Translate this page into:
Blister blight a threatened problem in tea industry: A review
⁎Corresponding author. krish_paper@yahoo.com (Krishnendu Acharya)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Tea is one of the most consumed beverages and is produced from the tender leaves of the tea plant. Various biotic and abiotic factors are directly related to tea productivity. Among the biotic factors the most destructive one is the blister blight disease of tea caused by an obligate parasitic fungus Exobasidium vexans Massee. The pathogen attacks the tender leaves of the tea plant which directly interferes with the economic growth of the tea growing countries as tea has tremendous export value. Numerous studies have identified the symptoms, epidemiology of the pathogen and its control strategies. Application of protectant and eradicant fungicides have shown promising results for controlling blister blight but overuse of chemical pesticides causes phytotoxicity, residual effects, thus use of microbial biocontrol agents are gaining more impetus. Different integrated disease management strategies along with modern emerging management approaches like elicitor mediated defense responses, development of transgenic tea plant, transcriptome study that induce many R-genes which ultimately provide innate immunity in tea plants. This review presents up-to-date information on blister blight disease which would help the future researchers to understand the host-pathogen interaction and the effective control measures to be adopt in a better way.
Keywords
Biocontrol
Camellia sinensis
Disease cycle
Epidemiology
Exobasidium vexans
1 Introduction
Tea [Camellia sinensis (L.) O. Kuntze] is one of the oldest known beverages and is a perennial monoculture crop that are cultivated both large- and small-scale plantations (Banerjee, 1983; Kachhawa and Kumawat, 2018). Tea is famous worldwide as it has tremendous health benefits, stimulating property and relatively low-cost drink (Shimizu et al., 2012). Tea plants have been placed in the family Camelliaceae with 82 species of the genus Camellia. Taxonomists describe the heterogeneous origin of the current day tea plants to three separate taxa, namely ‘China’ Camellia sinensis (L.) O. Kuntze,‘Assam’ C. assamica (Masters) Wight, and ‘Cambod’ C. assamica ssp. Lasiocalyx (Planex ex Watt) Wight. Tea is manufactured from young shoots of the tea plant; hence, the leaf diseases are of great concern. Given the economic importance of tea, any threats to yield are of great importance. Among various factors that hamper tea production, blister blight caused by a fungus Exobasidium vexans Massee is one of the most widespread diseases of tea (Muraleedharan and Chen, 1997). Blister blight is a leaf disease of tea that mainly attacks the tender leaves and is considered the most threaten disease of cultivated tea (Punyasiri et al., 2005; Sowndhararajan et al., 2013).
The tea industry is one of the oldest organized trades in India. The national economy of many countries situated between latitudes 41°N and 16°S is largely dependent upon the production of tea (Hazarika et al., 2009). Tea production is an important component of Indian agricultural production and gross domestic product (GDP). According to International Tea Committee, India is the second largest producer of tea after China, which account for 25% of global tea production and during the last five years the overall production of tea in India has increased by 10 percent (Tea Board of India, 2019). Blister blight is capable of causing enormous crop loss throughout the tea growing regions of Asia, especially in India, Sri Lanka, Indonesia and Japan. Since the pathogen attacks harvestable tender shoots, it inflicts a global yield loss of 40% (Gulati et al., 1993; Basu et al., 2010). In field conditions without any control measures an estimated loss of 50 and 33% has been reported in South India and Sri Lanka, respectively (VenkataRam, 1968; de Silva et al., 1997). In addition, the disease adversely affects the quality of made tea and it is even exceeded the economic threshold level (ETL) 35% which was calculated using the mathematical formula (ETL = C/PDK × 100) where, P = Price of tea/ kg, D = The loss in tea yield associated with one per cent blister blight infection, K = The reduction in disease due to fungicide application, C = The cost of blister control /ha/season (Radhakrishnan and Baby, 2004).
Literature survey reveals that existence of blister blight disease was first reported from upper Assam, India in the year 1868 (Watt and Mann, 1903) and had caused devastating damage in 1906 (Mann, 1906). Till 1908 it was considered that the disease was endemic to Assam region only but suddenly appeared in the Darjeeling district in 1908 (McRae, 1910) and consequently it became the major problem of all tea plantations of Asian Countries (Lujaerajumnean and Tummakate, 1987). Later, the disease was well documented from Japan and Formosa between 1912 and 1922 (de Weille, 1960), Sri Lanka in 1947 (Tubb, 1947) and Indonesia in 1949 (Reitsma and Van Emden, 1950). The disease has also been reported from Java, Sumatra and Malaysia (Chandramouli, 1992a) (Fig. 1).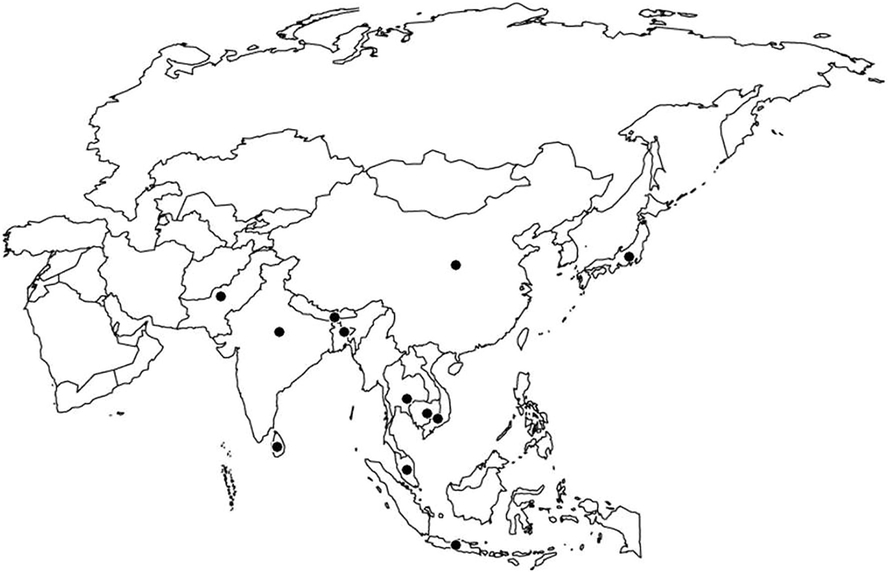
Geographical distribution of blister blight of tea. Black dots indicate the places of blister incidence.
