Translate this page into:
Bombax ceiba extract and its metabolites as α-glucosidase inhibitors for diabetes
⁎Corresponding author. azharrasul@gcuf.edu.pk (Azhar Rasul)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Alpha-glucosidase inhibitors characterize a major class of Type II antidiabetic drugs and play a significant role in lowering postprandial hyperglycemia. Currently, the market offers a limited number of synthetic inhibitors, highlighting the necessity for the discovery of new and potent compounds with enhanced efficacy in this area. For this purpose, an already established library of 51 plant extracts was screened against α-glucosidase, among which Bombax ceiba extract exhibits significant α-glucosidase inhibitory activity (IC50; 1.95 ± 0.29 µg/mL) as compared to acarbose (IC50; 3.14 ± 0.49 µg/mL). Moreover, in order to investigate the specific phytochemicals responsible for this activity, a literature-based library of 78 compounds from B. ceiba were curated and subsequently screened against α-glucosidase using molecular docking. The selection of hit compounds was evaluated on the base of computational tools. Out of these 78 compounds, nine potent compounds (Pelargonin, Simalin B, Linarin, Rutin, Nicotiflorin, Simalin A, Mangiferin, Quercetin and Apigenin) exhibited best binding affinities with α-glucosidase. These phytochemicals exhibited favorable binding energy, hydrogen bonding, and protein–ligand interactions as compared to acarbose. These results were further validated by in vitro α-glucosidase inhibition assay of commercially available phytochemicals. To the best of our knowledge, this report unveils B. ceiba as a highly effective inhibitor of α-glucosidase. The findings suggest that B. ceiba and its metabolites exhibit promising characteristics for the development of leading drugs in the field of anti α-glucosidase medications, which could play a crucial role in the management of diabetes.
Keywords
Bombax ceiba
α-glucosidase
Simalin A
Simalin B
Acarbose
In silico
Enzymatic assay
1 Introduction
Diabetes mellitus (DM) is among the swiftly burgeoning global health emergencies. In 2021, diabetes mellitus (DM) accounted for 537 million cases and resulted in 6.7 million fatalities. Projections indicate that these numbers will escalate to over 643 million cases by 2030 and reach a staggering 783 million cases by 2045 (Sun et al., 2022). Approximately 90 % individuals who have diabetes but remain undiagnosed lived in low and middle-income countries. Pakistan is the third most affected country with DM after China and India. About 33 million people in Pakistan are living with diabetes. This disease is not only an everyday health challenge but a financial one as well. The estimated global cost for diabetes is 825 billion USD annually (Febrian et al., 2023).
Currently available diabetes medications target various pathways and enzymes which take part in glucose homeostasis, with the aim of normalizing blood glucose levels. Among these, α-glucosidase inhibitors are an intriguing class of drugs. These enzymes assist in the digestion of dietary carbohydrates and breaking them down into glucose in small intestine. Consequently, this process results in an elevation of blood glucose levels. By reversibly inhibiting α-glucosidase enzymes postprandial hyperglycemia can be effectively reduced (Derosa and Maffioli, 2012). In contrast to other medications that maintain blood glucose levels, such as sulfonylureas, meglitinides, and insulin, α-glucosidase inhibitors do not cause obesity or hypoglycemia (Hossain et al., 2018). Moreover, urthermore, there are reports indicating that α-glucosidase inhibitors have the potential to reduce the risk of type II diabetes by 35.6 %. Importantly, this effect remains consistent across diverse patient populations, regardless of age, gender, or body mass index (BMI), thus highlighting their efficacy (Daou et al., 2022). Additionally, α-glucosidase inhibitors have also vaso-protective efficacy by lowering postprandial glucose levels, that is associated with endothelial abnormality, heart disease, and stroke (Matsui et al., 2001, Joshi et al., 2015). Although commercially available α-glucosidase inhibitors effectively lower blood sugar levels, they can potentially lead to gastrointestinal discomfort, diarrhea, and flatulence. (Akmal and Wadhwa, 2022). So, there is need to develop safer and novel natural inhibitors of α-glucosidase to manage diabetes mellitus.
Plants have thus long been one of the most reliable resources for medicines to treat diseases. Plant-based anti-diabetic medications have been used extensively from the earliest days since they are far more affordable and safer than synthetic drugs (Alam et al., 2022). To address the goals of this study, we have initially screened plant extracts library by using an in vitro based enzyme inhibitory assay for the α-glucosidase inhibitors identification. B. ceiba commonly known as red silk-cotton tree, belongs to the family Bombacaceae (Rameshwar et al., 2014). Approximately 250 species are found in this family (Rani et al., 2016). In Unani system of medicine, more emphasized to the use of gummy exudate known as mochras. This whole plant is used in different traditional medical systems. It has a number of therapeutic uses including Burning Micturition, Dysentry, Spermatorrhoea, Vaginal Discharge, Stomatitis, Diarrhoea, Haemoptysis, Dribbling of Urine, Bed Wetting, Menorrrhagia, Loosen Tooth, Bleeding Gums, and Blood Diseases. (Shukla et al., 2020).
B. ceiba showed different pharmacological activities such as anti-inflammatory, asthma, small-pox boils, wound healing, hypotensive, anti-oxidant, anti-pyretic, anti-analgesic and hepatoprotective activity (Rani et al., 2016).
In this study we explore first time anti-hyperglycemic properties of B. ceiba bark and its metabolites which showed significantly higher α-glucosidase inhibition than control (acarbose). Moreover, this work intends to investigate the enzyme kinetics and protein–ligand interactions of literature-based phytochemicals library of B. ceiba. We found nine potent hits including Pelargonin, Simalin B, Linarin, Rutin, Nicotiflorin, Simalin A, Mangiferin, Quercetin, and Apigenin on the base of lowest docking score (Joshi et al., 2014, Verma et al., 2015). Additionally, enzyme-based screening of commercially available potent hits was also carried out to validate their inhibitory efficacy against α-glucosidase. Through our computational and in vitro investigations, we have gathered substantial evidence demonstrating that B. ceiba and its metabolites exhibit remarkable ability to selectively target α-glucosidase. These findings may be useful for the development of new anti-diabetic drugs designed to effectively reduce hyperglycemia.
2 Materials and methods
2.1 Preparation of plant extracts library
In this study, we utilized a pre-existing library consisting of 51 plant extracts, which had been previously reported in our research article (Rasul et al., 2021). These plant samples were collected from the local flora of Pakistan. Prior to the extraction process, the plants were thoroughly washed, dried, and then subjected to the extraction procedure using a Soxhlet apparatus. For this purpose, a Whatman 1 pore size filter paper thimbles with 50 g of each plant’s powder were prepared and placed in thimble cup. In a Soxhlet flask, 250 mL of methanol was added and ran through five cycles. The crude extracts were prepared by collecting and evaporating the filtrate using a rotating evaporator. These extracts were stored at −20 °C until their further use.
