Translate this page into:
Biosynthesized zinc oxide nanoparticles (ZnO NPs) using actinomycetes enhance the anti-bacterial efficacy against K. Pneumoniae
⁎Corresponding authors at: State Key Laboratory of Biocontrol, Guangdong Provincial Key Laboratory of Plant Resources and Southern Marine Science and Engineering Guangdong Laboratory (Zhuhai), School of Life Sciences, Sun Yat-Sen University, Guangzhou 510275, PR China (W.-J. Li), Department of Biotechnology, K. S. Rangasamy College of Technology, Tiruchengode, Namakkal District, Tamil Nadu 637 215, India (B M Gnanamangai). mythumithras@gmail.com (Balasubramanian Mythili Gnanamangai), liwenjun3@mail.sysu.edu.cn (Wen-Jun Li)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Nanoparticles include a varied range of particulate materials, which exhibit at least one of its dimensions in the order of 1–100 nm. Biological methods are commonly employed for nanoparticle synthesis of which microbes play a major role owing to their cost efficiency when compared to the existing chemical and physical methods. Nanoparticles have unique physical, chemicals and optical properties than that of their bulk or commonly found forms. The study here elaborates on the potential of biologically synthesized zinc oxide nanoparticles from actinomycetes species isolated from the rhizosphere of soil in India. Zinc oxide is hexagonal in shape and of permanent white colour. Actinomycetes are commonly found gram positive bacteria in soil but appear like fungi with powdery mass. Innthis study catinomycete genus Streptomyces sp. was used to synthesize the zinc oxide nanoparticles (ZnONPs). In this study of synthesized nanoparticle was used to various biomedical application antibacterials and anti-biofilms against pathogenic microbes. Firstly, UV-spectrometer was used to analyse the ZnO NPs, which exhibited its resonance between 350 and 400 nm. The studies of the biosynthesized ZnO NPs against multi drug resistant K. pneumoniae revealed 200 µg/mL as Biofilm inhbition BIC with 88% biofilm degradation potential and 92% hydrophobicity index due to the effect of ZnO NPs influence.
Keywords
Nanoparticles
Actinomycetes
ZnO NPs
Anti-bacterial activity
Microbial inactivation
Minimum inhibition concentration
1 Introduction
Nanotechnology provides promising technological tools through which a broad range of novel materials can be synthesized and that seem to have a huge potential in the field of biomedical and life sciences. Nanoparticles are considered as the starting points based on which macro structures of novel materials and devices can be. One of the most exciting method for the synthesis of nanoparticle seem to be the one which employ microorganisms. Microbial process mediated nanoparticle synthesis provide materials of unusual physical, chemical, optical, and electronic properties. Biological synthesis of nanoparticles with the help of microorganisms like actinomycetes has been well reported for many nanomaterials.
Actinomycetes which are commonly found in soil are a group of gram positive filamentous bacteria (Prudence et al., 2020). They are well studied organisms owing to their soil degrading properties and also as a potent source of antibiotics. The mechanism that actinomycetes follow to convert metallic oxides to metal oxide nanoparticles is not clearly known. Of the available biological methods for synthesis of ZnO NPs. microbial methods are advantageous compared than cell cultures or utilizing plants sources due to their scalability, efficiency and life cycle of organisms (Mohd Yusof et al., 2019). This study is aimed at microbial synthesis of ZnO nanoparticles from actinomycetes species.
Application of ZnO has been reported in several physical and optical processes including waste water treatment, food package and antimicrobial agent (Sabir et al., 2014). ZnO NPs are recognized as safe or GRAS, nontoxic and bio compatible particles (Zhang et al., 2013). The biological method for the synthesis of ZnO NPs is advantageous as it simple method and retains intact antimicrobial activity. Depending on the nature of these particles, they have a vast potential of antimicrobial activity against pathogenic microorganisms. The biological synthesis of nanoparticle is viable and cost effective and safe synthesis route according to biomedical application which confer potential and more of functional.
There is a drastic rise in the problems like unresponsiveness to heavy doses of multiple drugs by antibiotic resistance pathogens. The research direction on new compound search is a hard travel to go through millions of novel compounds. The alternative is to use the existing techniques with simple modification that can give enough period to harness the problem.
Ability of the pathogens to survive in consortia is another paradigm to work on where the biofilm ability of the pathogens provides the amicable environment to host the destruction of health tissues and progression of the disease development. Genetic transformation of the pathogen by altering the gene responsible may be possible in making evolutionary changes (Tiwari et al., 2018). Rather using simple extrovert molecule like ZnO with safe entitlement can be of great applicable to the biofilm degradation potential (Shkodenko et al., 2020).
With all consideration scope, the study was performed to use an efficient actinomycetes mediated synthesis route to synthesize the ZnO nanoparticles. One such envisage is proposed here to use the biosynthesized ZnO NPs as potential agent to treat as antibacterial agent against K. pneumoniae, a Multi Drug Resistant (MDR) strain especially for degradation of biofilm ability.
2 Methods and methodology
2.1 Chemicals
All the chemicals used are off 99% pure AR grade from Hi Media. This includes starch casein nitrate medium, sodium hydroxide and zinc sulphate. The glass materials used are off borosil grade and sterilized.
2.2 Collection of sample
The rhizosphere soil sampling was done based on the soil data of Tamil Nadu for rice crop growing areas indicating the richness of Zinc in the soil. In order to exploit variable microbial diversity available at various stages of crop growth, collection of rhizosphere soil samples from the agricultural field in Kokkarayanpettai, Tiruchengode fields with the coordinates 11.376413, 77.870188 of various rice plants.