Considering the importance of blister blight disease and the associated pathogen around the world, reviewing the various aspects of this disease could be helpful for the future researcher to understand the disease in a better way. The present review deals with blister blight disease epidemiology, symptoms, biochemical responses during host-pathogen interaction and possible disease management strategies.
2 The pathogen
Exobasidium vexans Massee is an obligate parasitic fungus systematically placed under Exobasidiaceae, Exobasidiales, Exobasidiomycetes, Basidiomycota. The fungus was described by Massee, the Mycologist of Kew Botanical garden (Massee, 1898). A transverse section through blister region of leaf showed that mycelium grows intercellularly and produces finger like houstoria which penetrate the leaf parenchyma cells. The hymenium forms below the epidermis on the lower surface of the infected leaf. A palisade of paraphyses and basidia arises, forcing up the epidermis which forms the blister and then ruptures (Booth, 1983) (Fig. 2). Paraphyses are single, septate and apically rounded. The basidia are clavate, generally bear two sterigmata and are 30–35 × 5–6 μm. Basidiospores are unicellular at first, but a septum develops on maturity; they are ellipsoid, initially hyaline and 13–27 × 4.3–6.5 μm (Fig. 2).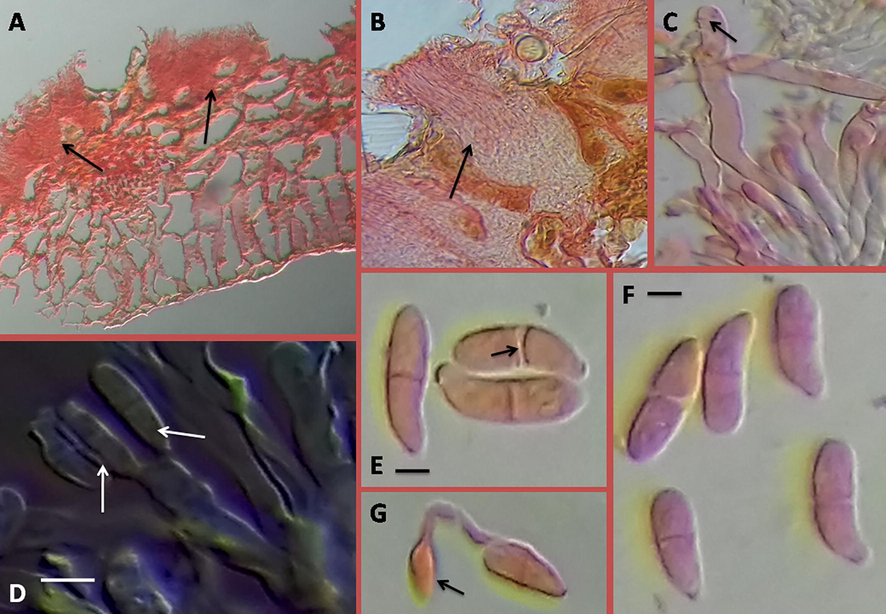
Exobasidium vexans. A-B, Section through the infected leaf showing hymenium where numerous paraphyses and basidia forcing up the epidermis (black arrow). C, An apically rounded paraphyses (black arrow). D- Basidia with basidiospores (white arrows show attached basidiospores with basidium); bar = 10 µm. E-F, matured basidiospores with distinct septation (black arrow); bar = 5 µm. G, germinating spore with germ tubes bearing appressoria at the tip (black arrow).