2.2 Inhibitory activity of α-glucosidase
The α-glucosidase inhibitory activity was assessed using protocol based on breakdown principle of the p-Nitrophenol glucopyranoside (PNPG), as described by Yırtıcı and Ergene with minor modification (Yırtıcı et al., 2022). In this experiment, 10 µL of each sample (prepared in DMSO) with different concentrations was added with 40 µL α-glucosidase (0.5 U/mL) from Saccharomyces cerevisiae (from Sigma Aldrich). Then 120 µL phosphate buffer (0.1 M with pH 7.4) was added in each well. Following a five-minute incubation period, 40 µL of substrate solution (5 mM) was added in each well, which was then incubated again at 37 °C for 30 min. To stop the reaction, 30 µL of Na2CO3 (0.1 M) was added. The absorption of p-nitrophenol was measured at 405 nm using INNO microplate reader. Acarbose (10 mM) was employed as control in this experiment. The inhibitory percentage was calculated using the following formula: To calculate IC50 value microdilution was done.
2.3 Phytochemical library preparation
The three dimensional SDF structures of the B. ceiba phytochemicals were retrieved from the PubChem database, accessed on May 02, 2023 (https://pubchem.ncbi.nlm.nih.gov) for the phytochemical library. Acarbose was used as control compound for result comparison. Ligand’s ability to bind with α-glucosidase target site was evaluated using the in silico ligand-target docking approach. Molegro Virtual Docker (MVD) version 6.0 was used for molecular docking, and the MolDock Score tool was used for scoring. The natural ligands found in the crystal structure served as the central docking zone. Following the procedure outlined by Thomsen and Christensen in 2006, the compounds subjected to re-docked within the alpha glucosidase crystal structures to evaluate the validity of the docking experiments (Thomsen and Christensen, 2006).
2.4 Molecular docking of phytochemicals
α-Glucosidase’s 3D structure was retrieved from Protein Database (PDB) (ID: 3A4A). By molecular Dynamics Visualization (MDV), the 3D structure was improved through 3D protonation, energy minimization, and the removal of solvent and ligand residues. Using the computational ligand-target docking approach, the ability of ligands to bind with the target sites of α-glucosidase was evaluated. The most favorable docking pose was identified to be the conformation with minimal binding energy. Using Discovery Studio Visualizer (DSV) 2021 (Accelrys Software Inc., San Diego, CA, USA), the potential interactions between ligands and proteins were examined (Thomsen and Christensen, 2006). The active site residues of the α-glucosidase bond (HIS112, ASP69, ARG442, GLU277, and ARG213) were chosen using the site discovery function in the MVD program (Sadiqa et al., 2022). The compounds with the highest binding affinities were chosen for further analysis after docking was completed. MolDock Score is designed using the GEMDOCK energy function consisting of electrostatic, steric, and hydrogen-bonding potentials. This is a suitable approach for flexible and hybrid dockings. Additionally, GEMDOCK is an automatic system that generates all related docking variables, such as atom formal charge, atom type, and the ligand binding site of a protein. Although the program gives results with different parameters, we used the MolDock Score. The MolDock scoring function consists of functions with a hydrogen bonding term, and charge schemes between small molecules and proteins (Yang and Chen, 2004).
2.5 Drug likeness and ADMET analysis of compounds
By utilizing structural similarities identified in previous experimental research, ADMET prediction systems enable us to make accurate forecasts regarding certain pharmacokinetic and drug-like attributes associated with substances. The selected compounds with the highest docking results were proceeded to ADMET analysis by utilizing ADMET lab 2.0 (https://admetmesh.scbdd.com). The hit compounds' physicochemical properties were determined (Dong et al., 2018, Xiong et al., 2021).
2.6 Statistical analysis
Each experiment was carried out three times using Microsoft Excel 2016 to obtain results that showed the mean and standard deviation (mean ± SD). Graphs were created using GraphPad Prism 8.0.2.
3 Results
3.1 Screening of plant extracts library against α-glucosidase
A library of 51 extracts of different parts of 35 plants were initially screened against α-glucosidase at 50 µg/mL and obtained results has been presented in Table 1. From these extracts, 19.6 % (10 plant extracts) showed high (>80 %) inhibition against α-glucosidase, 25.4 % (13 plant extracts) exhibit moderate (41 %-70 %) and 54.9 % (28 plant extracts) represent insignificant and low (0–40 %) inhibitory activity. Potent plant extracts from first screening were further screened at lower concentration (10 µg/ml) to find the most effective plant against α-glucosidase. After secondary screening B. ceiba bark was found highly effective against α-glucosidase and further tested at different concentrations (0.5, 1, 2, 4, 8, 16, 32 µg/mL) against α-glucosidase by using PNPG substrate. B. ceiba exhibited higher inhibition with IC50 1.95 ± 0.29 µg/mL, as compared to acarbose control 3.14 ± 0.49 µg/mL which clearly indicated significant inhibitory potential of B. ceiba as compared to acarbose (Fig. 1). Here +++ indicating above 80 % inhibition, ++ for 61 %-79 % and + for below 40–60 % and – for below 40 %.
Sr. No.
Scientific names
Common names
Family
Parts
Extract no.
α-glucosidase activity
1
Aloe barbadensis
Aloe vera
Asphodelaceae
Complete plant
1
+++
2
Azadirachta indica
Indian lilac
Meliaceae
Leaves
2
+++
3
Nerium oleander
Oleander
Apocynaceae
Leaves
3
_
4
Albizia lebbeck
Lebbeck
Fabaceae
Leaves
4
+
Seed
5
+
Flowers
6
+
Seed coat
7
+
5
Momordica charantia
Bitter gourd
Cucurbitaceae
Seeds
8
++
vegetable
9
+
6
Cyamopsis tetragonoloba
Guar gum
Fabaceae
Seeds
10
_
7
Oxalis corniculata
Wood-sorrel
Oxalidaceae
Whole plant
11
_
8
Cassia fistula
Golden shower
Fabaceae
Leaves
12
++
Bark
13
+++
9
Ageratum conyzoides
Goat weed
Asteraceae
Complete plant
14
_
10
Dalbergia sissoo
Indian rosewood
Fabaceae
Seeds
15
_
Bark
16
+
11
Chenopodium album
Lamb’s quarters
Amaranthaceae
Entire plant
17
_
12
Bombax ceiba
Cotton tree
Bombacaceae
Bark
18
+++
Leaves
19
+++
13
Cicer arietinum
Chickpea (white)
Fabaceae
Seeds
20
+
Chick pea (black)
Seeds
21
+
14
Smilax china L.
China root
Smilacaceae
Root
22
+
15
Helianthus annuus
Sun flower
Asteraceae
Seeds
23
+++
16
Peganum harmala
Wild Rue
Nitrariaceae
Whole Plant
24
_
17
Litchi chinensis Sonn.
Lychee
Sapindaceae
Seeds
25
++
Bark
26
+++
Leaves
27
++
18
Eucalyptus camaldulensis
Himalayan poplar
Myrtaceae
Bark
28
+++
19
Cyperus esculentus
Water grass
Cyperaceae
Flowers
29
+++
20
Artemisia absinthium
Common wormwood
Asteraceae
Whole plant
30
_
21
Ferula assa-foetida
Heng
Umbelliferae
Resin
31
_
22
Lawsonia inermis
Henna
Lythraceae
Leaves
32
_
23
Fagonia arabica
Dhamasa
Zygophyllaceae
Whole plant
33
_
24
Solanum nigrum
Black night shade
Solanaceae
Complete plant
34
++
25
Mangifera indica L.
