Translate this page into:
Biosynthesis of silver nano particles (AgNPs) from blue green algae (Arthrospira platensis) and their anti-pathogenic applications
⁎Corresponding author. jasimsalman@uobabylon.edu.iq (Jasim Mohammed Salman)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The increase in bacterial infections and the emergence of antibiotic resistance has led to the development of bio-derived and environmentally friendly nanomaterials to counteract bacterial activity, and silver nanoparticles AgNPs are a promising way to achieve this. The present work aimed to synthesize AgNPs from the blue-green algae Athrospira Platensis and evaluate their antibacterial efficacy against Enterococcus faecalis, Staphylococcus aureus, Proteus mirabilis, and Pseudomonas aeruginosa isolated from human urinary tract infections. The results were shown and confirmed by peak UV spectra at 450. Through SEM and DLS analyses, the shapes of the AgNPs were determined to be spherical and with sizes ranging from 15 to 100 nm. The zeta potential values of AgNPs were found to be −20 ± 3.2 mV indicating the absence of AgNPs aggregates. Synthetic AgNPs produced by A. platensis showed a strong antibacterial effect against bacteria isolated from human urinary tract infections. The diameters of the inhibitory zone for S. aureus E. faecalis P. mirabilis and P. aeruginosa 14 mm, 16 mm, 15 mm, and 12 mm, respectively. AgNPs showed the highest activity against E. faecalis strain, with the diameter of the inhibitory zone reaching 16 ± 0.2 mm at a concentration of 800 μg/mL.
These results will be a useful resource for studying the effects of man-made nanoparticles on the environmental safety and represent a promising step toward realizing AgNP-based antimicrobial drug production.
Keywords
Antibacterial Activity
AgNPs
Eco-friendly
Biosynthesis
Pathogens
Blue green algae
1 Introduction
Nanomaterials with sizes between one and one hundred nanometers have gained significant interest in various fields, including biomedicine, energy storage, catalysis, sensors, etc. (Khan et al., 2019; Aljeldah et al., 2023). This is mostly because of their extraordinary chemical and physical qualities, which include amazing optical qualities, great biocompatibility, and chemically stable properties. (Hashemi et al., 2020; Ying et al., 2022). AgNPs have drawn much attention, especially in biomedicine, due to their well-documented, wide-ranging antibacterial properties and exceptional efficacy (Rajkumar et al., 2021). Ag- nanoparticles exhibit significant bactericidal capabilities and demonstrate substantial toxicity toward microorganisms (Gibala et al., 2021). AgNPs achieve this by inducing damage to DNA, deactivating enzymes, inducing reactive oxygen species (ROS), and damaging membrane proteins (Xu et al., 2020).
The creation of nanoparticles can occur through three distinct pathways: chemical, physical, and microbiological. In general, physical and chemical procedures are more costly, hard to set up, and fraught with danger. In contrast, the biological pathway has an environmentally friendly synthesis process, a cost-effective technique, and NPs that are physiologically compatible (Choudhary et al., 2023).
Microalgae are a superior option for AgNP synthesis because they develop faster and create more biomass at a cheaper cost than other species (Dhaka et al., 2023; Obaid et al., 2023). Since algae are rich in bioactive organic compounds including polysaccharides, amino acids, carbohydrates, pigments, and secondary metabolites (Salman et al., 2023), they are ideal candidates for the biosynthesis of AgNPs. These active chemicals function as reducing agents to generate AgNPs, the size and shape of which can be manipulated (Alassali et al., 2016).Algae can efficiently amass high levels of heavy metals and convert them into different and diverse forms(Salman et al., 2022),due to these significant characteristics, algae are used in the creation and advancement of various nanomaterials, such as Ag, Fe, and Cu (Mukherjee et al., 2021). Over 20 distinct species of green microalgae are utilized for the synthesis of AgNPs, and nearly all microalgae species are capable of synthesizing AgNPs of diverse sizes and shapes (Rajkumar et al., 2021). The hydrophilic surface groups of carboxyl, sulfate, and hydroxyl that the algae create in their silver nanoparticles contribute to their distinctive application; they might be used in medicinal applications since the algae don't produce any harmful or toxic compounds.