3 Disease cycle
Blister blight has a multiple disease cycle with a relatively short fungal life cycle of 11–28 days (Fig. 3) (Table 1). Since the pathogen does not have any known alternate or collateral host, its mode of survival during off season deserves attention. The perennating mycelia persist within the branches and on necrotic blister lesions of the tea bushes or may produce thick walled spores (Petch, 1923; Sugha, 1997; Ajay et al., 2009). During favorable climatic conditions the mycelium become active and grows intercellularly for some time before the hymenium develops below the epidermis on the under-surface of the tender tea leaves. It is not yet clear whether the phenomenon of somatogamy that resulted in dikaryotization or not. During this phase mycelia grows rapidly within the host tissue; host tissue develop blister like structure and just below lower epidermis, hyphae are arranged compactly to form the hymenial layer. Then lower epidermis breaks to expose the hymenium where clavate basidia are produced which bears two, occasionally three and rarely four sterigmata bearing solitary basidiospores (Booth, 1983). In addition to basidia, the hymenial layer possesses conidiophores bearing two-celled conidia (Subba Rao, 1946). The wind-borne spores on lodging on the surface of a susceptible host tissue, germinates under favorable conditions mainly when the atmosphere is humid with minimum relative humidity of 80% (Reitsma and van Emden, 1950). Infection occurs through the formation of appressoria and direct penetration of the cuticle. After penetration visible sign of infection appears as a translucent spot, results in the development of characteristic convex lesions (blisters) on the under-surface of the young leaves (Punyasiri et al., 2005). It has been reported that a mature blister lesion can produce two million spores in 24 h (Huysmans, 1952).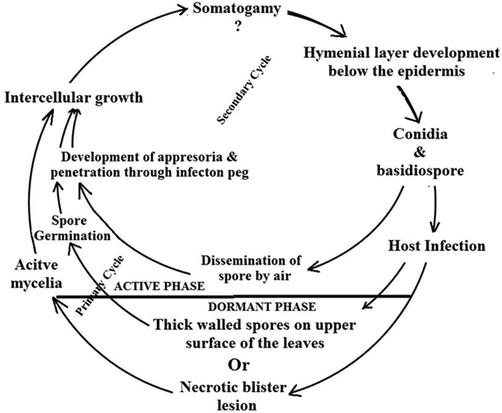
Disease cycle of Exobasidium vexans.
Development Phase
Duration
From Sporulation to germination
2 h to 5 days
From germination to entry (infection)
2 to 9 days
From entry to appearance of translucent spot (blister)
3 to 10 days
From germination to outbreak of basidia
6 to 9 days
Duration of life cycle: germination to sporulation
11 to 28 days
Spore discharge period
Upto 8 days
4 Symptoms
Symptoms of blister blight were described in details for the first time by Petch (1923). The young and tender shoots show susceptibility to the pathogen and develop symptoms. The first indication of blister blight disease is a small, pale-green, pale-yellow or pinkish, translucent spot on the tea leaf which is clearly seen against the darker green colour when the leaf is held against the light (Fig. 4). These tiny spots are referred as first stage of the disease (Reitsma and van Emden, 1949; Punyasiri et al., 2005). The circular spots enlarge until they reach a diameter of 3–12.5 mm. On the upper side of the leaf, the spots slowly become sunken into a shallow depression and on the under-side, they become correspondingly convex, forming the typical blister lesion (Boekhout, 1991). The concave upper surface of the lesion is smooth and shiny, whereas the lower convex surface is at first dull, then grey and finally pure white, due to a dense, velvety growth on which the spores are produced. On severity, tea leaves become folded or irregularly rolled, blister lesions reached to the mid-rib and the margin.
Symptoms of blister blight in tea. A. Infected leaves in tea bush, B. Various degree of infection, C. Typical symptoms of infection (close view).
The disease also affects the tender and young green stems. On the stem initially a pale-yellow spot appears, this spot gradually elongates and encircles the whole stem which becomes slightly swollen at this point and ultimately the place turns grey. Finally, the stem bends over and breaks off at the affected spot and consequently the leaves and buds above the diseased part wither and necrotize (Petch, 1923). When young stems are infected the damage become more serious, as the infected stem breaks off and dies back, retarding growth and reducing crop production (Arulpragasam, 1992).