Mango
Anacardiaceae
Pulp
35
+
Seed coat
36
++
Bark
37
+++
Peels
38
++
Seed
39
++
Leaves
40
++
26
Asphodelus tenuifolius
Wild onion
Asphodelaceae
Whole plant
41
++
27
Linum usitatissimum
Flax seeds
Linaceae
Seeds
42
_
28
Coriandrum sativum
Coriander
Apiaceae
Seeds
43
_
29
Citrullus colocynthis
Desert bitter gourd
Cucurbitaceae
Fruit
44
_
30
Acacia farnesiana
Thorn Mimosa
Fabaceae
Seeds
45
_
31
Trigonella foenum-graecum L.
Fenugreek
Fabaceae
Seed
46
_
32
Punica granatum
Pomegranate
Lythraceae
Peels
47
++
Seed
48
++
33
Cucumis melo agrestis
Wild melon
Cucurbitaceae
Leaves
49
_
34
Calotropis procera
Sodom apple
Apocynaceae
Leaves
50
_
35
Citrus maxima
Chinese grapefruit
Rutaceae
Bark
51
++
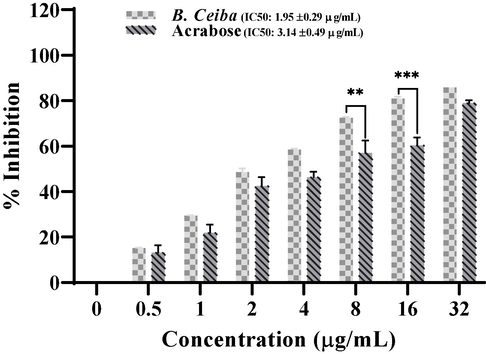
Percentage α-glucosidase inhibition with increased concentrations of B. ceiba extract and acarbose. The obtained IC50 was 1.95 ± 0.29 µg/mL for B. ceiba. The experiment is repeated in triplicates with mean ± standard deviation. Where (*) for p < 0.05, (**) for p < 0.005, (***) for p < 0.0005.
3.2 Identification of α-glucosidase inhibitors from b. Ceiba via in silico-based screening
Docking studies on seventy-eight phytochemicals of B. ceiba was done to evaluate their affinities with substrate binding sites of α-glucosidase (ID: 3A4A). The binding sites and respective details for each compound using the Molegro Virtual Docker (MVD) program package at α-glucosidase binding site are exhibited in Fig. 2. Already reported inhibitor compound acarbose served as a control in this experiment. The docking scores obtained with the α-glucosidase binding site are shown in Table 2. Out of 78 phytochemicals nine compounds (Pelargonin, Simalin B, Linarin, Rutin, Nicotiflorin, Simalin A, Mangiferin, Quercetin, and Apigenin) were found highly potent with best binding score. Pelargonin, Simalin B, Linarin, Rutin, Nicotiflorin, Simalin A, Mangiferin, Quercetin, Apigenin and acarbose at the α-glucosidase cavity scored were found to be −174.28 Å, −161.08 Å, −146.68 Å, −142.24 Å, −134.14 Å, 125.94 Å, 110.57 Å, −102.5 Å, −102.45 Å and −107.89 Å respectively.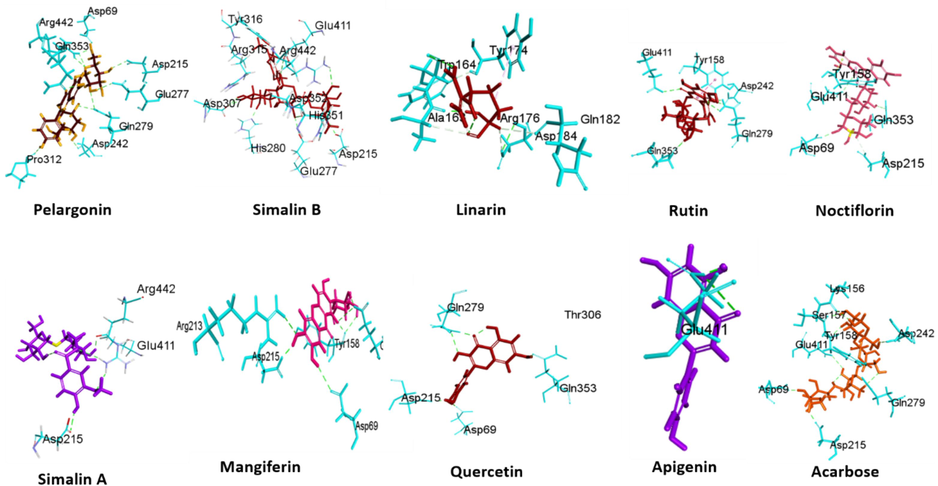
Three-dimensional representation depicting the selected docked complex with maltase-glucoamylase, an α-glucosidase enzyme.
Alpha-glucosidase binding activity
Compounds
PubChem CID
MolDock Score
HBond
Pelargonin
441,772
−174.28
−22.81
Simalin B
102,217,963
−161.08
−10.41
Linarin
5,317,025
−146.68
−16.15
Rutin
5,280,805
−142.24
−16.56
Nicotiflorin
5,318,767
−134.14
−12.29
Simalin A
102,217,962
−125.94
−15.39
Mangiferin
5,281,647
−110.57
−9.42
Quercetin
5,280,343
−102.50
−10.98
Apigenin
5,280,443
−102.45
−8.57
Acarbose
41,774
−107.89
−16.98
Table 3 illustrate the interaction details among nine hit phytochemicals and amino acids residues at binding cavity. The top hit compound, Pelargonin binds with the α-glucosidase binding complex through conventional hydrogen bond (GLN279, ARG442 and POS1), Carbon Hydrogen Bond (POS1) and Pi-doner hydrogen bond with ASP303. It also showed hydrophobic interactions, Pi-Pi Stacked and Pi-Alkyl with PHE303 and POS1 respectively. Second hit Simalin B, binds with the α-glucosidase binding complex through conventional hydrogen bond (ASP215, GLU411, ARG442 and POS2), Carbon Hydrogen Bond ARG315 and POS2. It also showed hydrophobic interactions Alkyl (POS2), and Pi alkyl (TYR72, TYR158, PHE314, TYR316, HIS351 and POS2). Here, T represent Types, C represents Category, D represents Distance (Å), H = Hydrophobic interactions E represents Electrostatic interactions, Pi-S: Pi Sigma, Pi-Pi S represents Pi-Pi Stacked, Pi-A = Pi-Alkyl, Pi-DHB = Pi-Donor Hydrogen Bond, Ca.HB = Carbon Hydrogen Bond, Pi-C = Pi-Cation
Pelargonin
Simalin B
Linarin
Rutin
T
C
Interacting residues
D
T
C
Interacting residues
D
T
C
Interacting residues
D
T
C
Interacting residues
D
Hydrogen Bond