Athrospira platensis is a free-floating filamentous cyanobacterium that belongs to the blue-green microalgae group. It is characterized by multicellular trachoma in a left-open helix and a cylindrical (Mahdia et al., 2012; Al-Sheikh et al., 2024).
Several studies have reported important antibacterial properties of silver nanoparticles produced from microalgae, For instance,Chaetoceroscalcitrans, Chlorella salina, and Tetraselmis gracilis act as a antibacterial agent against pathogens as Klebsiella spp., Pseudomonas aeruginosa, Proteus vulgaris (Merin et al., 2010).
2 Materials and methods
2.1 Production of extract A. platensis
An algae sample has been acquired from Lab. of Advance Ecology. The extract was prepared according to the methodology reported by El-Chaghaby et al., 2019. A. platensis powder (0.5 g) was used with 5 ml of alcohol (ethanol), then 45 ml of deionized water was append, with a pH value of 9 (by adding sodium carbonate or sodium hydroxide), and the blend was warmed at room temperature for 2 h using ultrasound. Lastly, the mixture was filtered using filter paper (Millipore 0.45 μm) to get the final extract.
2.2 Biosynthesis of Ag- NPs
AgNPs were produced from A. platensis extract by following methods that El-Chaghaby et al. (2019) had previously described. The extract is diluted, the pH is adjusted by adding sodium carbonate or sodium hydroxide, and then a (1 mL) silver nitrate solution (0.01 M) is added using deionized water. To collect AgNPs, they were added to the mixture dropwise and mixed for 24 h at 500 rpm utilizing a magnetic stirrer. Following that, it was rinsed and centrifuged for 30 min at 12,000 RCF. The sediment is then kept at ambient temperature for further experiments. The optical change of the silver nitrate solution to yellow was a sign of the creation of AgNPs.
2.3 Quantitative analysis to absorbance of AgNPs
Used a UV–visible spectrophotometer (Lamda 365, PerkinElmer) to monitor the production of AgNPs. The absorbance of the AgNPs created via biological synthesis was evaluated utilizing UV–visible spectroscopy across the 300–450 nm wavelength range, Fig. 1.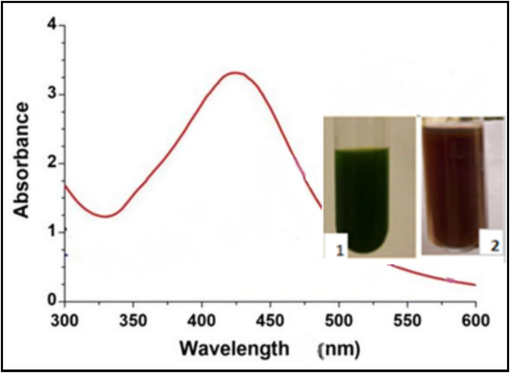
The UV–Vis absorption spectra of AgNPs extracted from A. platensis (1: without AgNo3, 2: with AgNPs).