5 Biochemical changes in the host during pathogenesis
Tolerant and susceptible variety of tea shows various degrees of biochemical changes during blister blight infestation. Rajalakshmi and Ramarethinam (2000) showed in C. assamica during blister blight infection the amount of polyphenol oxidase (PPO) and peroxidase (PO) were increased significantly whereas the level of phenyl alanine ammonia lyase (PAL) decreases. Similar observation was made by Chakraborty et al. (2002) during E. vexans interaction with ‘China’ variety of tea where PAL activity decreases and PO activity increases significantly whereas no significant changes were observed in PPO activity. An important stress marker (proline) and total phenol content also increased during infection. Alteration in flavonoid biosynthesis pathway was recorded during interaction (Punyasiri et al., 2001). Biochemically it has been observed that resistant cultivar contain high levels of epicatechin whereas the susceptible one contains high level of epigallocatechin gallate. The proanthocyanidin level also increased to significant level (Punyasiri et al., 2004). Resistance is directly related to the higher level of epicatechin and derivatives of proanthocyanidin composition (Punyasiri et al., 2001, 2005) During E. vaxans infection in a susceptible tea cultivar TRI- 2025 showed a shift of 2,3-trans to 2,3-cis of the proanthocyanidin (Punyasiri et al., 2004). Premkumar et al. (2008) observed a drastic reduction in sugar, nitrogen, proteins, amino acids, polyphenols etc. in infected tea leaves. Jeyaramraja et al. (2010) showed that the tea clone SA-6 was resistant to the E. vexans infection that might be due to the presence of higher deposition of epicuticular wax, thicker cuticular layer and constitutive expression of cutinase. Chakraborty et al. (2015) measured the regulation of defense enzyme by force inoculation of E. vaxans in a susceptible (B-668) and tolerant (TV-26) tea variety which showed differential expression of defense enzymes viz. chitinase, glucanase and peroxidase. Mur et al. (2015) made a detail metabolomic approach to understand the changes in metabolome profile between early, middle and late stage of infection with healthy one in tea of North Indian-Assam variety-TV9. They reported changes in antioxidant molecules, antimicrobial compounds and flavanoids during disease progression. E. vexans infection drastically affects the SA-JA-caffeine defence network during pathogenesis. In recent times several reports have highlighted nitric oxide (NO) as an important signaling molecule in plant defense (Acharya et al., 2005). During pathogenesis in the susceptible tea cultivar this NO production level decrease via increase of Km (Michaelis constant) and decrease of Vmax (maximum rate of reaction) signifies its importance as a signaling molecule in the susceptibility of tea plants towards blister blight infection (Chandra et al., 2012). Inhibition of NO production by l-NAME or by scavenging of NO by cPTIO in the tea leaves decreased in the activity of defense enzyme, PR- proteins, antioxidant enzyme and also decrease in the accumulation of total phenolics (Chandra et al., 2015a)
6 Epidemiology
Monsoon is the favorable time for infection, sporulation and spore dispersal. Different climatic condition in relation to disease severity was well documented by many workers (Gadd and Loos, 1950; Homberg, 1953; Visser et al., 1961; Kerr and Shanmuganathan, 1966; Kerr and Rodrige, 1967a; Kerr and Rodrigo, 1967b). Spore germinates well when the relative humidity (RH) is more than 80% and water film is available on the leaf surface (Gadd and Loos, 1950; Reitsma and van Emden, 1950; Huysmans, 1952). Retardation of germ-tube growth was observed when the RH reaches below 80% (de Weille, 1959). A moderate attack of blister blight occurs when RH exceeded 83% and persists for 10–14 days but if the same condition extended for 20–24 days a serious attack resulted (Huysmans, 1952). Relative humidity is directly and sunshine is inversely related to the severity of blister blight incidence (Chandramouli, 1992b). Visser et al. (1961) observed that an average of 3.5 h of sunshine per day over 5 days is enough to reduce the disease to a satisfactory level. Temperature above 32 °C is lethal for the basidiospores (Satyanarayana et al., 1974) while sporulation was prevented at 35 °C (Venkataram and Chandramouli, 1976).
7 Control measures
This disease can be managed through an integrated approach inclusive of cultural, chemical and biological methods:
7.1 Cultural practices
Various cultural operations like adequate weed control, changes in plucking and pruning regimes, lane cutting, shade patter and the careful choice of planting material has been practiced for controlling blister blight disease (Hudson and Muraleedharan, 1998). Since the pathogen infects only tender shoots, efforts were directed to reduce the disease severity by adopting early pruning and hand plucking (Eden, 1947). Collection and destruction of blister leaves in a huge scale along with chemical spraying have been found to be an effective control measure (Barthakur and Dutta, 2005). Severely infected tender young tea plants should be pruned immediately. At the onset of monsoon and during plucking the shade trees are pruned which allows sunlight to fall on the tea plants that causes reduction in disease incidence.
7.2 Chemical practices
Blister blight disease incidence above 35% has been found to cause significant crop loss and this is considered to be the threshold limit of disease incidence (Kerr and de Silva, 1968). Agnihothrudu and Chandramauli (1990) have made an extensive review on chemical control of blister blight disease. Controlling blister blight during the early part of the outbreak has not been highly effective due to the lack of knowledge on the biology of pathogen, availability of suitable fungicides and spraying technique. Huge number of fungicides were tested to control the disease but only very few chemical fungicides found to be effective (Baby, 2002). Both contact and systemic fungicides are used to control tea diseases. Application of copper fungicides like copper oxychloride (50% w/w @ 1:400) at seven days’ interval can help to manage the incidence of blister blight in the field below the threshold limit (Venkataram and Chandramouli, 1983). A combination therapy of nickel chloride hexahydrate with copper oxychloride gives better protection from the disease in comparison to copper oxychloride or nickel chloride alone (Venkataram and Chandramouli, 1983; Sugha and Singh, 1990). Copper in colloidal form when mixed with low metallic copper content (15%) were found to be effective at one-third of wettable dispersible powders (de Jong, 1954; Jayaraman and Venkataram, 1959). Systemic fungicides like Hexaconazole, Propiconazole and Baycor are also recommended as foliar spray at 15 days’ interval (Chandramouli, 1992b; Chandramouli and Premkumar, 1995, 1997). Ergosterol biosynthesis inhibitors (EBIs) applied to infected plants suppressed sporulation, reduced the size of spores and their viability, inhibited spore germination and provided some control of the disease (Baby et al., 2004).