Conventional Hydrogen Bond
GLN279
2.62
Hydrogen Bond
Conventional Hydrogen Bond
ASP215:OD1
2.65
Hydrogen Bond
Conventional Hydrogen Bond
THR306:HG1
2.51
Hydrogen Bond
Co. HB
GLN279
2.43
GLN279
2.80
GLU411:OE2
2.55
ARG315:HN
1.74
GLN279
2.39
ARG442
2.33
ARG442:HH21
2.36
POS4:H17
2.35
POS5
1.72
ARG442
1.84
POS2:H20
2.26
POS4:H18
1.63
POS5
1.51
ARG442
2.23
POS2:H20
2.87
POS4:H19
2.16
POS5
2.32
POS1
2.09
POS2:H24
1.81
POS4:H20
2.37
POS5
2.13
POS1
2.43
POS2:H25
1.67
POS4:H21
2.70
POS5
2.13
POS1
2.41
POS2:H26
1.66
POS4:H25
1.59
Ca. HB
POS5:H6
2.87
POS1
2.43
POS2:H27
2.03
POS4:H25
2.15
POS5:H8
2.80
POS1
1.71
POS2:H28
2.42
Ca. HB
PHE314
2.51
POS5:H10
2.48
POS1
2.33
POS2:H29
1.63
POS4
1.39
POS5:H11
2.78
Ca. HB
POS1:H2
2.77
Ca. HB
ARG315:HD1
2.69
POS4
2.96
H
Pi -S
POS5:H5
2.45
POS1:H3
2.29
POS2:H5
1.62
POS4
2.75
Alkyl
POS5:C12
4.16
POS1:H10
2.28
POS2:H14
2.43
E
Pi- Anion
GLU277
3.81
Pi-Pi T-shaped
TYR158
4.19
POS1:H14
3.06
POS2:H15
2.63
PHE178
5.06
Pi-D HB
ASP307
3.51
POS2:H16
2.76
H
Pi-Pi S
PHE303
5.27
POS2:H18
2.97
PHE303
4.44
POS2:H40
2.41
H
Pi-Pi S
PHE303
4.07
E
Pi-Cation
ARG442:NH1
3.49
Pi-Alkyl
POS1
4.59
H
Alkyl
POS2:C25
3.65
PHE303
4.57
Pi-Anion
GLU277:OE2
4.61
POS2:C26
4.39
Pi-Alkyl
TYR72
5.14
ASP352:OD1
4.75
Pi-Alkyl
TYR72
3.32
TYR158
3.80
GLU411:OE2
4.83
TYR158
3.70
PHE178
4.18
PHE314
3.81
POS4
5.05
H
Pi-Pi T-shaped
TYR72
5.74
TYR316
4.11
HIS351
4.35
POS2
4.85
Nicotiflorin
Simalin A
Mangiferin
Quercetin
T
C
Interacting residues
D
T
C
Interacting residues
D
T
C
Interacting residues
D
T
C
Interacting residues
D
Hydrogen Bond
Co. HB
ARG442:HH12
2.54
Hydrogen Bond
Conventional Hydrogen Bond
GLN279:HE22
1.76
Hydrogen Bond
Conventional Hydrogen Bond
ARG213:HH21
2.41
Hydrogen Bond
Conventional Hydrogen Bond
GLN279:HE21
2.66
POS6:H17
2.67
ARG442:HH11
2.50
GLU411:OE2
3.35
GLN279:HE22
1.88
POS6:H19
1.75
ARG442:HH12
2.59
POS3:H8
1.68
ARG315:HE
2.20
POS6:H21
1.88
POS4:H15
1.84
POS3:H9
2.66
ARG442:HH12
2.62
POS6:H29
2.30
POS4:H16
1.91
POS3:H13
1.94
POS2:H7
1.79
Ca. HB
ARG315:HD2
2.39
POS4:H17
2.44
POS3:H17
2.76
POS2:H8
2.15
POS6:H1
2.53
POS4:H25
2.24
POS3:H18
1.70
POS2:H9
1.65
POS6:H8
2.67
Ca. HB
POS4:H2
2.37
Ca. HB
ARG315:HD1
2.29
POS2:H10
1.60
POS6:H9
1.97
POS4:H6
1.48
POS3:H1
2.45
H
Pi-Pi T-shaped
TYR72
5.74
Pi-Lone Pair
TYR158:O
2.91
POS4:H9
1.86
POS3:H1
2.81
E
Pi-Cation
ARG442:NH1
3.49
Others
Pi-Pi S
TYR158
5.35
POS4:H27
2.51
POS3:H4
2.18
Pi- Anion
GLU277:OE2
4.61
H
Pi-Alkyl
PHE178
4.88
Pi-D HB
GLU277:OE2
3.78
H
Pi-Pi T-shaped
TYR158
5.44
ASP352:OD1
4.75
POS6
5.11
H
Pi-Pi Stacked
PHE178
5.07
TYR158
5.02
GLU411:OE2
4.83
Pi-Alkyl
TYR72
3.54
Pi-Alkyl
POS3
5.46
HIS351
4.73
POS4
4.99
Apigenin
Acarbose
T
C
Interacting residues
D
T
C
Interacting residues
D
Hydrogen Bond
Conventional Hydrogen Bond
ARG442:HH12
2.52
Hydrogen Bond
Conventional Hydrogen Bond
GLN279:HE22
2.59
POS3:H8
1.99
POS1:H11
2.25
POS3:H9
1.87
POS1:H26
2.50
Ca. HB
ARG315:HD2
2.39
POS1:H28
2.84
E
Pi-Cation
ARG442:NH1
1.82
POS1:H37
2.21
H
Pi-Pi S
PHE178
3.94
POS1:H38
1.58
Pi-Pi T-shaped
TYR72
5.68
POS1:H39
1.66
Pi-Alkyl
POS3
5.07
POS1:H40
1.68
POS1:H41
2.17
POS1:H42
2.15
POS1:H43
1.69
Ca. HB
POS1:H1
2.38
POS1:H8
1.61
POS1:H9
2.58
POS1:H15
2.33
POS1:H16
2.82
POS1:H23
2.99
POS1:H35
2.00
Pi-D HB
POS1:H36
2.46
H
Pi −S
POS1:H17
2.68
Linarin being third potent hit, binds with the α-glucosidase binding complex through conventional hydrogen bond (THR306, ARG315, and POS4), Carbon Hydrogen Bond (PHE314 and POS4). It also showed electrostatic interaction (Pi-anion) with GLU277 and hydrophobic interactions, Pi-Pi S and Pi-Alkyl with PHE303 and TYR72, TYR158, PHE178, POS4 respectively. The interaction detail between the nine selected compounds (Pelargonin, Simalin B, Linarin, Rutin, Nicotiflorin, Simalin A, Mangiferin, quercetin and apigenin) and amino acids residue at the substrate binding site of α-glucosidase are presented in Table 3.