2.4 Evaluation of particle size distribution and morphology of AgNPs
AgNPs particle size distribution and morphology have been assessed using a Zeta potential analyzer (Zeta sizer Nano ZS, Worcestershire, UK), a “Transmission Electron Microscope (TEM) (JEM-1400Flash Electron Microscope), and Dynamic Light Scattering (DLS)”, Fig. 2.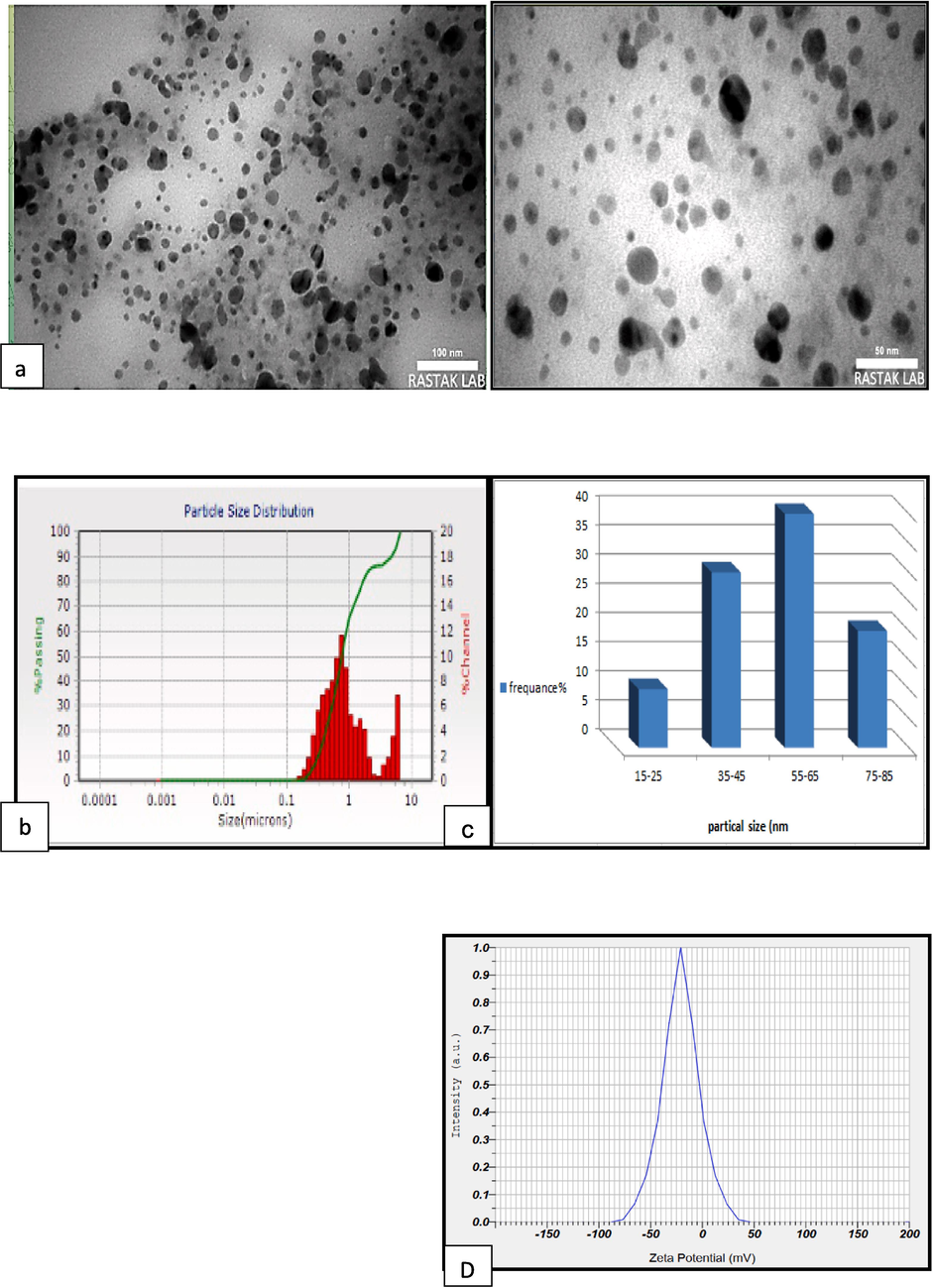
(a)TEM image of Ag NPs (50 and100nm), (b) particle size distribution histogram of SNPs obtained from TEM image(c) Particle size distribution of the SNPs produced by biosynthesis using DLS,and (d) Zeta potential analysis of surface charge of AgNPs.