7.3 Biological practices
Fungicides with different modes of action are used for controlling various foliar pathogens of tea, though fungicide have shown some promising results but phytotoxicity and residual effects are the prime problems beside the environmental pollution and human health hazards (Muraleedharan and Chen, 1997; Ajay and Baby, 2010). Moreover, repeated use of toxic fungicide develops resistance against the target pathogen as well as it increases production cost of tea (Goldman et al., 1994). As tea is made from tender leaves of tea plants, the chances of remaining pesticide residue contaminating the brewed tea are very high. It is a global concern to minimize the chemical residue in tea. Many international regulatory authorities such as environmental protection agency (EPA), German Laws (GL), European Economic Commission (EEC/EC), food and agricultural organization (FAO), world health organization (WHO) etc. have fixed the maximum residue limits (MRL) values for tea growing countries but the acceptable values are highly variable between countries (Barooah, 2008; Abd El-Aty et al., 2014). In India the authorities fixed MRL values at < 0.1 mg/kg for most commonly used pesticides in tea (Gurusubramanian et al., 2008; Barooah et al., 2011). In this context, search continues for alternative strategies that are eco-friendlier to control the tea diseases. There is a pressing need in tea industry either for exclusively utilizing biological products in disease management or for reducing the use of chemicals by supplementing with biological products in integrated management practices. In recent years biological management of tea diseases gaining more importance than the application of chemical fungicides which causes adverse environmental and human health hazards (Baby et al., 2004; Saravanakumar et al., 2007; Sanjay et al., 2008). Antagonists like Trichoderma harzianum, Gliocladium virens, Serratia marcescens, Pseudomonas fluorescens and Bacillus subtilis are experimentally used in controlling blister blight of tea (Acharya et al., 2001; Premkumar, 2002; Premkumar and Baby, 2005). These antagonists are applied as formulations based on talc/ vermicompost but the results were not encouraging. Use of Pseudomonas fluorescens and Bacillus subtilis as liquid culture supplemented with ammonium sulphate and salicylic acid enhanced bio-efficacy especially when the disease incidence is less (Premkumar, 2003). A phylloplane bacterium Orchobacterium anthropi BMO-111 achieved better performance when compared with the conventional chemical fungicides copper oxychloride and hexaconazole (Sowndhararajan et al., 2013). Balasuriya and Kalaichelvan (2000) showed that the spore germination of E. vexans is inhibited by the extract of Glomerella cingulata. Liquid culture filtrate of G. cingulata was effective in controlling blister blight (Premkumar, 2001) but ineffective when used as talc based bioformulation (Premkumar, 2002). Four rounds application of B. subtilis at 7 days interval showed 40% reduction where as two strains of actinomycetes showed 50% reduction on blister blight incidence which was reported from North East India (Barthakur et al., 2002; Sarmah et al., 2005). Saravanakumar et al. (2007) showed application of PGPR strain P. fluorescens Pf1 at 7 days’ intervals consistently reduced the disease incidence of blister blight.
8 Emerging areas in the management of blister blight
Elicitor mediated improvement of innate immunity in plants is now become an alternative and eco-friendly approach for crop protection (Acharya et al., 2011; Chandra et al., 2015b; Chakraborty and Acharya, 2016). The effect of chemical elicitors Acibenzolar-S-methyl (ASM) in inducing resistance in tea plants against blister blight disease showed reduced severity of disease (Ajay and Baby, 2010). In another study foliar application of abiotic elicitor (calcium chloride) at a concentration of 1% and biotic elicitors chitosan (0.01%) and chitosan nanoparticle (0.001%) reduced blister blight incidence around 71, 66 and 75% respectively over the untreated plants during the peak season (Chandra et al., 2015b, 2017; Chandra, 2017). All these elicitors showed significant increase in the level of defense molecule like β-1,3 glucanase, PO, PPO, PAL and phenolics. Chandra et al. (2014a, 2014b, 2017)); Chandra (2017) also demonstrated that the elicitor mediated improvement of innate immunity in tea is regulated through nitric oxide signaling. Progression in gene technology and the use of Agrobacterium mediated transformation have made it possible to integrate foreign gene into crop plants for the development of disease-resistant varieties (Yamamoto et al., 2000; Jia et al., 2010; Yuan et al., 2012). A transgenic tea plant resistant to blister blight disease was developed in which the expression of chitinase gene is much higher to hydrolyse the cell wall of E. vexans (Jeyaramraja and Meenakshi 2005). Transcriptome study showed that specific sites and sequence motifs, ubiquitously conserved in upstream regions of genes that are upregulated during SAR or R-mediated defense (Maleck et al., 2000; Zipfel et al., 2004). However, only few genes regulating blister blight resistance has been identified (Bhorali et al., 2012). Jayaswall et al. (2016) for the first time reported detailed defense responsive expressions against the blister blight in tea. The overall results reveled activation of R genes, defense related enzymes, retrotransposons, transcription factors and other defense molecules provide immunity in resistant geneotype of tea.
9 Conclusion
Widespread research over fifty years on several aspects of blister blight solved many important problems. In conclusion, more investigations are required to develop a holistic approach which will help the researcher to design an integrated management strategy by combining all the possible system like legalization of application of fungicides through forecasting systems, limiting their dosages with botanicals, implementing biological control agents, coupled with the novel cultural practices for the control of blister blight.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Residues and contaminants in tea and tea infusions: A review. Food Addit. Contam. Part A.. 2014;31(11):1794-1804.