3.3 Physicochemical properties of potent hits via computational analysis
Hit compounds were selected on the base of their lowest docking score values and significant binding interactions with α-glucosidase. The top hits were chosen for further evaluation of their physicochemical properties and drug-likeness. The radar plot depicted in Fig. 3 provides a clear indication that the hit compounds possess suitable physicochemical properties for oral provision. While some compounds deviated slightly from the rules, as shown in Table 4, all these phytochemicals obey Pfizer rule’s requirements. According to this rule, the molecular weight (MW) should be in range of 100 ∼ 600 g/mol, the logarithm of the partition coefficient (MlogP) should be < 5, the hydrogen bond acceptors number (nHA) should be < 10, and the number of hydrogen bond donors should be in range of 0 ∼ 7. Furthermore, these compounds demonstrated drug-like properties in terms of medicinal chemistry characteristics, as they complied with the drug rules of Pfizer criteria. This suggests that these phytochemicals have the potential to serve as viable drug candidates.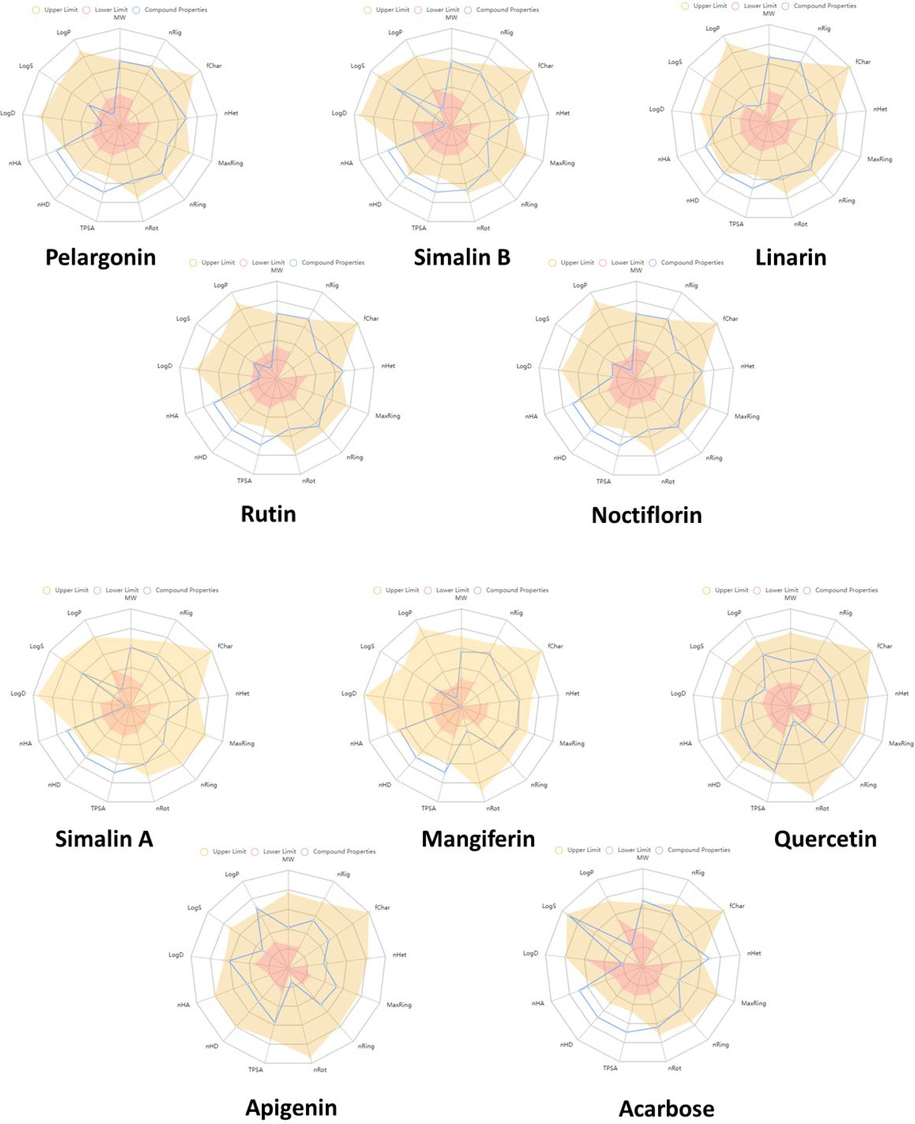
Showing the physicochemical properties of potent hits in radar plot. Brown area represents the upper limit, the blue area represents the compound's property, and the pink area represents the lower limit of the physicochemical property.
Physicochemical properties
Optimal
Pelargonin
Simalin B
Linarin
Rutin
Nicotiflorin
Simalin A
Mangiferin
Quercetin
Apigenin
Acarbose
Molecular Weight (MW)
100 ∼ 600
595.17
624.23
592.18
610.15
594.16
492.15
422.08
302.040
270.050
645.250
nHA
0 ∼ 12
15
17
14
16
15
14
11
7
5
19
nHD
0 ∼ 7
10
8
7
10
9
8
8
5
3
14
nRot
0 ∼ 11
7
10
7
6
6
8
2
1
1
9
nRing
0 ∼ 6
5
4
5
5
5
3
4
3
3
4
MaxRing
0 ∼ 18
10
6
10
10
10
6
14
10
10
6
nHet
1 ∼ 15
15
17
14
16
15
14
11
7
5
19
nRig
0 ∼ 30
29
24
30
30
30
19
23
18
18
24
TPSA
0 ∼ 140
250.52
244.91
217.97
269.43
249.2
225.06
201.28
131.360
90.900
321.170
logS
−4 ∼ 0.5
−2.732
−0.687
−3.890
−3.928
−3.952
−1.085
−3.626
−3.671
−3.606
0.377
logP
<5
−0.921
−2.046
0.386
−0.763
−0.553
−1.977
−0.521
2.155
3.307
−4.370
logD
1 ∼ 3
0.748
−0.283
1.792
0.695
1.052
0.181
0.039
1.767
2.704
−2.523
Medicinal chemistry
Pfizer Rule
Accepted
Accepted
Accepted
Accepted
Accepted
Accepted
Accepted
Accepted
Accepted
Accepted
3.4 ADMET profiling of hit compounds
We proceeded to carry out ADMET profiling (Absorption, Distribution, Metabolism, Excretion and Toxicity) study for the nine selected compounds against alpha glucosidase using ADMET lab 2.0 online platform. The ADMET profiling of compounds are presented in Table 5. Accurate ADMET profiling plays important role in ensuring the safe drug delivery. All these phytochemicals cannot cross blood brain barrier (BBB) except Simalin A and also do not cause any cardiovascular toxicity (hERG) and Skin irritation. Among all these active compounds Apigenin and Quercetin showed high gastrointestinal absorption. On the other hand, Linarin, Rutin and Nicotiflorin showed positive AMES mutagenicity while Pelargonin, Simalin B, Simalin A, Apigenin did not exhibit carcinogenicity and safer α-glucosidase inhibitors. In the context of classification endpoints, the prediction probabilities undergo a transformation into six distinct symbols to facilitate interpretation. The probability range of 0–0.1 is denoted by '---', while the interval of 0.1–0.3 is represented as '--'. Similarly, the range 0.3–0.5 is symbolized by '-', and 0.5–0.7 is expressed as '+'. Moving towards higher probabilities, the interval 0.7–0.9 is indicated by '++', and finally, the range 0.9–1.0 is conveyed through the symbol '+++'. This categorization system offers a concise and standardized representation of prediction confidence levels across different probability thresholds.