2.5 Antimicrobial effects of Ag NPs
The antimicrobial impact of Bio-AgNps on four common pathogenic bacterial isolates was evaluated using the well diffusion method. These isolates included Gram-positive S. aureus,and E. faecalis, along with Gram-negative P. mirabilis, and P. aeruginosa. They were obtained from the Microbiology Laboratory. Initially, Muller Hinton agar plates were wiped of 0.5 MacFarland bacterial suspensions, and then 100 µL of each of the following concentrations of bio-Ag “(800 µg\ml, 400 µg\ml, 200 µg\ml, and 100 µg\ml)” were aseptically transferred on inoculated plates, separately. Finally, the dishes were kept in a lab incubator at 37 °C for an entire day. Transparent areas around every well represent the different inhibitory concentrations of nanoparticles as a result of the responses of different bacterial strains Fig. 2.
3 Result and discussion
3.1 Analyzing of AgNPs by UV–visible spectroscopy
As soon as silver nitrate was added, the A. platensis suspension's color altered. A. platensis' dark green hue was transformed to brown, signifying the synthesis of AgNPs. The hue was altered to brown as a result of surface plasmon resonance (SPR) stimulation, both of which are crucial for verifying the synthesis of AgNPs (Moraes et al., 2021). The formation of AgNPs was verified by performing spectroscopic analysis in the 300–600 nm wavelength range, using an ultraviolet spectrophotometer. According to Fig. 2, the nanoparticles produced through biosynthesis exhibited the maximum absorption at a wavelength of 450 nm, and which is in line with many studies that which is in line with many studies that indicating that the SPR peak falls within the wavelength range between 410 and 450 nm, as a signature of biosynthesis nanoparticles (Yasin et al., 2013; Rashad et al., 2019).
3.2 Analysis of AgNPs by Transmission electron microscopy (TEM)
TEM is utilized in this work to identify the shape, surface, size, and topology of the biosynthesized AgNPs (Al-Jalda et al., 2023). Image J software was utilized to analyze and capture a picture of the TEM. The AgNPs that were produced had a spherical form and varied in size from 18 to 100 nm, according to the TEM examination. Sizes of 50 and 100 nm were photographed, and the highest peak frequency was recorded at about 55–65 nm, as in Fig. 2 (a & b). “Dynamic light scattering (DLS)” is an instrument utilized to analyze aggregation dynamics and assess the size and dispersion of nanoparticles. DLS is currently, a commonly utilized and effective way for assessing particle sizes range from 1 to 100 nm range (Raza et al., 2016; Hodoroaba et al., 2019). The current findings show that the average particle size of Ag NPs was 58.682 ± 1.56 nm, with a variety of sizes of 18 to 100 nm, as in Fig. 2-c.
The results indicated that there were no clear agglomerations and that the nanoparticles were uniformly distributed and had a spherical shape. Antibacterial activity of AgNPs was significantly influenced by the size and shape of the particles. Specifically, smaller spherical nanoparticles are more efficient against microbes (Menichetti et al., 2023). The phenomenon can be accounted for by the greater surface area to volume ratio, which leads to the increased binding surface area of nanoparticles (Skandalis et al., 2017). Multiple research have shown a positive correlation between the size of nanoparticles and their effectiveness in penetrating and eliminating germs (Bruna et al., 2021; Ong& Nyam, 2022).
The current investigation aligns with earlier research on the possible applications of green algae. A. platensis for the synthesis of variously sized and shaped AgNPs. As, Doshi et al. (2007) synthesized spherical AgNPs with sizes ranging from 2 to 8 nm using the algae A. platensis. In a related study, Mahdia et al. (2012) reported employing A. plantesis to synthesize silver nanoparticles, with an average size of approximately 12 nm. Fatima et al. (2020), revealed the possibility of synthesizing silver nanoparticles utilizing Portieriahor nemanii, which have a spherical shape and an average size from 70 to 75 nm.