- [CrossRef] [Google Scholar]
- Signaling role of nitric oxide in the induction of plant defense by exogenous application of by abiotic inducer. Arch. Phytopathol. Plant Protect.. 2011;44:1501-1511.
- [CrossRef] [Google Scholar]
- Biocontrol of blister blight of tea by Pseudomonas fluorescence. J. Hill Res.. 2001;14(2):110-111.
- [Google Scholar]
- Nitric oxide: A common antipathogenic factor of plants. Indian J. Exp. Biol.. 2005;43:100-103.
- [Google Scholar]
- Agnihothrudu, V., Chandramauli, B., 1990. Blister blight of tea and its control, in: Proceeding International Conference on Tea Research. Global Perspective. pp. 75-86
- Induction of systemic resistance to Exobasidium vexans in tea through SAR elicitors. Phytoparasitica. 2010;38:53-60.
- [Google Scholar]
- Survival of Exobasidium Vexans, the Incitant of Blister Blight Disease of Tea, During Off season. Int. J. Appl. Agric. Res.. 2009;4(2):115-123.
- [Google Scholar]
- Disease control in Asia. In: Wilson K.C., Clifford M.N., eds. Tea Cultivation to Consumption. London: Chapman & Hall; 1992. p. :353-374.
- [Google Scholar]
- Effect of ergosterol biosynthesis inhibitors on blister blight disease, the tea plant and quality of made tea. Crop Prot.. 2004;23:795-800.
- [Google Scholar]
- Baby, U.l., 2002. An overview of blister blight disease of tea and its control. J. Plant. Crops 30(2), 12.
- Is there potential in natural tea-phylloplane microorganisms in the control of blister blight leaf disease of tea. Planter: Kuala Lumpur. 2000;76(892):409-417.
- [Google Scholar]
- Arthropod accumulation on tea in young and old habitats. Ecol. Entomol.. 1983;18(2):339-342.
- [Google Scholar]
- Pesticide residues in tea and their intake assessment using brew factor. J. Tea Sci.. 2011;31(4):289-294.
- [Google Scholar]
- Residue limits of agrochemicals and heavy metals in tea. In: Ghosh P., ed. Field management in tea (Darjeeling 2008). Nagarkata Sub-Station, West Bengal, India: Tea Research Association; 2008. p. :234-246.
- [Google Scholar]
- Disease management in tea. In: Field Management in Tea. Jorhat, Assam: Tea Research Association; 2005. p. :179-181.
- [Google Scholar]
- Barthakur, B.K., Dutta, P., Sarmah, S.R., Begum, R., Kalita J.N., Singh K., 2002. Effect of certain native microbials in controlling diseases of tea. Two and a Bud 49, 54-54.
- Bhorali, P., Gohain, B., Gupta, S., Bharalee, R., Bandyopadhyay, T., Das, S.K., Agarwal, N., Singh, H.R., Bhagawati, P., Bhattacharyya, N., Ahmed, P., Borchetia, S., S. Sarma, S., Das S., 2012. Molecular analysis and expression profiling of blister blight defenserelated genes in tea. Indian J. Genet. 72(2), 226-233. DOI: 10.13140/2.1.2468.3527.
- A revision of ballistoconidia-forming yeasts and fungi. Stud. Mycol.. 1991;33:1-19.
- [Google Scholar]
- Booth, C., 1983. Exobasidium vexans. CMI Descriptions of Pathogenic Fungi and Bacteria, No. 779. CAB International, Wallingford, UK.
- Biochemical responses of tea plants induced by foliar infection with Exobasidium vexans. Indian Phytopathol.. 2002;55(1):8-13.
- [Google Scholar]
- Ex vivo analysis of formulated bio-elicitors from a phytopathogen in the improvement of innate immunity in host. Arch. Phytopathol. Plant Protect.. 2016;49:485-505.
- [CrossRef] [Google Scholar]
- Chakraborty, N., Chandra, S., Acharya, K., 2015. Sublethal heavy metal stress stimulates innate immunity in tomato. Sci. World J. 2015: Article ID 208649, http://dx.doi.org/10.1155/2015/208649.
- Nitric oxide production mediates chitosan-triggered immunity and resistance to Exobasidium vexans in Camellia sinensis. India: University of Calcutta; 2017. PhD Thesis
- Nitric oxide functions in tea plant disease resistance response, in: International Symposium on “Emerging Trends in Free Radicals. In: Antioxidants and nutraceuticals on Health, Disease and Radiation Biology. West Bengal, India: University of Calcutta; 2012. p. :211.
- [Google Scholar]
- Abiotic elicitor mediated improvement of innate immunity in Camellia sinensis. J. Plant Growth Regul.. 2014;33:849-859.
- [CrossRef] [Google Scholar]
- Induction of defense response against blister blight by calcium chloride in tea. Arch. Phytopathol. Plant Protect.. 2014;47:2400-2409.
- [CrossRef] [Google Scholar]
- Chitosan nanoparticles: a positive modulator of innate immune responses in plants. Sci. Reporter. 2015;5:15195.
- [CrossRef] [Google Scholar]
- Chitosan induced immunity in Camellia sinensis (L.) O. Kuntze against blister blight disease is mediated by nitric oxide. Plant Physiol. Biochem.. 2017;115:298-307.