Category
Property
Pelargonin
Simalin B
Linarin
Rutin
Nicotiflorin
Simalin A
Mangiferin
Quercetin
Apigenin
Acarbose
Absorption
Caco-2>
−5.15−6.4/Low
−6.3/Low
−6.0/Low
−6.3/Low
−6.2/Low
−6.3/Low
−6.2/ Low
−5.2/High
−4.8/High
−6.1/ Low
PgN-Inhibitor
−−−
−−−
−−−
−−−
−−−
−−−
−−−
−−−
−−−
−−−
PgN-Substrate
+++
+++
+++
+++
+++
+++
+
−−−
++
++
HIA
+++
+++
+++
+++
−−−
+++
+++
−−−
−−−
+++
Distribution
PPB
73.8 %
23.9 %
71.1 %
83.8 %
83.48 %
26.05 %
84.9 %
95.4 %
97.2 %
8.2 %
BBB
−
−
−−
−−
−−
+
−−−
−−−
−−−
−
Metabolism
CYP2D6 Inhibitor
−−−
−−−
−−−
−−−
−−−
−−−
−−−
−
++
−−−
CYP2D6 Substrate
−−
−−
−−
−−
−−
−−
−−
−−
++
−−−
CYP3A4 Inhibitor
−−−
−−−
−−−
−−−
−−−
−−−
−−−
−
++
−−−
CYP3A4 Substrate
−−−
−−−
−−−
−−−
−−−
−−−
−−−
−−−
−−
−−−
CYP2C9 Inhibitor
−−−
−−−
−−−
−−−
−−−
−−−
−−−
+
+
−−−
CYP2C9 Substrate
−−
−−−
−
−−
+
−
−−
+
+++
−−−
CYP2C19 inhibitor
−−−
−−−
−−−
−−−
−−−
−−−
−−−
−−−
+
−−−
CYP1A2 Inhibitor
−−−
−−−
−−−
−−−
−−−
−−−
−−−
+++
+++
−−−
CYP1A2 Substrate
−−−
−−
−−−
−−−
−−−
−−−
−−−
−−
−−
−−−
CYP2C19 Substrate
−−−
+
−−
−−−
−−−
−−−
−−−
−−−
−−−
−−−
Excretion
Clearance
1.4/ Low
0.99/ Low
1.2/ Low
1.3/ Low
1.21/ Low
1.4/ Low
3.17/ Low
8.2/ Moderate
7.0/ Moderate
0.37/ Low
Toxicity
hERG
−−−
−−−
−−−
−−−
−−−
−−−
−−−
−−−
−−−
−−−
DILI
+++
−−
+++
+++
++
+
+++
+++
++
+++
H-HT
−−−
−−−
−−−
−−−
−−−
−−−
−−
−−−
−−−
−−
FDAMDD
−−−
−−−
−−−
−−−
−−−
−−−
−−−
−
−
−−−
Ames
−
−−
++
++
+++
−−
++
+
−
−−−
3.5 In vitro α-Glucosidase inhibition assay for bioactive compounds
Commercially available phytochemicals were further tested against α-glucosidase to validate our in silico results. For this purposes Apigenin, Quercetin and Mangiferin were used against α-glucosidase to determine their inhibitory activity. The inhibitory effect of the Apigenin, Quercetin and Mangiferin was compared with B. ceiba extract. B. ceiba showed IC50 at a concentration of 1.95 ± 0.29 µg/mL, on the other hand Apigenin, Quercetin and Mangiferin showed higher inhibition against α-glucosidase with IC50 values at a lower concentration of 0.83 ± 0.07 µg/mL, 0.96 ± 0.01 µg/mL and 1.47 ± 0.16 µg/mL respectively Fig. 4.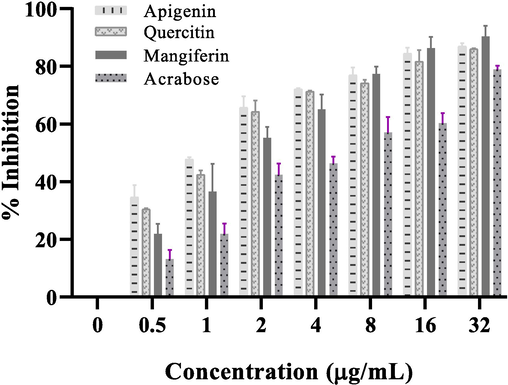
α-Glucosidase inhibition (%age) by increasing concentrations of Apigenin, Quercetin and Mangiferin as compared to acarbose. The resulting IC50 values are 0.83 ± 0.07 µg/mL for Apigenin, 0.96 ± 0.01 µg/mL for Quercetin and 1.47 ± 0.16 µg/mL for Mangiferin. The experiment was repeated in triplicates (n = 3) mean values ± Standard deviation.
4 Discussion
Plants and their derived compounds have long been used as a valuable source of medicines for the treatment of diseases. In particular, plant-based anti-diabetic medications have been widely utilized since ancient times due to their affordability and safety as compared to synthetic drugs. (Alam et al., 2022). In this study we screened 51 extracts of different parts of 35 plants against α-glucosidase. Glucosidases catalyze dietary carbohydrates from the small intestine and facilitate their absorption, specifically their glucose contents (Ahamad et al., 2011). From this screening, B. ceiba was found highly potent inhibitor of α-glucosidase. Different studies have shown that B. ceiba bark is a chief source of flavonoids, as compared to phenolic compounds where Apigenin and Quercetin are common flavonoids in B. ceiba extract and have anti-diabetic potential due to intestinal α-glucosidases inhibition (Vaghasiya et al., 2011, Hassan, 2018, Depani et al., 2019). PNPG substrate was used to investigate the inhibitory potential of B. ceiba against α-glucosidase. Similar results also reported by Hung et al.,2019 that, the isolated compounds from Root extract of Bombax malabarica showed alpha glucosidease activity of shorealactone with IC50 values 224 μM, l-epicatechin 5-O-β-D-xyloside with IC50 values 345 μM, and 2-C-[β-D-apiosyl-(1 → 6)]- β-D-glucosyl]-1,3,6-trihydroxy-7-methoxyxanthone with IC50 values 285 μM (Lam et al., 2019).