Zeta potential is a surface charge potential measurement that can be utilized to assess the stability of a nanoparticle in aqueous solutions (Karmakar, 2019). The current study's value was −20 ± 3.2 mV, indicating that the produced nanoparticles were negatively charged in a dispersed medium, causing the particles to repel one another, preventing agglomeration (Chen et al. 2016). The results were similar to the study by Nagar and Devra (2019), which revealed that the zeta potential was –22.4 mV.
3.3 Antimicrobial activity of Ag NPs
The results of a recent study show, that Ag NPs made with A. platensis extract have good antibacterial qualities against pathogenic strains of both Gram-positive and Gram-negative bacteria, including P. aeruginosa,P. mirabilis, and S. aureus and E. faecalis, Fig. 3-a, b, this may be because AgNPs have multiple, simultaneous mechanisms of action, and in incorporation with antibacterial agents like organic compounds or antibiotics, they have demonstrated synergistic impacts against pathogenic bacteria (Bruna et al., 2021).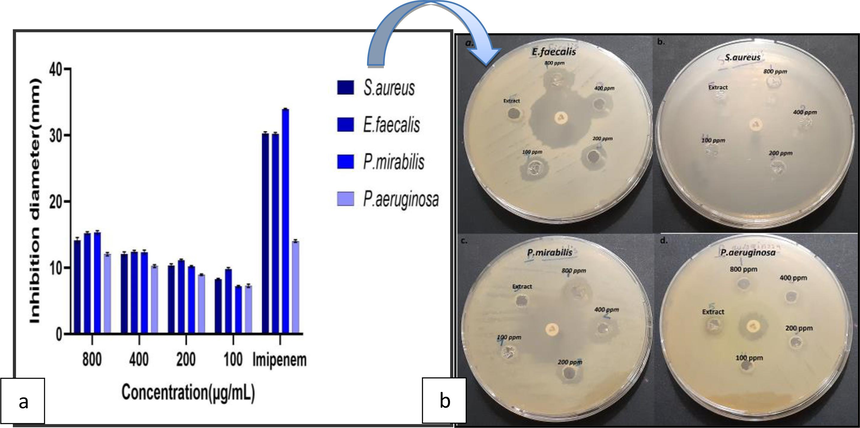
(a). Petri dishes showing the zone of inhibition of AgNPs on S. aureus, E. faecalis, P. mirabilis, and Pseudomonas aeruginosa. (b) Antibacterial activity of the AgNPs.
The findings of this investigation align with several studies that have validated the efficacy of AgNPs derived from algae as an inhibitor of bacteria, both gram-positive and gram-negative, such as studies conducted by(Mukundan et al., 2017; Bamal et al., 2021; Bruna et al., 2021; Dhaka et al., 20230). The study currently uses concentrations different (100, 200, 400, and 800 μg/ml) of AgNPs from A.platensis. The findings indicated that the maximum inhibition zone for E. faecalis measured 16 ± 0.2 mm at an 800 μg/ml concentration, while the minimum inhibition zone for P. mirabilis measured 7 ± 0.1 mm at a 100 μg/ml concentration (Table 1), because AgNPs able to penetrate bacterial cell walls, alter the makeup of cell membranes, and lead to cell death (Xu et al., 2020). The susceptibility of the bacteria used in this investigation may be the cause of the variance in inhibition rate. It is consistent with many studies that confirm the effective role of AgNPs as antibacterial agents. For example, Sinha et al. (2015) reported that AgNP extract from the algae Pithophora oedogonia has good antibacterial activity against a wide range of bacteria such as Shigella flexneri, Micrococcus luteus, E. coli, B. subtilis, S. aureus and Vibrio cholerae, and the largest zone of inhibition was seen in P. aeruginosa was (17.2 mm). According to Aboelfetoh et al. (2017), AgNPs synthesized from the alga Caulerpa serrulata had potent antimicrobial activities, exhibiting a minimal inhibition area of 10 mm at 50 μL against S. typhi and a substantial inhibition area of 21 mm at 75 μL against E. coli.
