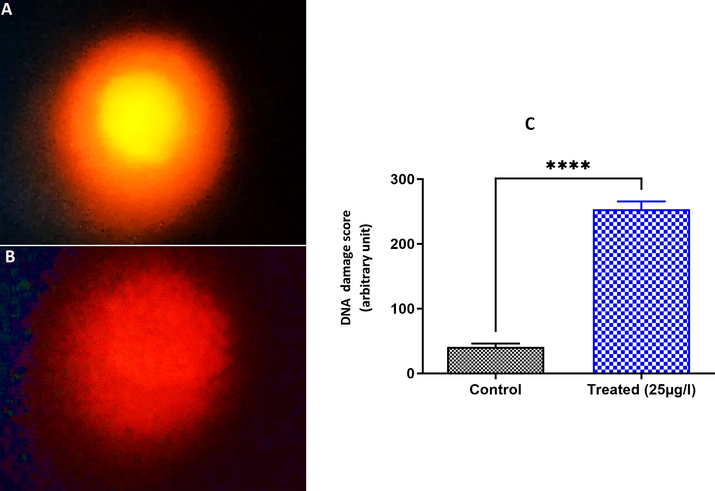
DNA damage score in testis cells of E. johanssoni showing a higher score of DNA alteration in the 25 µg L−1 treatment (B) compared to the control group (A). C. Relative fluorescence intensity of DNA damage levels in the control and exposed groups. Values are expressed as the mean ± standard deviation (SD) from at least three separate experiments. (*) P < 0.05 and (***) P < 0.001 indicate a significant difference from the control.
4 Discussion
This study was designed to evaluate the response of the testis in the freshwater leech E. johanssoni to BTEX toxicity and elucidate the mechanisms inducing damage. Histopathology is now increasingly being used as a biomarker of environmental stress, and histopathological responses have been reported as valuable markers of toxicity (Akinsanya et al., 2019; Jia et al., 2019; Zhang et al., 2020). In this study, light microcopy examination of E. johanssoni testes revealed severe histological alterations and extensive necrosis with complete loss of normal cyst structure. Our findings were consistent with previous studies that focused on the ability of BTEX to induce obvious testicular histopathological damage in the freshwater leech Limnatis nilotica exposed to 1.4 and 2.8 mg L−1 BTEX (Khaled et al., 2016; Khaled et al., 2017). It has been reported that xylene exposure induces changes in testicular tissue rates manifested by a decrease in epithelial height of the seminiferous tubule and Sertoli cells (Gules and Eren, 2010). Similar exposure to benzene led to histopathological lesions in the testes of Sprague Dawley rats characterized by cytoplasmic vacuolization, pyknosis of nuclei, chromatolysis, desquamation and dissolution of germ cells in the tubular lumen (Singh and Bansode, 2011). In the freshwater fish Heteropneustes fossilis, exposure to linear alkyl-benzene sulfonate (LAS) caused significant testicular histopathological changes, leading to a decrease in reproductive potential (Kumar et al., 2007). Surprisingly, leeches appeared to be highly susceptible to aquatic toxicity, as the testicular effects observed in this study were caused by a wide range of chemical contaminants other than BTEX. Indeed, it has been reported that the structure and the development of reproductive tissue in the freshwater leech Nephelepsis obscura were strongly impaired after cadmium exposure revealing reduced ova and spermatozoa genesis and also a marked reduction of ovisacs, epididymis and testisacs masses (Davies et al., 1995; Westcott, 1997). On the other hand, exposure of the leech Poecilobdella viridis to reserpine and chlorpromazine induced decrease in ovary and testis indices (Rao et al., 1983).