2.3 Isolation of actinomycetes
Serial dilution of soil sample was carried out using sterile distilled water (Agadagba et al., 2014) up to 10-6 and was plated in starch casein nitrate agar plates. 0.1 mL was taken from each dilution and was spread onto plates containing starch casein nitrate agar medium. The plates were incubated at 37 °C for 72hrs until the appearance of actinomycetes colonies. As per the study of (Sharma et al., 2014) colonies appearing as dry, powdery and typically pigmented as green, orange, pink, yellow and white powder nature and they were for further use for future
2.4 Morphological identification
Morphological identification of soil isolates is the first step in confirming the actinomycetes species. It includes both naked eye observation or macroscopic observation and microscopic observation of colonies on the agar plates. Macroscopic characterization on the colonies was performed based on the following parameters such as shape, size, colour and mycelial growth. Also, the spore production was monitored based on the previous report of Muthu et al. (2013). Finally, the microscopic observations of important characters were see and confirmed as actinomycetes (Kumar et al., 2010).
2.5 Microbial synthesis of ZnO NPs
The well cultured actinomycete culture was inoculated into starch casein nitrate broth and maintained at room temperature for 4–5 days. For synthesis of ZnO NPs, the samples of zinc sulphate (0.1 M) plus sodium hydroxide solution (0.4) were taken together in a 50 mL test tube, and 50 mL of actinomycete culture was also added into the same tube and maintained in shaker incubator at 40 °C for 15 min to form the ZnO NPs. The flask was then heated in a microwave oven for 1–2 min followed by letting it cool for 1 h. The nanoparticles would settle down on its own. The appearance of white colour deposits on the bottom of the flask would confirm the formation of ZnO nanoparticles. After this, ZnO nanoparticles were washed with deionized water and centrifugation was carried out at 3000 rpm for 10 min. The centrifugation was repeated till a clear supernatant was obtained. The pellet was collected in a small plate and it was dried in a muffle furnace at 400 °C for 8 h or till it appeared totally dry (Fig. 1). Thus ZnO NPs were produced in a powdery form as described by Mishra et al., (2013).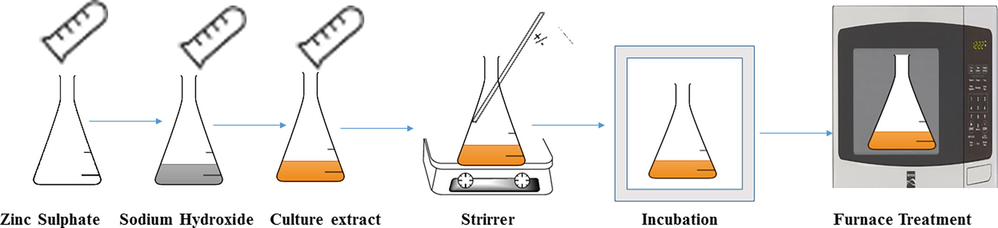
Scheme of synthesis of ZnO using actinomycetes.
2.6 Physico-chemical analysis of ZnO NPs
2.6.1 UV– visible spectroscopy
Biologically synthesized ZnO nanoparticles were subjected to UV–visible spectroscopy to observe the excitation spectra. It was measured using ultraviolet–visible spectrophotometer (Shimadzu, Japan), operated at the resolution of 1 nm. An absorbance spectra scan of 300–500 nm was carried out for the re-suspended nanoparticles on deionized water on the Hitachi double beam spectrophotometer to confirm the reduction of nanoparticles (Santhoshkumar et al., 2017) (Mishra et al., 2013) (Dobrucka and Długaszewska 2016).
2.6.2 X-Ray diffractometer (XRD)
The formed ZnO NPs was clearly confirmed by using X-ray diffraction instrument. Particles diameter was calculated using by Debby Scherrer's formula or Scherrer equation.
2.6.3 Fourier transform infrared spectroscopy (FTIR)
Next to XRD, the FTIR was scanned for detect the binding efficiency of ZnO NPs. By employing the FTIR spectrophotometer, structural information can be elucidated from its various vibrational modes. Dried ZnO NPs powder was directly used for FTIR analysis. Scanned FTIR result was noted based on the frequency at 400–4000 cm−1 with 4 cm -1.
2.6.4 DLS with Zeta potential study
The ZnO nanoparticles were re-suspended in aqueous solution and it was then filtered through a 0.22 µm syringe filter. The size distribution of the nanoparticles was measured using dynamic light scattering technique. Particle size, size distribution and Zeta potential effect of ZnO NPs was scanned using Malvern zeta sizer ZS ver 6.32 (Malvern Instrument, Malvern, UK).
2.6.5 Elemental dispersion analysis of X-Ray (EDAX)
EDAX was used to analyse the elemental composition of the synthesized ZnO nanoparticle. The presence or absence of the confirmative peaks of ZnO NPs was evaluated using EDAX.
2.6.6 Scanning electron microscopy (SEM)
The surface morphology of the nanoparticles was determined using scanning electron microscopy. Zinc oxide nanoparticle were dispersed in absolute ethanol under ultrasonic stirring followed by dropping some of the solution onto the glass slide and evaporating the solvent at room temperature. Then these specimens were coated with a thin gold layer through physical vapour deposition of about 3 mm thickness in vacuum before subjecting for SEM analysis.
2.6.7 Transmission electron microscopy (TEM)
Transmission electron microscope image gives the information about morphology and the size of ZnO nanoparticle. For preparation of TEM, the sample was coated with copper grid and viewed the shape and size of the ZnO NPs. The samples were prepared from very dilute dispersion of the particle in 2-propanol. In addition, the SAED electron diffraction was also viewed for detect the morphology of ZnO NPs
2.7 Biological activity analysis
2.7.1 Bacterial inactivation by ZnO NPs
The in vitro inhibition experiment of agar well diffusion method was performed against K. pneumoniae using the effect of ZnO NPs. Prepared muller hint agar was spread with staled K. pnemoniae culture and wells were punctured into the agar using gel borer. Different concentration (25–100 µg/mL) of ZnO NPs was inoculated into the punctured wells, and incubated overnight with room atmosphere. Multi-drug resistant character of K. pneumonia was confirmed by using the disc of cefotaxime. After overnight, the zones present around the wells of the ZnO NPs were measured. All the study carried out for bacterial inactivation of ZnO were in triplicates and the results were statistically analyzed using SPSS v.21.