Bacterial strains
Inhibition diameter (mm)
800(µg/ml)
400 (µg/ml)
200(µg/ml)
100(µg/ml)
Impanel
S.aureus
14 ± 0.4
12 ± 0.3
10 ± 0.2
8.3 ± 0.1
30 ± 0.25
E.faecalis
16 ± 0.2
12 ± 0.2
11 ± 0.1
10 ± 0.2
30 ± 0.1
P.mirabilis
15 ± 0.3
12 ± 0.3
10 ± .0.1
7 ± 0.1
33 ± 0.1
P.aeruginosa
12 ± 0.2
10 ± 0.2
8.9 ± 0.1
7 ± 0.3
14 ± 0.1
Current findings indicate that AgNPs had a good inhibitory impact on bacterial growth described above. This effect was attributable to the interaction between silver ions and cellular components of bacteria, thus inhibiting bacterial growth. (More et al., 2023). AgNP penetration into a cell can cause increased oxidative stress, modify signal transduction pathways, and alter ATP production (Mohammad et al., 2022), on other hand; among all the geometric shapes, spheres have the least amount of surface area. As is the natural propensity of materials, spherical shapes ensure the lowest possible level of energy in existence (Arif & Uddin, 2021). Some scientists have suggested that silver nanoparticles can attach themselves to the bacterial cell wall, based on electrostatic interactions between positively charged silver ions and the negatively charged cell membrane surface, which includes amino, phosphate, and carboxyl groups, this interaction may allow the nanoparticles to penetrate the cell membrane, leading to structural alterations and increased permeability, and this causes the breakdown of the membrane, additionally AgNPs have been shown in numerous studies to possess antibacterial properties against a range of microorganisms resistant to antibiotics (Joshi et al., 2020; Jin et al., 2023).
Soleimani and Habibi Berkohi (2017) in their study on the effect of AgNPs isolated from Chlorella vulgaris algae, have found that these nanoparticles have a good inhibitory effect on Staphylococcus aureus. In a study, Kumar et al. (2023) used the alga Chlorella minutissima to generate AgNPs and found that they inhibited a variety of Gram-positive and Gram-negative bacteria as E. faecalis and P. mirabilis, S. aureus and P. aeruginosa, it has been isolated from urinary tract infections. While the study (Gorman & Golovanov, 2022), found that AgNPs were more efficient in inhibiting Gram-positive bacteria than Gram-negative bacteria, attributed to differences in the peptidoglycan and LPS layer compositions in the cell walls between the two bacterial species. This finding contradicts the research conducted by Meikle et al. (2020). Table 2 presents the minimal inhibitory concentration (MIC) for the AgNPs, the MIC values for E.feacalis, S.aureus, P.mirabilis, and P.aeruginosa are 100, 200, 100, 200 μg/mL respectively. The minimum bactericidal concentration (MBC) for each of the indicated bacteria was determined was found to be 200, 200, 100 and 400 μg/ml. The findings of a recent study suggest that AgNPs exhibit high efficacy in suppressing the proliferation of urinary tract infections. Nevertheless, additional examination of their dimensions and utilization of different bacteria is necessary to achieve optimal outcomes.