The most important parameters used to measure the health of aquatic life and the mechanism of toxicity in aquatic organisms are oxidative stress indicators (Kumari et al., 2014; Wei and Yang, 2015). To the best of our knowledge, no previous research has investigated oxidative stress in leeches. In fact, this animal invertebrate group was poorly used as a bioindicator for bioassessment and monitoring of water quality (Moser et al., 2009). Interestingly, lipid peroxidation, which was determined indirectly by measuring the production of MDA, is considered as a sensitive biomarker to assess oxidative stress status and tissue damage level, as MDA represents the end product formed during the decomposition of poly-unsaturated fatty acids mediated by free radicals (Giera et al., 2012). Our results showed a significant increase in MDA levels in the leech E. johanssoni exposed to BTEX, suggesting that these hydrocarbons exert their toxicity by peroxidating membrane polyunsaturated fatty acids. This notion has also been explored in the catfish Clarias gariepinus and the earthworm Eudrilus eugeniae, and an increase in MDA levels after exposure to BTEX was identified (Eseigbe et al., 2013; Doherty, 2014), whereas the same hydrocarbon caused lipid peroxidation injury in the sea cucumber Apostichopus japonicus (Jiang et al., 2014). The described increase in MDA contents is due to the inhibitory effect on the mitochondrial electron transport system, which stimulates the generation of intracellular reactive oxygen species (ROS) (Stohs et al., 2001). This result is evident due to the chemical characteristics of BTEX, which includes highly lipid soluble compounds that readily accumulate at high levels in the tissue of aquatic organisms, triggering the release of ROS. Consequently, BREX impacts vital cellular functions either alone or through metabolites (Cheng et al., 2015). In fact, it has been previously reported that testicular tissue is one of the main targets of ROS due to its high content of polyunsaturated membrane lipids (Mishra and Acharya, 2004). ROS serve as cell signaling molecules for normal biological processes (Auten and Davis, 2009). However, in response to external environmental stimuli, an imbalance between the production of ROS and the capability of the cell to ensure an effective antioxidant response is defined as oxidative stress (Ray et al., 2012). The increase in MDA levels in our findings indicate the generation of ROS, which is consistent with earlier research that found that BTEX toxicity is mediated by ROS produced during the metabolism of these components (Singh et al., 2010).
The antioxidant enzymes SOD, CAT and GPx have powerful detoxification roles against injurious ROS and they represent the first line defense antioxidants against ROS attack (Ighodaro and Akinloye, 2018). In particular, SOD catalysis the dismutation of superoxide anions into oxygen and hydrogen peroxide, CAT and GPx breakdown hydrogen peroxide (H2O2) to water and oxygen (Ran et al., 2007). In the exposed leeches, we noted a significant decrease in SOD, CAT and GPx activities after 26 days of exposure to BTEX, suggesting that BTEX exposure could impair the antioxidant defense system. Under normal conditions, ROS are quickly detoxified by the antioxidant defense system (Juan et al., 2021). However, under BTEX exposure for 26 days, excessive ROS are generated, which may destroy the cell membrane and form lipid peroxides and oxidized proteins. This process may lead to a disturbance in the balance between the prooxidants and antioxidants. Thus, the detoxification function of antioxidant enzymes was inhibited. It was reported that many factors could reflect the state of antioxidant enzymes such as the intensity, the duration of the stress applied and the vulnerability of the exposed organisms, hence, the activity of antioxidant enzymes may be increased or decreased under chemical stress (Otitoloju and Olagoke, 2011). It has also been noted that BTEX attacks the antioxidant system, including superoxide dismutase (SOD), glutathione peroxidase (GPx), and catalase (CAT) (Singh et al., 2009; Maksoud et al., 2019; Pajaro-Castro et al., 2019), which is consistent with our findings.
It is well established that excess ROS generation after treatment with chemical environmental pollutants is closely related to DNA damage (Rowe et al., 2008; Cheng et al., 2015; Zhang et al., 2020). Using a comet assay, our results showed an increase in DNA damage in the testes of exposed leeches, which is consistent with previous studies that focused on the genotoxicity of BTEX (Khaled et al., 2016; Khaled et al., 2017). Indeed, a previous study revealed that BTEX induces DNA damage in isolated human lymphocytes resulting from the induction of DNA strand breaks (Chen et al., 2008). Exposure to toluene, ethylbenzene and xylene caused a stress response and DNA damage in the earthworm E. fetida (Liu et al., 2010). Interestingly, a study on benzene exposure revealed induced DNA damage and increased ROS production in fetal liver cells (Tung et al., 2012), whereas exposure to benzoquinone, a metabolite of benzene, disrupted fetal topoisomerase IIα, an essential enzyme for DNA repair (Holmes and Winn, 2019).
In summary, the present study displayed that exposure to BTEX induced histopathological alterations, oxidative stress and DNA damage in the testes of the freshwater leech E. johanssoni. The measurement of these biochemical responses can be used as a useful biomarker to appraise the risk of effects of environmental pollutants on the health status of exposed animals.
Author contributions
-
Khaled: conceptualize, carried out the experiments and write the first draft, I. Saidi: carried out the experiments, H. Ferjani: carried out the experiments, R. Ben Ahmed: analyzed the data and write the first draft, A. Alrezaki: analyzed the data, F. Guesmi: analyzed the data and write the first draft, H. Bouzenna: Analyzed the data and write the first draft, A.H. Harrath: designed the experiments and finalized the final version.