2.7.2 Microbroth dilution experiment
Biofilm inhibition ability of ZnO NPs was evaluated in 96-well polystyrene plate against K. pneumoniae to detect the minimum inhibition concentration ZnO NPs. 50 µL of nutrient broth was added into the 96-well plate and followed by 10 µL of bacterial culture. Then, 10–100 µg/mL concentration of ZnO NPs was added into all the wells except control well, and makes a final volume of 300 µg/mL using nutrient agar. The plate was maintained in room atmosphere overnight without contamination. Next day, the turbidity range of the wells were observed visibly and calculated the colonies based on the live/dead condition using microscope. Consecutively, to identify the minimum inhibition concentration percentages, O.D values of all the wells were measured at 540 nm and calculated the percentage using below formula and the results reported were performed thrice and statistically analyzed using SPSS v.21.
3 Result and discussion
3.1 Actinomycetes culture from soil isolation
The collected soil samples were subjected to actinomycetes culture isolation. Actinomycetes colonies were isolated by serial dilution method using starch casein nitrate agar medium. Five isolate having white aerial mycelium was isolated and they were further used for ZnO NPs synthesis. The five isolate which were isolated from the soil sample colonies, were designated as HP01, HP02, HP03, HP04, HP05. They were subjected to synthesis of ZnO NPs. Similarly, Rajamanickam et al., (2012) has used actinomycetes for the biosynthesis of zinc nanoparticle for antibacterial food packaging. Previously, actinomycete mediated metal oxide nanoparticles was very effective against cancer cells and pathogenic bacteria (Golinska et al., 2014). The supported evidences of Aeruginosa et al. (1982) and Balagurunathan et al., (2011) were reported that the green synthesized nanoaprticles have more inhibition role against various bacteria. Therefore, anti-bacterial inhibition study was proved that the actinomycete mediated ZnO NPs has improved anti-bacterial activity and it is an excellent bio source for nanoparticles synthesis.
3.2 Identification of actinomycetes
Microscopic examination of Gram stained actinomycetes isolates were performed. The actinomycetes exhibited purple colour under 100x magnifications which depicts the filamentous nature of gram positive bacteria (Fig. 2a and b). Similar studies were done to confirm the nature of actinomycetes earlier by Sharma et al., (2014). Muthu et al., (2013) isolated Isoptericola variabilis from Cauvery river soil sample and confirmed them as actinomycetes by their powdery nature and stained structures.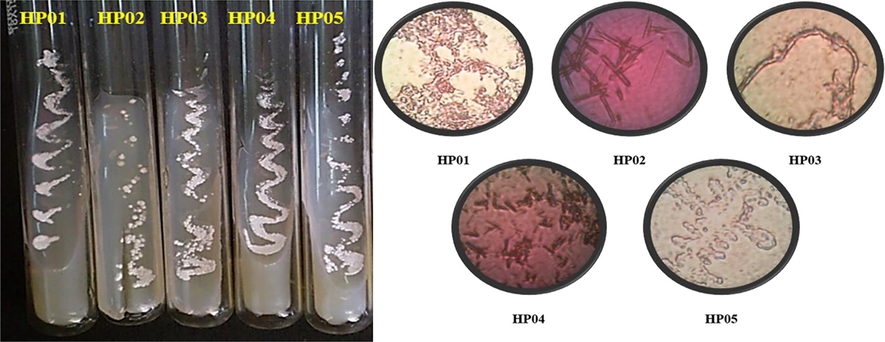
Isolated actinomycetes (a) Microscopical features (b) of the isolated actinomycetes.
3.3 Microbial synthesis of zinc oxide nanoparticles
The microbial synthesis of ZnO nanoparticles was performed by using culturing actinomycetes on starch casein nitrate broth. Microscopic identification yielded five isolates, which were then evaluated for zinc oxide nanoparticle formation. The white precipitate was formed with the two isolates indicating the synthesis of ZnO nanoparticles (Streptomyces enisocaesilis). The culture extract treated with (0.1 M) zinc sulphate at 37 °C for 96hr, gave a yield of 30 mg zinc oxide nanoparticles. Actinomycetes mediated the synthesis of ZnO nanoparticles from ZnSO4. The possible reaction for the biological synthesis is routed well below
ZnSO4 + 2NaOH + Culture extract Zn(OH)2 ↓ (White Precipitate) + Na2SO4
It reduced the particles from micro to Nano size with the help of enzymes and by the metabolic reactions of the actinomycete isolates. The nano sized zinc oxide has much higher potential than the micron size zinc oxide. Similarly, Datta et al., (2017) has synthesized ZnO NPs from the leaf extract of Parthenium hysterophorus and Ibrahem et al., (2017) produced ZnO nanoparticles from Aspergillus niger. Mishra et al., (2013) has reported the synthesis ZnO NPs using Lactobacillus sporangens and established the presence of nanoparticles by examining the formation of white powdery precipitate at the bottom of the flask. In another study by Rajabairavi et al., (2017), performed the biosynthesis of zinc oxide nanoparticle using endophytic bacteria Sphingobacterium thalpophilum. Synthesis of zinc oxide nanoparticles were mediated by using terpenoid fractions of Andrographis peniculata leaves by Kavitha et al., (2017).
3.4 ZnO nanoparticle characterization
3.4.1 UV–Visible spectroscopy analysis
The absorption spectra of Zinc oxide NPs as observed by the UV visible spectrophotometer was used as a confirmation for the presence of ZnO NPs in the colloidal solution.