Inhibitory concentration (IC50) was 1.95 ± 0.29 µg/mL in B. ceiba, represent higher inhibition than already reported α-glucosidase inhibitor acarbose (IC50: 3.14 ± 0.49 µg/mL). There are different plants such as Allium sativum (Eidi et al., 2006), Gymnema sylvestre (Spasov et al., 2008), Citrullus colocynthis (Gurudeeban and Ramanathan, 2010), Trigonella foenum greacum (Renuka et al., 2009), Momordica charantia (Chaturvedi, 2012), Ficus bengalensis (Gayathri and Kannabiran, 2008), Syzygium cumini (Kumar et al., 2008) etc. already have been reported showing better antidiabetic potential than commercially available medicines. The demand for novel drugs with enhanced effectiveness and reduced toxicity remains persistent. The drug discovery and development procedure is highly expensive and time killing. In addition to the obstacles encountered during target validation and hit identification, clinical trials frequently show a significant failure rate due to factors such as insufficient pharmacokinetics, limited efficacy, and high toxicity (Chang et al., 2023). The field of drug design has experienced a significant transformation with the introduction of in silico analysis, which has led to improved efficiency and cost reduction compared to traditional drug design methods. The integration of advanced databases, software, and tools in bioinformatics has played a crucial role in the exploration and dissemination of numerous novel therapies and applications (Musuamba et al., 2021). In this study, a collection of phytocompounds obtained from PubChem IDs was subjected to docking simulations with α-glucosidase to assess their potential as α-glucosidase inhibitors. Out of these compounds nine compounds were selected as α-glucosidase inhibitors by their minimum energy and top MolDock scores. Recent investigations have highlighted the potential interactions of these bioactive phytochemicals and their significant hydrophobic contact with α-glucosidase. The docking analysis revealed binding energies of Pelargonin, Simalin B, Linarin, Rutin, Nicotiflorin, Simalin A, Mangiferin, Quercetin, Apigenin at the α-glucosidase cavity scored were found to be −174.28 Å, −161.08 Å, −146.68 Å, −142.24 Å, −134.14 Å, 125.94 Å, 110.57 Å, −102.5 Å, and −102.45 Å respectively, along with the formation of several hydrogen bonds. These compounds exhibited binding interactions with residues such as HIS112, ASP69, ARG442, GLU277, and ARG213 of α-glucosidase, consistent with previous studies highlighting their strong inhibitory activity and binding capabilities of with α-glucosidase (Yan et al., 2014). Additionally, a comprehensive evaluation of these compounds based on the “Rule of Five” (Ro5) was conducted to check their drug-likeness and molecular properties (Chen et al., 2020). Advanced high-performance ADMET profiling analyses have emerged as valuable tools in early-stage drug discovery, facilitating the identification of active lead compounds (Ferreira and Andricopulo, 2019). The ADMET compound profiling conducted in this study confirmed the favorable absorption properties of all the compounds, without producing any adverse effects. Various models assessing P-glycoprotein substrates, blood–brain barrier (BBB) penetration, and gastrointestinal uptake were employed to evaluate the ADMET-related characteristics of these potential compounds. Notably, all these phytochemicals showed significant gastrointestinal absorption while showing limited BBB penetration except Simalin A, suggesting a reduced risk of harmful or adverse side effects compared to acarbose. It was observed that all these phytochemicals exhibited susceptibility with P-gp substrate except Quercetin. P-glycoproteins play a crucial role in transporting drugs to targeted organs (Elmeliegy et al., 2020). Furthermore, Apigenin and Quercetin exhibited positive inhibition against CYP1A2 and CYP2C9, indicating the potential for drug-drug interactions with these enzymes. Importantly, these phytochemicals didn’t exhibit toxicity, such as skin sensitization, mutagenesis and cardiotoxicity as non-inhibitors of hERG (Priest et al., 2008). Among potent compounds, commercially available phytochemicals, Apigenin, Quercetin, and Mangiferin, also accessed by in vitro enzyme inhibitory assay to validate in silico results. these compounds exhibited significant inhibitory activity with the IC50 values 0.83 ± 0.07 µg/mL, 0.96 ± 0.01 µg/mL, and 1.47 ± 0.16 µg/mL respectively, which were notably less than acarbose. Li et al., (2009) also reported Quercetin and Rutin, that these compounds showed higher anti-diabetic results than acarbose (Li et al., 2009). There are chemically prepared inhibitors already reported with strong α-glucosidase inhibitory activity than standard drug (Rashid et al., 2022). Many α-glucosidase inhibitors have shown efficacy at lower concentrations (IC50) compared to the positive control, indicating their potential therapeutic value (Yin et al., 2014). Previous studies have reported similar findings in Aegles marmelos and Syzygium cumini, where they demonstrated the ability to inhibit α-glucosidase and GLUT4 expression in adipocytes and contribute significantly to regulating blood glucose levels in individuals with diabetes (Anandharajan et al., 2006). In light of potential side effects and the higher costs associated with synthetic compounds, there is a preference for natural bioactive substances as potential candidates for drug development (Nisbet and Moore, 1997, Nisar et al., 2018). These findings could be useful for the development of novel therapeutic approaches by B. ceiba and its active metabolites for managing diabetic hyperglycemia via targeting α-glucosidase. Although two compounds Simalin A and B reported very first time in this study as α-glucosidase modulators yet their biochemical and in vitro evaluation is strongly recommended for their validation as α-glucosidase inhibitors.
5 Conclusion
In this study, in vitro enzymatic assay screening of 51 plant extracts was performed. The screening results showed that B. ceiba bark extract exhibited strong α-glucosidase inhibition with IC50 value 1.95 ± 0.29 µg/mL. To the best of our knowledge, this is first report discloses Bombax ceiba as potent α-glucosidase inhibitor. Literature based library of 78 phytochemicals of B. ceiba was prepared and these compounds were docked with α-glucosidase binding site. Based on the in silico data, nine potent hits were identified with best binding affinities and showed favorable ADMET properties. Among these, Simalin A and B were identified as α-glucosidase modulators through virtual screening for the first time. Although, their in-vitro and biochemicals studies are strongly recommended to ensure α-glucosidase as the prime molecular target of these compounds. Furthermore, commercially available three phytochemicals, Apigenin, Quercetin and Mangiferin, were validated as α-glucosidase inhibitors using in vitro enzymatic assay with IC50 value 0.83 ± 0.07 µg/mL, 0.96 ± 0.01 µg/mL, 1.47 ± 0.16 µg/mL respectively. On the base of these findings, it is suggested that B. ceiba bark extract and its metabolites may holds the potential as a natural resource for managing hyperglycemic conditions in diabetic patients via targeting α-glucosidase. As α-glucosidase is a rate-limiting enzyme of intestinal carbohydrate digestion; therefore, it is recommended to evaluate the inhibitory efficacy of these compounds on the other key proteins involved in intestinal carbohydrate digestion. Additionally in vivo studies are recommended to validate their effectiveness and safety. Subsequently, clinical trials should be conducted to evaluate their potential as antidiabetic agents in diabetic patients.
Funding
This study was supported by a grant from Punjab Higher Education Commission (PHEC) under the project No. PHEC/ARA/PIRCA/20316/13.
CRediT authorship contribution statement
Mudassir Hassan: Conceptualization, Software, Validation, Writing – original draft, Writing – review & editing. Azhar Rasul: Conceptualization, Supervision, Writing – review & editing. Farhat Jabeen: Investigation, Writing – review & editing. Salma Sultana: Formal analysis, Writing – review & editing. Maria Manan: Methodology, Writing – review & editing.
Acknowledgement
The authors gratefully thanks to the Punjab Higher Education Commission (PHEC) for providing funding grant (PHEC/ARA/PIRCA/20316/13) and the Department of Zoology Government College University Faisalabad for providing lab access.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Review on role of natural αlpha-glucosidase inhibitors for management of diabetes mellitus. Int. J. Biomed. Res.. 2011;2:374-380.
- [Google Scholar]
- Alpha Glucosidase Inhibitors. StatPearls [Internet]. StatPearls Publishing; 2022.
- Antidiabetic phytochemicals from medicinal plants: prospective candidates for new drug discovery and development. Front. Endocrinol.. 2022;13
- [Google Scholar]
- In vitro glucose uptake activity of Aegles marmelos and Syzygium cumini by activation of Glut-4, PI3 kinase and PPARγ in L6 myotubes. Phytomedicine. 2006;13(6):434-441.