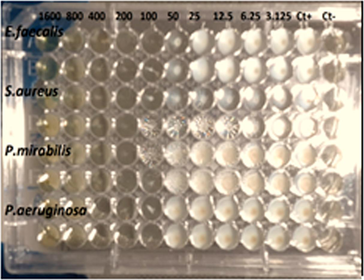
Bacterial strains
E.faecalis
S.aureus
P.mirabilis
P.aeruginosa
Minimum inhibition concentration (µg/Ml)
100
200
100
200
Minimum bacterial concentration (µg/Ml)
200
400
200
400
4 Conclusion
The algae A. platensis has been utilized in this study to biosynthesize silver nanoparticles, and the resulting AgNPs showed good efficacy in inhibiting pathogenic bacteria, including P. mirabilis, S. aureus, P. aeruginosa and E. faecalis. In general, these results indicate the possibility of applying this type of biosynthetic nanomaterial as an antimicrobial agent to prevent urinary tract infections. It represents a promising step towards realizing the production of AgNPs-based antimicrobial drugs. Further research can be directed toward improving manufacturing conditions, understanding the mechanisms of AgNPs synthesis, and controlling nanoparticle size. In conclusion, algal extract is considered a sustainable, efficient, and environmentally friendly method for the biosynthesis of AgNPs, due to its high metal accumulation capacity, rapid growth rate, and abundant organic content, including carbohydrates.
CRediT authorship contribution statement
Zahraa H. Obaid: Writing – original draft, Software, Resources, Methodology, Formal analysis, Data curation. Sarab A. Juda: Writing – original draft, Resources, Methodology, Formal analysis, Data curation. Ashwak F. Kaizal: Methodology, Formal analysis, Data curation. Jasim Mohammed Salman: Writing – review & editing, Writing – original draft, Supervision, Project administration, Investigation.
Acknowledgements
The researchers extend their thanks and appreciation to ERSC, University of Babylon, for their support.
Author Contributions
The authors collaborated to finish this project. Z.H.O. wrote the text and the explanation of the findings, while S. A. J. conducted the experiments and sample collection. J. M. S. wrote, edited, and rewrote the draft after reviewing the material and making necessary revisions. The final paper was approved by all authors.
Funding for the study
This study did not receive any specific grants from funding agencies public.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Eco-friendly synthesis of silver nanoparticles using green algae (Caulerpa serrulata). Reaction optimization, catalytic and antibacterial activities. Environ. Monit. Assess.. 2017;189(7)
- [CrossRef] [Google Scholar]
- Synergistic antibacterial potential of greenly synthesized silver nanoparticles with fosfomycin against some nosocomial bacterial pathogens. Infection and DrugResistance. 2023;125–142
- [CrossRef] [Google Scholar]
- Silver nanoparticles biosynthesis, characterization, antimicrobial activities, applications, cytotoxicity and safety issues: An updated review. Nanomaterials. 2021;11(8):208.
- [CrossRef] [Google Scholar]
- Silver nanoparticles and their antibacterial applications. Int. J. Mol. Sci.. 2021;22(13):7202.
- [CrossRef] [Google Scholar]
- Sustainable phyco-fabrication of silver nanoparticles using Coelastrellaterrestris and their multiple downstream applications. Biocatalysis and Agricultural Biotechnology.. 2023;53:102854
- [CrossRef] [Google Scholar]
- A review on biological synthesis of Silver nanoparticles and their potential applications. Results in Chemistry.. 2023;101108
- [CrossRef] [Google Scholar]
- Bioremediation potential of live and dead Spirulina: spectroscopic, kinetics and SEM studies. Biotechnol. Bioeng.. 2007;96(6):1051-1063.
- [CrossRef] [Google Scholar]
- Assessment of phytochemical components, proximate composition and antioxidant properties of Scenedesmus obliquus, Chlorella vulgaris and Spirulina platensis algae extracts. Egyptian Journal of Aquatic Biology & Fisheries.. 2019;23(4):521-526.
- [CrossRef] [Google Scholar]
- Biosynthesis of silver nanoparticles using red algae Portieriahornemannii and its antibacterial activity against fish pathogens. Microb. Pathog.. 2020;138:103780
- [CrossRef] [Google Scholar]
- Lipopolysaccharide structure and the phenomenon of low endotoxin recovery. Eur. J. Pharm. Biopharm. 2022
- [CrossRef] [Google Scholar]
- Green synthesis of silver nanoparticles using Teucriumpolium leaf extract and assessment of their antitumor effects against MNK45 human gastric cancer cell line. J. Mol. Struct.. 2020;1208:127889
- [CrossRef] [Google Scholar]
- Hodoroaba, V. D., Unger, W., Shard, A. (Eds.). ,2019. Characterization of nanoparticles: measurement processes for nanoparticles. Elsevier.eBook ISBN: 9780128141830.