Based on the available colloidal nature, whether the ZnO NPs was present or not was initially confirmed by UV spectrometer and result was proved that the ZnO NPs peaks were available at 330 nm and 430 nm. This shows that from five samples only two samples produced ZnO NPs. The sample HP01 and HP05 showed peak value @330 nm & 430 nm (Fig. 3) respectively, confirmed that the synthesized product was zinc oxide nanoparticle. The absorption peak was similar to that of result obtained by Ibrahem et al., (2017); Mishra et al., (2013). Balraj et al., (2017) who has obtained the absorption peak at 364 nm which were in agreement under the range of zinc oxide nanoparticle analysis. Similarly Santhoshkumar et al., (2017) has obtained the absorbance peak at 380 nm. From the above studies, we concluded the presence of ZnO nanoparticles in the biosynthesized samples.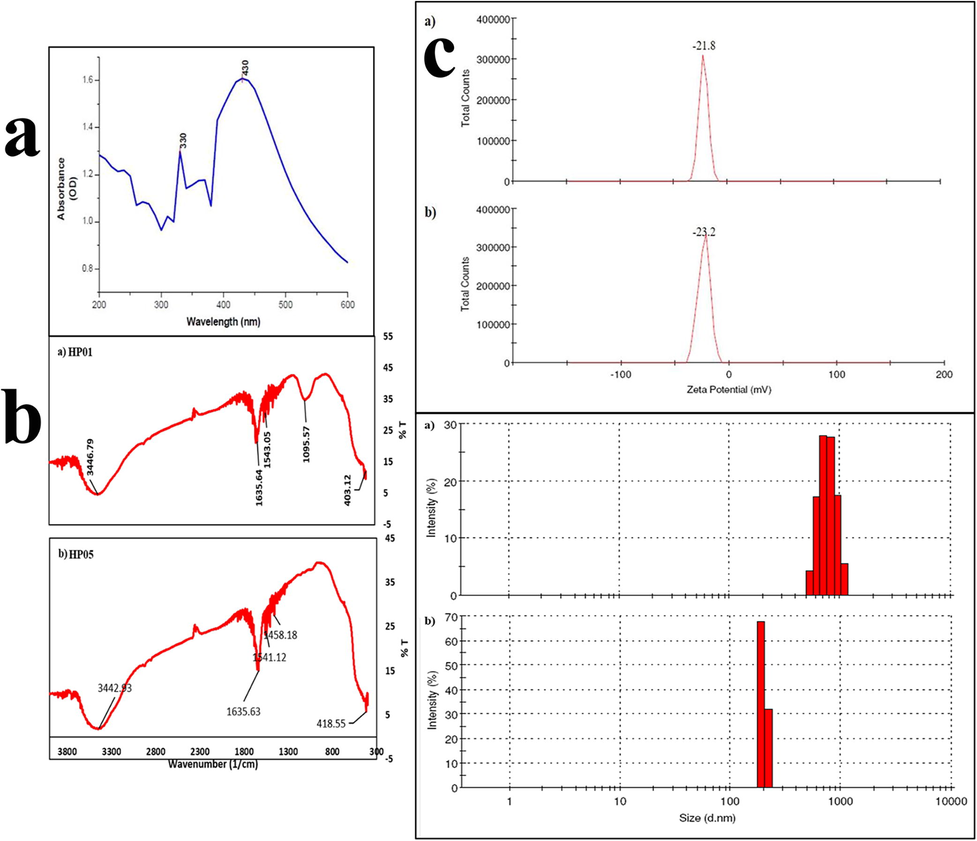
UV absorption spectra (a), FTIR analysis (b) and Zeta potential (c) of ZnO NPs from HP01 and HP05.
3.4.2 Ftir
It is a well-established fact that the surface to volume ratio for the nanoparticle is higher than their bulk counterparts FTIR results would yield conclusive results on the presence or absence of various vibrational modes of synthesized ZnO NPs. The FTIR spectra of the synthesized zinc oxide nanoparticle are in the range of 4000 to 400 cm−1. The FTIR analysis was carried out for both HP01 and HP05 isolates respectively (Fig. 3b). For the sample HP01 the FTIR profile of zinc oxide nanoparticles affirmed the presence of multiple peaks at 3446, 1635, 1543, 1095 and 403 cm−1. Similarly, for HP05 the presence of multiple peaks at 3442, 1635, 1541, 1458 and 418 cm−1 was confirmed. The broadened appearance of intense bands with O–H bond group was confirmed at 3442 and 3446 cm−1. Presence of OH peaks indicates the presence of residual moisture irrespective of heating and drying of samples. The peak bands at 1635, 1541, 1543 and 1458 cm−1 represents C = O stretching of COO and CHO moiety and C-O stretching was confirmed at 1095 cm−1. 403–418 cm−1 of the bonds were confirms zinc oxide bonds. Thus the FTIR results confirm the formation of zinc oxide nanoparticle. Similarly, the results from Datta et al., (2017) and Mishra et al., (2013) have shown at 424 cm−1 and 462.25 cm−1 with ZnO NPs. Al-Dhabi and Arasu (2018) has obtained peak at 417 cm−1 for the zinc oxide nanoparticles which was produced by green approach. Santhoshkumar et al., (2017) has described the peaks for the ZnO nanoparticle in the wavelength range of 500–4000 cm−1 which has been synthesized using plant leaf extract. Baliah et al., (2018) revealed a peak between 3421 cm−1-–677 cm−1 for the ZnO NPs synthesized using onion pulp extract.