- [CrossRef] [Google Scholar]
- Chitosan nanoparticles: A new tool for sustainable organic cultivation. In: National seminar entitled “New Horizons in Biotechnology”. Haldia, West Bengal, India: Haldia Institute of Technology; 2015. p. :25.
- [Google Scholar]
- Studies on the blister blight (Exobasidium vexans Massee, Hymenomycetes, Exobasidiales) disease affecting tea in southern India. Coimbatore, India: Bharathiar University; 1992. PhD Thesis
- Blister blight control – New recommendations. UPASI Handbook of Tea Culture, Section. 1992;13:1-5.
- [Google Scholar]
- Hexaconazole - a novel fungicide in tea blister management. UPASI Tea Scientific Department Bullet.. 1995;48:59-66.
- [Google Scholar]
- Comparative evaluation of fungicide spray schedules against blister blight (Exobasidium vexans Massee) disease of tea. Pestology. 1997;11:19-21.
- [Google Scholar]
- Report of the Chief Scientific Officer, II. Research and Advisory. Ann. Report UPASI Scientific Department Bullet.. 1954;54:4-7.
- [Google Scholar]
- The adaptation of tea cultivation to the occurrence of tea blister blight. Arch. Tea Cult.. 1959;20:161-192.
- [Google Scholar]
- Blister blight Exobasidium vexans in tea and its relationship with environmental conditions. Neth. J. Agri. Sci.. 1960;8(3):183-210.
- [Google Scholar]
- The effects of hand plucking with special reference to blister blight. Tea Quarterly. 1947;19:105-109.
- [Google Scholar]
- Further observations on the spore growth of Exobasidium vexans. Trans. Brit. Mycol. Soc.. 1950;33:19-21.
- [Google Scholar]
- Molecular and cellular biology of biocontrol Trichoderma spp. Trends Biotechnol.. 1994;12:478-482.
- [Google Scholar]
- Economic yield losses caused by Exobasidium vexans in tea plantations. Indian Phytopathol.. 1993;46:155-159.
- [Google Scholar]
- Pesticide usage pattern in tea ecosystem, their retrospects and alternative measures. J. Environ. Biol.. 2008;29(6):813-826.
- [Google Scholar]
- Observations on climate in relation to the control of Exobasidium vexans Massee. Bergculture. 1953;22:345-353.
- [Google Scholar]
- Impact of cultural operations on blister blight control. Planters' Chronicle. 1998;93(8):360-361.
- [Google Scholar]
- Bestrijding van blister blight (Exobasidium vexans) in thee op. Sumatra. Bergcultures. 1952;21:419-464.
- [Google Scholar]
- Control of blister blight of tea in southern India. Ann. Rep. UPASI Sci. Dep. Tea Sect.. 1959;59:28-35.
- [Google Scholar]
- Transcriptome analysis reveals candidate genes involved in blister blight defense in tea (Camellia sinensis (L) Kuntze) Sci. Rep.. 2016;6:30412.
- [CrossRef] [Google Scholar]
- Jeyaramraja, P.R., Meenakshi, S., 2005. Agrobacterium tumefaciens mediated transformation of embryogenic tissues of tea (Camellia sinensis (L.) O. Kuntze). Plant Mol. Biol. Report. 23, 299a–299i.
- Role of physical barriers and chitinase in conferring blister blight resistance to Camellia sinensis (L.) O. Kuntze. Res. J. Parasitol.. 2010;5:166-173.
- [Google Scholar]
- Enhanced resistance to fungal pathogens in transgenic Populus tomentosa Carr. by overexpression of an nsLTP-like antimicrobial protein gene from motherwort (Leonurus japonicus) Tree Physiol.. 2010;30:1599-1605.
- [Google Scholar]
- Oligonychus coffeae: Red spider mite of tea: A review. J. Entomol. Zool. Stud.. 2018;6(3):519-524.
- [Google Scholar]
- Kerr, A., de Silva, R.L., 1968. Epidemiology of tea blister blight Exobasidium vexans Massee. Symp. 1st International Congress in Plant Pathology, London.
- Epidemiology of tea blister blight Exobasidium vexans Massee III spore deposition and disease prediction. Trans. Brit. Mycol. Soc.. 1967;50:49-55.
- [Google Scholar]
- Epidemiology of tea blister blight (Exobasidium vexans). IV Disease forecasting. Trans. Brit. Mycol. Soc.. 1967;50:609-614.
- [Google Scholar]
- Epidemiology of tea blister blight Exobasidium vexans Massee II. The diurnal and seasonal periodicity of spores in air over a tea estate. The Tea Quarterly. 1966;37:175.
- [Google Scholar]
- The transcriptome of arabidopsis thaliana during systemic acquired resistance. Nat. Genet.. 2000;26(4):403-410.
- [CrossRef] [Google Scholar]
- A Report on the outbreak of blister blight of tea inDarjeeling District. Bullet. Agric. Res. Inst. Pusa. 1910;18:1-20.
- [Google Scholar]
- The development of tea blister caused by Exobasidium vexans in tea (Camellia sinensis) correlates with the reduced accumulation of some antimicrobial metabolites and the defence signals salicylic and jasmonic acids. Plant Pathol.. 2015;64:1471-1483.