Acknowledgements
The authors extend their appreciation to Researchers Supporting Project number RSP-2021/17, King Saud University, Riyadh, Saudi Arabia.
Funding
This Project was funded by the Researchers Supporting Project number RSP-2021/17, King Saud University, Riyadh, Saudi Arabia.
Conflicts of Interest
The authors declare no competing interests.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Availability of the data and materials
The data that support the findings of this study are available from the corresponding author, (Abdel Halim Harrath), upon reasonable request.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Aspidogastrea africanus Infections, comparative assessment of BTEX and heavy metals Bioaccumulation, and histopathological alterations as biomarker response in Chrysichthys nigrodigitatus (Lacépède, 1803) of Lekki Lagoon, Nigeria. Sci. Afr.. 2019;3:e00060.
- [Google Scholar]
- Toluene can disrupt rat ovarian follicullogenesis and steroidogenesis and induce both autophagy and apoptosis. Biology. 2021;10:1153.
- [Google Scholar]
- Toxicological profile for benzene. US Department of Health and Human Services: Public Health Service Atlanta; 2007.
- Toxicological Profile for Xylene. US Department of Health and Human Services: Public Health Service Atlanta, GA; 2007.
- ATSDR, A., 2000 Toxicological profile for toluene. USDoHaH Services, editor. Atlanta: US Public Health Service: 1-357.
- ATSDR, A., 2010. Toxicological profile for ethylbenzene. Agency for Toxic Substances and Disease Registry.
- Oxygen toxicity and reactive oxygen species: the devil is in the details. Pediatr. Res.. 2009;66(2):121-127.
- [Google Scholar]
- A case study of BTEX characteristics and health effects by major point sources of pollution during winter in Iran. Environ. Pollut.. 2019;247:607-617.
- [Google Scholar]
- Assaying for superoxide dismutase activity: some large consequences of minor changes in conditions. Anal. Biochem.. 1987;161(2):559-566.
- [Google Scholar]
- [30] Microsomal lipid peroxidation. In: Methods Enzymol.. Elsevier; 1978. p. :302-310.
- [Google Scholar]
- Ambient BTEX exposure and mid-pregnancy inflammatory biomarkers in pregnant African American women. J. Reprod. Immunol.. 2021;145:103305.
- [Google Scholar]
- Assessment of genotoxicity of methyl-tert-butyl ether, benzene, toluene, ethylbenzene, and xylene to human lymphocytes using comet assay. J. Hazard. Mater.. 2008;153(1-2):351-356.
- [Google Scholar]
- Effects of ammonia exposure on apoptosis, oxidative stress and immune response in pufferfish (Takifugu obscurus) Aquat. Toxicol.. 2015;164:61-71.
- [Google Scholar]
- Oxidative damage to DNA: do we have a reliable biomarker? Environ. Health Perspect.. 1996;104(suppl 3):465-469.
- [Google Scholar]
- Changes in reproductive potential of the leech Nephelopsis obscura (Erpobdellidae) as biomarkers for cadmium stress. Can. J. Zool.-Revue Canadienne De Zoologie. 1995;73(12):2192-2196.
- [Google Scholar]
- Histopathological and biochemical alterations in Eudrilus eugeniae (Kinberg 1867) as biomarkers of exposure to monocyclic aromatic hydrocarbons in oil impacted site. J. Basic Appl. Zool.. 2019;80:1-15.
- [Google Scholar]
- Doherty, V.F., 2014. Antioxidant Enzymes and Histopathological Biomarkers of Exposure to Monocyclic Aromatic Hydrocarbons in Clarias Gariepinus (Catfish) and Eudrilus Eugeniae (Earthworm).
- Histopathology alterations and lipid peroxidation as biomarkers of hydrocarbon-induced stress in earthworm, Eudrilus eugeniae. Environ. Monit. Assess.. 2013;185(3):2189-2196.
- [Google Scholar]
- Toxic effects of BTEX in water on Daphnia magna and Limnodrilus hoffmeisteri and safety assessment of the aquatic environment. Acta Sci. Circumstantiae.. 2009;29:1485-1490.
- [Google Scholar]
- Recent advancements in the LC-and GC-based analysis of malondialdehyde (MDA): a brief overview. Chromatographia. 2012;75(9-10):433-440.
- [Google Scholar]
- The effect of xylene and formaldehyde inhalation on testicular tissue in rats. Asian-Australas. J. Anim. Sci.. 2010;23:1412-1420.