3.4.3 X-ray diffractometer
XRD spectra comprehensively provide a greater insight about the crystallinity of a nanoparticle. The formation of crystalline zinc oxide nanoparticle can be affiliated to the broadening of the characteristic XRD spectra. The zinc oxide nanoparticles which is obtained from HP01 sample was evaluated to study its crystalline nature by XRD spectroscop (Fig. 4a)y. It can be ascertained from the results that the synthesized zinc oxide nanoparticles were in their pure phase devoid of impurities. The XRD of the synthesized zinc oxide shows broad peak at values of 2 theta are 32.3, 34.9, and 36.8 which are typical for the zinc oxide structure. Using the Debye-Scherrer’s equation
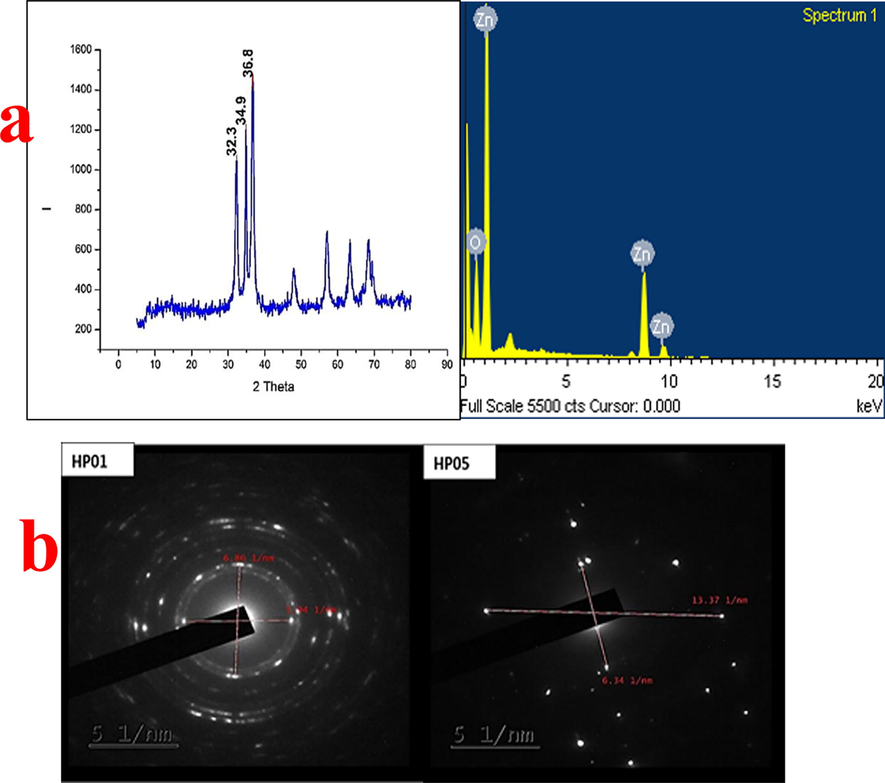
XRD and EDAX analysis of HP01 (a) and SAED analysis (b) of HP01 and HP05.
The particle size as determined for the zinc oxide NPs was found to be 11.76 nm, 14.58 nm and 11.57 respectively. Al-Dhabi and Arasu (2018) has used XRD analysis for the ZnO NPs synthesized by the green approach. In contrast, Khalil et al., (2014) has analysed with the Debye-Scherrer’s equation and obtained most intense peak at 2 theta (36.67°). Therefore, XRD result pattern of well-organized crystalline structure of ZnO NPs was indicated from sharp peak of the result. The XRD aided for identification of crystallinity and lattice nature of the nanoparticle.
3.4.4 Zeta potential
Zeta potential analysis was used as a primary tool to evaluate the surface charges acquired by ZnO NPs. This result in turn would provide further understanding on the colloidal stability of zinc oxide nanoparticles. Zeta potential value for zinc oxide nanoparticles was measured for the sample HP01 and HP05 and water as a dispersant in all. Zeta potential value for the sample was found to be −21.8mv and –23.2mv for HP01 and HP05 respectively (Fig. 3c). The observed high negative value between – 20mv to − 25mv indicates that the particles repel each other. Thus they can stay as colloids for extended time. The result was contradictory with Yedurkar et al., (2016) and the result of 49.19mv was obtained for ZnO NPs after zeta potential analysis using Ixora coccinea leaf extract. Thereby the high value of zeta potential is an indication for stability of intended formulation. Jafarirad et al., (2014) showed the result of zeta potential for zinc oxide nanoparticles was found to be – 20 to − 30mv. Therefore, the synthesized zinc oxide nanoparticle shows the stability against aggregation. Since 20mv will be sufficient to stop the aggregation.
3.4.5 Particle size analysis
Particle size analysis and size distribution analysis can be performed using various techniques. DLS or dynamic light scattering is one such widely used method used for understanding particle size and size distribution of nanoparticle. Molecules in suspension is subjected through a beam of light and the scattering of light due to Brownian movement is captured to understand the average particle size, distribution and poly dispersity index (PDI). The analyses were made for both HP01 and HP05 samples (Fig. 5). The result showed that the biosynthesized zinc oxide NPs had an average particle size of 903.3 nm and 321.3 nm for HP01 and HP05 respectively with the polydispersity index of 0.304 and 1.00. The Fig. 3 indicates the size distribution on intensity. Similarly, the particle size analysis was performed for zinc oxide nanoparticle which is synthesized by Marine Streptomyces sp. (Balraj et al., 2017).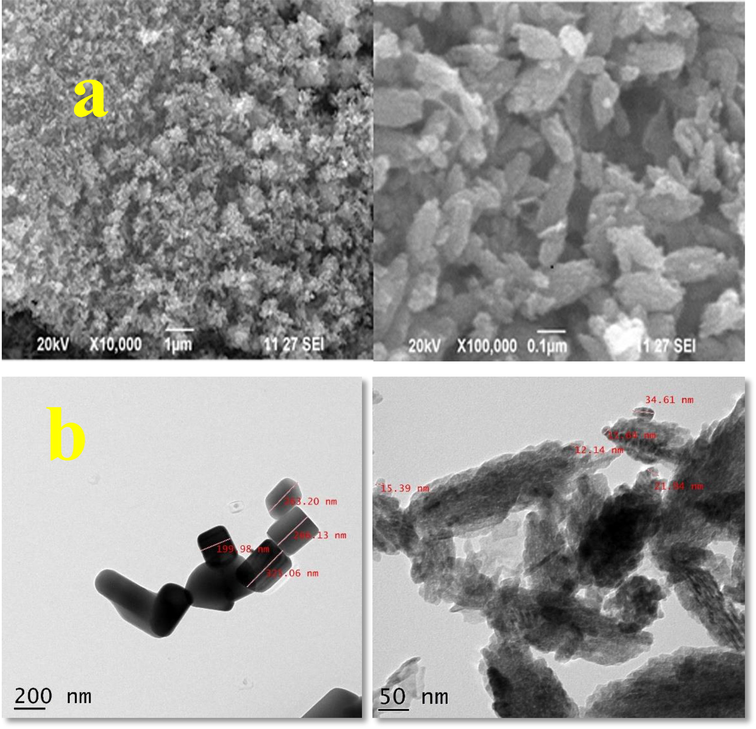
Morphological observation of SEM (a) and TEM (b) of biological synthesized ZnO NPs.