- [CrossRef] [Google Scholar]
- Pests and diseases of tea and their managements. J. Plant. Crops. 1997;25(1):15-43.
- [Google Scholar]
- The Diseases of the Tea Bush. London, UK: Macmillan; 1923. p. :220.
- Premkumar, R., 2001. Report of the plant pathology division. Annual Report of UPASI Tea Research Foundation pp. 32-33.
- Premkumar, R., 2002. Report of the plant pathology division. Annual Report of UPASI Tea Research Foundation pp. 35-36.
- Premkumar, R., 2003. Report of the plant pathology division. Annual Report of UPASI Tea Research Foundation pp. 38-39
- Blister blight control – A review of current recommendations. Planters’ Chronicle. 2005;101(5):26-36.
- [Google Scholar]
- Growth and photosynthetic and biochemical responses of tea cultivars to blister blight infection. Photosynthetica. 2008;46(1):135-138.
- [CrossRef] [Google Scholar]
- Preformed and induced chemical resistance of tea leaf against Exobasidium vexans infection. J. Chem. Ecol.. 2005;31:1315-1324.
- [CrossRef] [Google Scholar]
- Chemical and biochemical basis of the resistance and susceptibility of Sri Lankan tea cultivars to blister blight leaf disease (Exobasidium vexans) In: in: Proceedings of International Symposium on Tea Culture. 2001. p. :94-97.
- [Google Scholar]
- Flavonoid biosynthesis in the tea plant Camellia sinensis: Properties of enzymes of the prominent epicatechin and catechin pathways. Arch. Biochem. Biophys.. 2004;431:22-30.
- [CrossRef] [Google Scholar]
- Economic threshold level for blisterblight of tea. Indian Phytopathol.. 2004;57:195-196.
- [Google Scholar]
- The role of Exobasidium vexans Massee in flavonoid synthesis by Camellia assamica Shneider. J. Plant. Crops. 2000;28:19-29.
- [Google Scholar]
- De bladpokkenziekte can de thee in Indonesia. Arch. Voor Thee Cult.. 1949;17:71-76.
- [Google Scholar]
- Evaluation of fungicides and biocontrol agents against grey blight disease of tea in the field. Crop Prot.. 2008;27:689-694.
- [Google Scholar]
- PGPR-induced defense responses in the tea plant against blister blight disease. Crop Prot.. 2007;26:556-565.
- [Google Scholar]
- Microbial bioagents for controlling diseases of tea. Proc. Int. Tea Sympos. 2005:767-775.
- [Google Scholar]
- Nutraceutical approach for preventing obesity-related colorectal and liver carcinogenesis. Int. J. Mol. Sci.. 2012;13(1):579-595.
- [Google Scholar]
- Biocontrol potential of phylloplane bacterium Ochrobactrum anthropi BMO-111 against blister blight disease of tea. J. Appl. Microbiol.. 2013;114:209-218. Epub 2012 Oct 29
- [CrossRef] [Google Scholar]
- Subba Rao, M.K., 1946. Blister blight of tea in South India. Paper No. 4: United Planters' Association of Southern India. Coonoor, India.
- Perpetuation and seasonal build-up of Exobasidium vexans, causal agent of blister blight of tea in Himachal Pradesh. Trop. Sci.. 1997;37:123-128.
- [Google Scholar]
- Comparative efficacy of fungicides against blister blight of tea (Exobasidium vexans Massee) Indian J. Mycol. Plant Pathol.. 1990;20:211-219.
- [Google Scholar]
- Annual Bulletin of Statistics (September 2019), International Tea Committee. 2019. Retrieved from
- [Google Scholar]
- Control of blister blight of tea. A leaf disease of tea new to Ceylon. Tea Quart. 1947;19:43.
- [Google Scholar]
- Report of plant pathologist. Ann. Report UPASI Scientific Depart. Tea Sci. 1968:16-28.
- [Google Scholar]
- Systemic fungicides for integrated blister blight control. UPASI Tea Sci. Depart. Bullet.. 1976;33:70-87.
- [Google Scholar]
- Interaction of dosage, spray interval and fungicide action in blister blight disease control in tea. Crop Prot.. 1983;2:27-36.
- [Google Scholar]
- The influence of sunshine and rain on tea blister blight Exobasidium vexans Massee in Ceylon. Ann. Appl. Biol.. 1961;49:306-315.
- [Google Scholar]
- The pests and blight of tea plants. Calcutta: Government Press; 1903. p. :429.
- Transgenic grapevine plants expressing a rice chitinase with enhanced resistance to fungal pathogens. Plant Cell Rep.. 2000;19:639-646.
- [Google Scholar]
- Molecular cloning and characterization of PtrLAR3, a gene encoding leucoanthocyanidin reductase from Populus trichocarpa, and its constitutive expression enhances fungal resistance in transgenic plants. J. Exp. Bot.. 2012;63(7):2513-2524.
- [Google Scholar]
- Bacterial disease resistance in arabidopsis through flagellin perception. Nature. 2004;428(6984):764-767.
- [CrossRef] [Google Scholar]