- [Google Scholar]
- Ethylbenzene exposure disrupts ovarian function in Wistar rats via altering folliculogenesis and steroidogenesis-related markers and activating autophagy and apoptosis. Ecotoxicol. Environ. Saf.. 2022;229:113081.
- [Google Scholar]
- BTEX exposure assessment and quantitative risk assessment among petroleum product distributors. Ecotoxicol. Environ. Saf.. 2017;144:445-449.
- [Google Scholar]
- DNA damage and perturbed topoisomerase iiα as a target of 1, 4-benzoquinone toxicity in murine fetal liver cells. Toxicol. Sci.. 2019;171:339-346.
- [Google Scholar]
- First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alexandria J. Med.. 2018;54(4):287-293.
- [Google Scholar]
- Fluorene-9-bisphenol exposure induces cytotoxicity in mouse oocytes and causes ovarian damage. Ecotoxicol. Environ. Saf.. 2019;180:168-178.
- [Google Scholar]
- Effects of beneze, toluene, ethylbenzene, and xylene (BTEX) on lipid peroxidation in sea cucumber Apostichopus japonicus. Fish. Sci. (Dalian). 2014;33:15-21.
- [Google Scholar]
- The chemistry of reactive oxygen species (ROS) revisited: outlining their role in biological macromolecules (DNA, lipids and proteins) and induced pathologies. Int. J. Mol. Sci.. 2021;22:4642.
- [Google Scholar]
- Effects of oil-related environmental pollutants on gonads of the freshwater leech Limnatis nilotica (Annelida, Hirudinea) Invertebr. Reprod. Dev.. 2016;60(4):263-270.
- [Google Scholar]
- Impact of oil-related environmental pollutants on the ovary structure in the freshwater leech Erpobdella johanssoni (Johansson, 1927)(Clitellata: Hirudinea) Eur. Zool. J.. 2017;84(1):286-293.
- [Google Scholar]
- BTEX biodegradation in contaminated groundwater using a novel strain (Pseudomonas sp. BTEX-30) Int. Biodeterior. Biodegrad.. 2017;116:234-242.
- [Google Scholar]
- Antifertility effect of Benzene extract of flowers of Hibiscus rosa sinensis L. on reproductive system in male albino rats. Indian J. Appl. Pure Biol.. 2014;29:215-217.
- [Google Scholar]
- Histopathological changes in testis of the freshwater fish, Heteropneustes fossilis (Bloch) exposed to linear alkyl benzene sulphonate (LAS) J. Environ. Biol.. 2007;28:679-684.
- [Google Scholar]
- The applicability of oxidative stress biomarkers in assessing chromium induced toxicity in the fish Labeo rohita. In: BioMed Research International. 2014.
- [Google Scholar]
- Lead accumulation, oxidative damage and histopathological alteration in testes and accessory glands of freshwater crab, Sinopotamon henanense, induced by acute lead exposure. Ecotoxicol. Environ. Saf.. 2015;117:20-27.
- [Google Scholar]
- Joint action and lethal levels of toluene, ethylbenzene, and xylene on midge (Chironomus plumosus) larvae. Environ. Sci. Pollut. Res.. 2013;20(2):957-966.
- [Google Scholar]
- Oxidative stress and DNA damage in the earthworm Eisenia fetida induced by toluene, ethylbenzene and xylene. Ecotoxicology. 2010;19(8):1551-1559.
- [Google Scholar]
- Biochemical study on occupational inhalation of benzene vapours in petrol station. Respir. Med. Case Reports. 2019;27:100836.
- [Google Scholar]
- Mandani, P., Desai, K., Highland, H., 2013. Cytotoxic effects of benzene metabolites on human sperm function: an in vitro study, Int. Schol. Res. Notices.
- Application of the comet assay and detection of DNA damage in haemocytes of medicinal leech affected by aluminium pollution: A case study. Environ. Pollut.. 2009;157(5):1565-1572.
- [Google Scholar]
- Protective action of vitamins on the spermatogenesis in lead-treated Swiss mice. J. Trace Elem. Med Biol.. 2004;18(2):173-178.
- [Google Scholar]
- Moser W, Govedich F, Klemm D (2009) Annelida, Hirudinida (leeches).
- Oxidative DNA damage induced by toluene is involved in its male reproductive toxicity. Free Radic Res. 2003;37(1):69-76.