3.4.6 Energy dispersive X-ray analysis (EDAX)
EDAX analysis is commonly employed for elemental analysis of the synthesized nanoparticles. A scanning electron microscopic system is usually coupled with x-ray microanalysis system for evaluation of chemical composition. HP01 synthesized zinc oxide nanoparticles were subjected to EDAX analysis. The result of X-ray microanalysis of the zinc and oxygen compounds synthesized by actinomycetes is represented in Fig. 5 which showed the presence of optical absorption peak of the produced nanoparticle. The elemental composition of the ZnO nanoparticle showed the presence of 61.57% and 38.43% for oxygen and zinc respectively. Thus the nanoparticle synthesized can be considered as highly pure. In contrast, the zinc oxide NPs produced from plant extract with higher efficiency (Santhoshkumar et al., 2017). Similarly, the zinc oxide NPs synthesized using root extract of Zingiber officinale was taken to EDAX analysis. The elemental analysis of nanoparticle from root extract showed about 80% zinc and 19% oxygen in the case of Anand.
3.4.7 Sem
SEM technique elucidate, HPO1 based ZnO NPs morphology, size and shapeby SEM. The SEM image depicts that actinomycetes has enormous capacity to produce ZnO NPs that are definite with needle shape. SEM images were observed in different magnification (10,000 & 100,000). The SEM images of the synthesized ZnO NPs showed needle shaped structures of average particle size 321.3 nm (Fig. 5a). Similarly, the SEM analysis of zinc oxide NPs synthesized from Streptomyces species was reported to have spherical shape of average particle size as 16 – 25 nm (Shanmugasundaram and Balagurunathan, 2017). In contrast, zinc oxide synthesized using S. thalpophilum showed a triangular structures of uniform dimensions with an average size of 112 nm (Rajabairavi et al., 2017). Supportively, the spherical nature of ZnO NPs was confirmed by Santhoshkumar et al., (2017). The present study also revealed the rod and needle shaped structure for HP01 and HP05 respectively.
3.4.8 Selected area electron diffraction (SAED)
Selected area electron diffraction analysis is used for the analysis of crystalline nature of the nanoparticle. SAED analysis is made for two samples HP05 and HP01 respectively (Fig. 4b). Polycrystalline structures of the zinc oxide NPs was disclosed by bright spots. The inter-planar distance between the bright spots was calculated. The crystalline distance with inter-planar spacing values for HP05 are 0.32 and 0.15 Å and for HP01 are 0.29 and 0.34 Å respectively. They corroborated with the observations of XDR studies. The presence of lattice fringes of ZnO NPs represents the crystalline nature. SAED pattern displayed rings containing bright spot indicating that nanoparticles are polycrystalline with uniform shape and bigger grains. Reported measurements of structural parameters of synthesized ZnO NPs from waste Zn batteries were made from XRD spectra (Farzana et al., 2018). Lattice planar of the face centered cubic ZnO indicating the biogenic nanoparticles with SAED pattern shows well defined electron diffraction spot confirming the crystalline nature of the nanoparticle.
3.4.9 Transmission electron microscopy (TEM) analysis
The average particle size and distribution of zinc oxide NPs were estimated using transmission electron microscopy. Synthesized ZnO NPs was shown in TEM. And TEM analysis was done to the samples HP05 and HP01 (Fig. 5b). The diameter of the nanoparticle varies between 199 and 326 nm for HP05 sample and for HP01 the diameter of the nanoparticle varies between 12 and 35 nm. The synthesized zinc oxide NPs from HP05 showed higher size diameter than that of HP01 as observed from the results. The image confirmed the presence of needle shaped nanoparticles. Due to the inter particle interaction such as Van der Waals and other major intra molecular forces the ZnO NPs tend to aggregate and exist as soft agglomerates. Al-Dhabi and Arasu (2018) ruled out that the particle agglomeration is an issue as far as the application of ZnO NPs are concerned. They have confirmed that most applications of ZnO NPs are based on particle size and not on agglomerate size. Similarly, the TEM analyses was carried out for ZnO NPs by Satyajit Saha and Amit Kumar Bhunia (2014) and show the diameter varies between 20 and 40 nm. In contrast, the nanoparticles synthesized from waste Zn-c battery showed the diameter of ZnO NPs in the range of 10–40 nm with spherical shape (Farzana et al., 2018). Here the synthesized ZnO NPs from HP01 show the similar result.
3.5 Biological activity analysis
3.5.1 Anti-bacterial activity
In our result, the highest zone of inhibition 24 mm was exhibited against K. pneumoniae by 250 µg/mL concentration. In addition, the minimum zone of 12 mm zone was exhibited by 50 µg/mL concentration. Also, 50 µg/mL concentration is a rapid increasing level concentration. The results indicated that the biosynthesized ZnO NPs exhibited anti-bacterial activity and also showed inhibition ability at increasing concentration. Interestingly, the multi-drug resistant detection antibiotic and distilled water wells were shown no zone against K. pneumonia. It was suggested that the biosynthesized ZnO NPs possessed anti-bacterial activity across multi drug resistant bacteria at increasing concentration. The zone of inhibition and concentration dependent inhibition differentiation results were presented in Fig. 6. In mechanism, the negative charges of the bacterial surface were easily degraded by positive charges of the ZnO NPs due to the transfer of effective Zn+ ion molecules. After, the bacterial cell wall was damaged and porin channels were completely distracted (Praveena et al., 2020). Then it affected the teichoic acid and lipid bilayer in inside of the bacterial cells. The Zn+ ions was connected to the porin channels of bacteria and lost their antigenicity (Vinotha et al., 2019). Finally, the depletion of essential components in the ZnO NPs treated cells were killed and remained in decline phase. Based on the above mentioned mechanism, the observed results suggested that the biosynthesized ZnO NPs possessed anti-biofilm properties and it significantly caused damage of intracellular and extracellular bacterial components.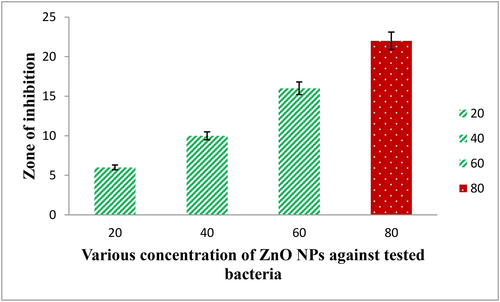
Antibacterial activity of ZnO NPs against tested bacteria.