- [Google Scholar]
- Antidiabetic potentials of Momordica charantia: multiple mechanisms behind the effects. J. Med. Food.. 2012;15(2):101-107.
- [Google Scholar]
- Analysis of the physicochemical properties of acaricides based on Lipinski's rule of five. J. Comput. Biol.. 2020;27(9):1397-1406.
- [Google Scholar]
- In vitro α-glucosidase inhibitory activity of Tamarix nilotica shoot extracts and fractions. PLoS One. 2022;17(3):e0264969.
- [Google Scholar]
- Ethnobotanical potential and phytochemical screening of Bombax ceiba L. Eur. J. Med. Plants.. 2019;29:1-8.
- [Google Scholar]
- α-Glucosidase inhibitors and their use in clinical practice. Arch. Med. Sci.. 2012;8(5):899.
- [Google Scholar]
- ADMETlab: a platform for systematic ADMET evaluation based on a comprehensively collected ADMET database. J. Cheminf.. 2018;10(1):29.
- [CrossRef] [Google Scholar]
- Antidiabetic effect of garlic (Allium sativum L.) in normal and streptozotocin-induced diabetic rats. Phytomedicine. 2006;13(9–10):624-629.
- [Google Scholar]
- Effect of P-glycoprotein (P-gp) inducers on exposure of P-gp substrates: review of clinical drug–drug interaction studies. Clin. Pharmacokinet.. 2020;59:699-714.
- [Google Scholar]
- Diabetes prediction using supervised machine learning. Procedia Comput. Sci.. 2023;216:21-30.
- [Google Scholar]
- ADMET modeling approaches in drug discovery. Drug Discov. Today.. 2019;24(5):1157-1165.
- [Google Scholar]
- Antidiabetic and ameliorative potential of Ficus bengalensis bark extract in streptozotocin induced diabetic rats. Indian J. Clin. Biochem.. 2008;23(4):394.
- [Google Scholar]
- Antidiabetic effect of Citrullus colocynthis in alloxon-induced diabetic rats. Inventi Rapid: Ethno Pharmacol.. 2010;1(1):1112-1115.
- [Google Scholar]
- Leaf litter of Bombax ceiba L. threatens plant cover and floristic diversity in a new urban ecosystem. Flora. 2018;242:22-30.
- [Google Scholar]
- Hossain, M.A., Pervin, R.J.N., T. I. f. Diabetes, et al., 2018. Current antidiabetic drugs: review of their efficacy and safety. Nutritional and Therapeutic Interventions for Diabetes and Metabolic Syndrome (Second Edition). 455-473.
- Simalin A and B: Two new aromatic compounds from the stem bark of Bombax ceiba. Phytochem. Lett.. 2014;7:26-29.
- [Google Scholar]
- Therapeutic potential of α-glucosidase inhibitors in type 2 diabetes mellitus: an evidence-based review. Expert Opin. Pharmacother.. 2015;16(13):1959-1981.
- [Google Scholar]
- Anti-diabetic activity of Syzygium cumini and its isolated compound against streptozotocin-induced diabetic rats. J. Med. Plants Res.. 2008;2(9):246-249.
- [Google Scholar]
- Chemical investigation on the root bark of Bombax malabarica. Fitoterapia. 2019;139:104376
- [Google Scholar]
- Comparative evaluation of quercetin, isoquercetin and rutin as inhibitors of α-glucosidase. J. Agric. Food Chem.. 2009;57(24):11463-11468.
- [Google Scholar]
- Scientific and regulatory evaluation of mechanistic in silico drug and disease models in drug development: Building model credibility. CPTPharmacometricsSystPharmacol.. 2021;10(8):804-825.
- [Google Scholar]
- Comparison of medicinally important natural products versus synthetic drugs-a short commentary. Nat. Prod. Chem. Res.. 2018;6(2):308.
- [Google Scholar]
- Will natural products remain an important source of drug research for the future? Curr. Opin. Biotechnol.. 1997;8(6):708-712.
- [Google Scholar]
- Role of hERG potassium channel assays in drug development. Channels. 2008;2(2):87-93.
- [Google Scholar]
- A Pharmacognostic and pharmacological overview on Bombax ceiba. Scholars Acad. J. Pharm.. 2014;3(2):100-107.
- [Google Scholar]
- Ethnomedicinal and pharmacological activities of Mochrus (Bombax ceiba Linn.): An overview. Cellmed.. 2016;6(1):2.1-2.9.
- [Google Scholar]
- Integrating pharmacological and computational approaches for the phytochemical analysis of Syzygium cumini and its anti-diabetic potential. Molecules. 2022;27(17):5734.
- [Google Scholar]
- Mangifera indica extracts as novel PKM2 inhibitors for treatment of triple negative breast cancer. Biomed Res. Int.. 2021;2021:1-11.
- [Google Scholar]
- Evaluation of the antidiabetic effect of Trigonella foenum-graecum seed powder on alloxaninduced diabetic albino rats. Int. J. PharmTech Res.. 2009;1(4):1580-1584.
- [Google Scholar]
- Identification of Novel Natural Inhibitors to Human 3-Phosphoglycerate Dehydrogenase (PHGDH) for Cancer Treatment. Molecules. 2022;27(18):6108.
- [Google Scholar]
- Review on traditional uses, biological activities, phytoconstituents of Bombax Ceiba Linn. Ace. 2020;62:5.
- [Google Scholar]
- Antidiabetic properties of Gymnema sylvestre (a review) Pharm. Chem. J.. 2008;42(11):626-629.
- [Google Scholar]
- IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract.. 2022;183:109119
- [Google Scholar]
- MolDock: a new technique for high-accuracy molecular docking. J. Med. Chem.. 2006;49(11):3315-3321.
- [Google Scholar]
- Phytochemical analysis of some medicinal plants from western region of India. Res. J. Med. Plant.. 2011;5(5):567-576.
- [Google Scholar]
- Comparative phytochemical study of stem bark versus small branches of Bombax ceiba Linn. using HPTLC–UV detection method. World J. Pharm. Res.. 2015;4(4):912-922.
- [Google Scholar]
- ADMETlab 2.0: an integrated online platform for accurate and comprehensive predictions of ADMET properties. Nucleic Acids Res.. 2021;49(W1):W5-W14.
- [CrossRef] [Google Scholar]
- α-Glucosidase inhibition by luteolin: Kinetics, interaction and molecular docking. Int. J. Biol. Macromol.. 2014;64:213-223.
- [Google Scholar]
- Yang, J.M., Chen, C.C., Function, and Bioinformatics, 2004. GEMDOCK: a generic evolutionary method for molecular docking. Proteins: Structure, Function, and Bioinformatics. 55 (2) 288-304.
- α-Glucosidase inhibitors isolated from medicinal plants. Food Sci. Human Wellness. 2014;3(3–4):136-174.
- [Google Scholar]
- Phytochemical composition, antioxidant, enzyme inhibition, antimicrobial effects, and molecular docking studies of Centaurea sivasica. S. Afr. J. Bot.. 2022;144:58-71.
- [Google Scholar]