- [Google Scholar]
- Guidelines for drinking water quality incorporating. First and Second Addenda, Recommendations, third edition, WHO, Geneva, Switzerland. 2008;1:491-492.
- [Google Scholar]
- Lipid peroxidation and antioxidant defense enzymes in Clarias gariepinus as useful biomarkers for monitoring exposure to polycyclic aromatic hydrocarbons. Environ. Monit. Assess.. 2011;182(1-4):205-213.
- [Google Scholar]
- Toxicity and expression of oxidative stress genes in Tribolium castaneum induced by toluene, xylene, and thinner. J Toxicol Environ Health, A. 2019;82(1):28-36.
- [Google Scholar]
- Water and sediment toxicity assessment by use of behavioural responses of medicinal leeches. Environ. Int.. 2003;28(8):729-736.
- [Google Scholar]
- The medicinal leech as a convenient tool for water toxicity assessment. Environmental Toxicology: An International Journal. 2004;19(4):336-341.
- [Google Scholar]
- Effect of environmental contaminants on mammalian testis. Curr Mol Pharmacol. 2014;7:119-135.
- [Google Scholar]
- Reduction in glutathione peroxidase 4 increases life span through increased sensitivity to apoptosis. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2007;62(9):932-942.
- [Google Scholar]
- Effect of tranquillisers on the reproduction of a freshwater leech, Poecilobdella viridis (Blanchard) Proceedings: Animal Sciences. 1983;92:323-331.
- [Google Scholar]
- Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal.. 2012;24(5):981-990.
- [Google Scholar]
- DNA damage-induced reactive oxygen species (ROS) stress response in Saccharomyces cerevisiae. Free Radical Biol. Med.. 2008;45(8):1167-1177.
- [Google Scholar]
- A review of assessment of benzene, toluene, ethylbenzene and xylene (BTEX) concentration in urban atmosphere of Delhi. International Journal of Physical Sciences. 2012;7:850-860.
- [Google Scholar]
- Genotoxicity and apoptosis in Drosophila melanogaster exposed to benzene, toluene and xylene: attenuation by quercetin and curcumin. Toxicol. Appl. Pharmacol.. 2011;253(1):14-30.
- [Google Scholar]
- Effects of co-exposure of benzene, toluene and xylene to Drosophila melanogaster: alteration in hsp70, hsp60, hsp83, hsp26, ROS generation and oxidative stress markers. Chemosphere. 2010;79(5):577-587.
- [Google Scholar]
- Induction of hsp70, hsp60, hsp83 and hsp26 and oxidative stress markers in benzene, toluene and xylene exposed Drosophila melanogaster: role of ROS generation. Toxicol. Appl. Pharmacol.. 2009;235(2):226-243.
- [Google Scholar]
- Benzene-induced histopathological changes and germ cell population dynamics in testes of Sprague Dawley rats. J. Environ. Biol.. 2011;32:687.
- [Google Scholar]
- Influence of oil-related environmental pollutants on female reproduction. Reprod. Toxicol.. 2017;71:142-145.
- [Google Scholar]
- Oxidative mechanisms in the toxicity of chromium and cadmium ions. J. Environ. Pathol. Toxicol. Oncol.. 2001;20(2):12.
- [Google Scholar]
- DNA double-strand breaks and DNA recombination in benzene Metabolite-Induced genotoxicity. Toxicol. Sci.. 2012;126(2):569-577.
- [Google Scholar]
- Oxidative damage of hepatopancreas induced by pollution depresses humoral immunity response in the freshwater crayfish Procambarus clarkii. Fish Shellfish Immunol.. 2015;43:510-519.
- [Google Scholar]
- The effects of low-level chronic cadmium exposure on a freshwater leech. University of Calgary; 1997.
- Effect of benzene, toluene, xylene on the semen quality and the function of accessory gonad of exposed workers. Ind. Health. 2001;39(2):206-210.
- [Google Scholar]
- Oxidative stress and genotoxicity of long-term occupational exposure to low levels of BTEX in gas station workers. Int. J. Environ. Res. Health. 2016;13(12):1212.
- [Google Scholar]
- Involvement of mitochondria-mediated apoptosis in ethylbenzene-induced renal toxicity in rat. Toxicol. Sci.. 2010;115:295-303.
- [Google Scholar]
- Effects of acute ammonia toxicity on oxidative stress, DNA damage and apoptosis in digestive gland and gill of Asian clam (Corbicula fluminea) Fish Shellfish Immunol.. 2020;99:514-525.
- [Google Scholar]