3.5.2 Minimum biofilm inhibition concentration (BIC)
The increasing inhibition concentration of ZnO NPs against biofilm forming K. pneumonia was proved by O.D values of spectrophotometry, and the differentiation of concentration dependent inhibition was shown in Fig. 7. Higher turbidity of biofilm culture was observed at 200 µg/mL concentration and at the same concentration; attachment of biofilm culture was not shown. Compared with untreated control, the decreased biofilm mould colonies were shown in increasing concentration. After 190 µg/mL, the confluent layer of biofilm was completely absent in the 24-well and suggested that the ZnO NPs completely arrested the biofilm growth. At 200 µg/mL concentration, biofilm as absent and pathogenicity of K. pneumonia in culture was not detected. Therefore, this concentration of 200 µg/mL concentration was considered as a BIC. This concentration was used for further in vitro and spectroscopic study. Before monitoring the spectroscopy analysis, the damaged cytoplasmic membrane was absorbed the more crystal violet stain compared with untreated control (Wilson et al., 2017; Rajivgandhi et al., 2018). Previously, extracellular components of polysaccharides, nucleic acid, proteins and other leaking materials are the basic principle materials for make biofilm formation in bacteria. If the crystal violet absorption was high in the cell membrane molecules, it indicates, the biofilm was damaged. The result was agreed by Megan O’Shaughnessy 2020 on P. aeruginosa and the intracellular materials here were fully compromised by treatment of ZnO NPs. Also, no attached cells on the 24-well plate walls were observed due to absence of crystal violet formation. ZnO NPs had the potential to degrade adherence cells as its concentration increases. Therefore, based on the result, ZnO NPs possessed biofilm degradation ability based on its concentration. When the concentration was high, the inhibition rate was gradually increased as well as bacterial biofilm was decreased. A study reported (Reyes-Torres et al., 2019), that the lowest concentration of biological mediated ZnO NPs retained improved anti-bacterial activity. Similar result was stated by Maruthupandy et al., (2018), the lack of growth at lowest concentration of ZnO NPs was detected against MDRs pathogens. Recently, (El-Nahhal et al., 2017) the better anti-bacterial efficacy of ZnO NPs displayed maximum inhibition zone against bacteria compared to chemical synthesis. Our result was correlated with previously reported articles and suggested that the biosynthesized ZnO NPs has more bactericidal against performed K. pneumoniae at minimum concentration.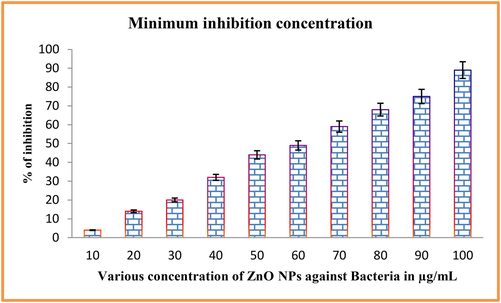
Minimum inhibition concentration of ZnO NPs against tested bacteria.
4 Conclusion
Synthesis of ZnO NPs for biomedical application is safe using biological synthesis (Actinomycetes). It can be used to improve the drug delivery and availability that are hindered due to other microbial interactions during the route of drug metabolism. The ZnO NPs being a stable structure and safe compound can be used as dental fillers with more activity of biofilm degradation that is stimulates the cavity development. Biological mediated synthesis confers safe route of synthesis for biomedical application. The present study has envisaged the activity of the biologically synthesized ZnO nanoparticle as an alternative compound against MDR strains. In future it can act as a safe biological and potential compound for safe and exact drug delivery vehicle in compromised cases of cancer treatment
Acknowledgements
All the authors gratefully acknowledge the National Natural Science Foundation of China (Project Approval Numbers: 41950410573, 91951205 and 31670009) and Postdoctoral Science Foundation of China (Project Approval Number: 2019 M663213) for financial support for this work. Wen-Jun Li was also supported by Introduction project of high-level talents in Xinjiang Uygur Autonomous Region. The authors extend their appreciation to the Researchers Supporting Project number (RSP-2021/70), King Saud University, Riyadh, Saudi Arabia. The authors are indebted to the Management of K.S.R Group of Institutions, CEO, and Principal of K. S. Rangasamy College of Technology, for the financial assistance and mentoring support during the study.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Health protection agency isolation of actinomycetes from soil. J. Microbiol. Res.. 2014;136(140)
- [CrossRef] [Google Scholar]
- Biosynthesis of gold nanoparticles by actinomycete streptomyces viridogens strain HM10. Indian J. Biochem. Biophys.. 2011;48:331-335.
- [Google Scholar]
- Synthesis and characterization of onion mediated silver doped zinc oxide nanoparticles. Int. J. Sci. Res. Sci. Eng. Technol.. 2018;4:111-120.
- [CrossRef] [Google Scholar]
- Synthesis and characterization of Zinc Oxide nanoparticles using marine streptomyces sp. with its investigations on anticancer and antibacterial activity. Res. Chem. Intermed.. 2017;43(4):2367-2376.
- [CrossRef] [Google Scholar]
- Green synthesis of zinc oxide nanoparticles using parthenium hysterophorus leaf extract and evaluation of their antibacterial properties. J. Biotechnol. Biomater.. 2017;07:3-7.
- [CrossRef] [Google Scholar]
- Biosynthesis and antibacterial activity of ZnO nanoparticles using trifolium pratense flower extract. Saudi J. Biol. Sci.. 2016;23(4):517-523.
- [CrossRef] [Google Scholar]
- Stabilization of nano-structured ZnO particles onto the surface of cotton fibers using different surfactants and their antimicrobial activity. Ultrason. Sonochem.. 2017;38:478-487.
- [CrossRef] [Google Scholar]
- Zinc oxide nanoparticles from waste Zn-C battery via thermal route: Characterization and properties. Nanomaterials. 2018;8(9):717.
- [CrossRef] [Google Scholar]
- Biogenic synthesis of metal nanoparticles from actinomycetes: biomedical applications and cytotoxicity. Appl. Microbiol. Biotechnol.. 2014;98(19):8083-8097.
- [CrossRef] [Google Scholar]
- Biosynthesis of zinc oxide nanoparticles and assay of antibacterial activity. Am. J. Biochem. Biotechnol.. 2017;13(2):63-69.
- [CrossRef] [Google Scholar]
- Jafarirad, S., Mehrabi, M., Rassul, E., 2014. Biological Synthesis of Zinc Oxide and Copper Oxide Nanoparticles 62–64.
- Synthesis and characterisation of zinc oxide nanoparticles using terpenoid fractions of andrographis paniculata leaves. Int. Nano Lett.. 2017;7(2):141-147.
- [CrossRef] [Google Scholar]
- Synthesis and characterization of ZnO nanoparticles by thermal decomposition of a curcumin zinc complex. Arab. J. Chem.. 2014;7(6):1178-1184.
- [CrossRef] [Google Scholar]
- Isolation and screening of soil actinomycetes as source of antibiotics active against bacteria. Int. J. Microbiol. Res.. 2010;2(2):12-16.
- [CrossRef] [Google Scholar]
- Biologically synthesized zinc oxide nanoparticles as nanoantibiotics against ESBLs producing gram negative bacteria. Microb. Pathog.. 2018;121:224-231.
- [CrossRef] [Google Scholar]
- Studies on the inhibitory activity of biologically synthesized and characterized zinc oxide nanoparticles using lactobacillus sporogens against staphylococcus aureus. J. Pure Appl. Microbiol.. 2013;7:1263-1268.
- [Google Scholar]
- Microbial synthesis of zinc oxide nanoparticles and their potential application as an antimicrobial agent and a feed supplement in animal industry: A review. J. Anim. Sci. Biotechnol.. 2019;10:1-22.
- [CrossRef] [Google Scholar]
- Isolation and identification of actinomycetes isoptericola variabilis from cauvery river soil sample. Int. J. Curr. Microbiol. App. Sci. 2013;2:236-245.
- [Google Scholar]
- Identification of a novel antibacterial protein from hemolymph of freshwater zooplankton Mesocyclops leuckarti. Saudi J. Biol. Sci.. 2020;27(9):2390-2397.
- [CrossRef] [Google Scholar]
- Advances in actinomycete research: An actinobase review of 2019. Microbiol. (United Kingdom). 2020;166:683-694.
- [CrossRef] [Google Scholar]
- Rajabairavi, N., Raju, C.S., Karthikeyan, C., Varutharaju, K., Nethaji, S., Hameed, A.S.H., Shajahan, A., 2017. Biosynthesis of novel zinc oxide nanoparticles (ZnO NPs) using endophytic bacteria Sphingobacterium thalpophilum, in: Springer Proceedings in Physics. 10.1007/978-3-319-44890-9_23
- Biosynthesis of zinc nanoparticles using actinomycetes for antibacterial food packaging. Int. Conf. Nutr. Food Sci.. 2012;39:195-199.
- [Google Scholar]
- Antibiofilm activity of zinc oxide nanosheets (ZnO NSs) using Nocardiopsis sp. GRG1 (KT235640) against MDR strains of gram negative Proteus mirabilis and Escherichia coli. Process Biochem.. 2018;67:8-18.
- [CrossRef] [Google Scholar]
- Synthesis of CuO and ZnO nanoparticles by a novel green route: Antimicrobial activity, cytotoxic effects and their synergism with ampicillin. Ceram. Int.. 2019;45(18):24461-24468.
- [CrossRef] [Google Scholar]
- Zinc oxide nanoparticles for revolutionizing agriculture: Synthesis and applications. Sci. World J.. 2014;2014:1-8.
- [CrossRef] [Google Scholar]
- Synthesis of zinc oxide nanoparticles using plant leaf extract against urinary tract infection pathogen. Resour. Technol.. 2017;3(4):459-465.
- [CrossRef] [Google Scholar]
- Bio-medically active zinc oxide nanoparticles synthesized by using extremophilic actinobacterium, Streptomyces sp. (MA30) and its characterization. Artif. Cells, Nanomedicine Biotechnol.. 2017;45(8):1521-1529.
- [CrossRef] [Google Scholar]
- Metal oxide nanoparticles against bacterial biofilms: Perspectives and limitations. Microorganisms. 2020;8:1-21.
- [CrossRef] [Google Scholar]
- Mechanism of anti-bacterial activity of zinc oxide nanoparticle against carbapenem-resistant acinetobacter baumannii. Front. Microbiol.. 2018;9:1-10.
- [CrossRef] [Google Scholar]
- Synthesis of ZnO nanoparticles using insulin-rich leaf extract: Anti-diabetic, antibiofilm and anti-oxidant properties. J. Photochem. Photobiol. B Biol.. 2019;197:111541.
- [CrossRef] [Google Scholar]
- Quantitative and Qualitative Assessment Methods for Biofilm Growth: A Mini-review. Technol: Res. Rev. J. Eng; 2017. p. :6.
- Biosynthesis of zinc oxide nanoparticles using ixora coccinea leaf extract—a green approach. Open J. Synth. Theory Appl.. 2016;05(01):1-14.
- [CrossRef] [Google Scholar]
- Biomedical applications of zinc oxide nanomaterials. Curr Mol Med.. 2013;13:1633-1645.
- [CrossRef] [Google Scholar]