- Altering sliver nanoparticles-induced inhibition to bacterial denitrification via visible light by regulating silver transformation and adaptive mechanism under anaerobic conditions. Chem. Eng. J.. 2023;452:139268
- [CrossRef] [Google Scholar]
- Interactions of gold and silver nanoparticles with bacterial biofilms: Molecular interactions behind inhibition and resistance. Int. J. Mol. Sci.. 2020;21(20):7658.
- [CrossRef] [Google Scholar]
- Particle size distribution and zeta potential based on dynamic light scattering: Techniques to characterize stability and surface charge distribution of charged colloids. In: In Book: Recent Trends in Materials Physics and Chemistry. Publisher: Stadium Press(India) Pvt Ltd.; 2019. p. :117-159.
- [Google Scholar]
- Nanoparticles: Properties, applications and toxicities. Arabian Journal of. 2019;chemistry.12(7),908–931
- [CrossRef] [Google Scholar]
- Chlorella minutissima-assisted silver nanoparticles synthesis and evaluation of its antibacterial activity. Systems Microbiology and Biomanufacturing.. 2023;1–10
- [CrossRef] [Google Scholar]
- Preparation, characterization, and antimicrobial activity of cubosome encapsulated metal nanocrystals. ACS Applied Materials &. 2020;interfaces.12(6):6944-6954.
- [CrossRef] [Google Scholar]
- Effect of Size, Shape and Surface Functionalization on the Antibacterial Activity of Silver Nanoparticles. Journal of Functional Biomaterials.. 2023;14(5):244.
- [CrossRef] [Google Scholar]
- High diversity of microalgae as a tool for the synthesis of different silver nanoparticles: A species-specific green synthesis. Colloid Interface Sci. Commun.. 2021;42:100420
- [CrossRef] [Google Scholar]
- Silver nanoparticles: bactericidal and mechanistic approach against drug resistant pathogens. Microorganisms.. 2023;11(2):369.
- [CrossRef] [Google Scholar]
- A review of green synthesis of metal nanoparticles using algae. Front. Microbiol.. 2021;12:693899
- [CrossRef] [Google Scholar]
- Comparative study of synthesized silver and gold nanoparticles using leaves extract of Bauhinia tomentosa Linn and their anticancer efficacy. Bull. Mater. Sci.. 2017;40:335-344.
- [CrossRef] [Google Scholar]
- A kinetic study on the degradation and biodegradability of silver nanoparticles catalyzed Methyl Orange and textile effluents. Heliyon.. 2019;5(3).DOI
- [CrossRef] [Google Scholar]
- Review on Toxicity and Removal of Pharmaceutical Pollutants Using Immobilised Microalgae. Ecological Engineering & Environmental Technology (EEET). 2023;24(6)
- [CrossRef] [Google Scholar]
- A green approach for the synthesis of silver nanoparticles by Chlorella vulgaris and its application in photocatalytic dye degradation activity. Environ. Technol. Innov.. 2021;21:101282
- [CrossRef] [Google Scholar]
- Antibacterial activity of silver nanoparticles biosynthesized using Spirulina platensis microalgae extract against oral pathogens. Egyptian Journal of Aquatic Biology and Fisheries.. 2019;23(5):261.
- [CrossRef] [Google Scholar]
- Size-and shape-dependent antibacterial studies of silver nanoparticles synthesized by wet chemical routes. Nanomaterials. 2016;6(4):74.
- [CrossRef] [Google Scholar]
- Salman, J.M., Kaduem, N.F., Juda, S.A.,2022. Algal immobilization as a green technology for domestic wastewater treatment. IOP Conference Series: Earth and Environmental Science, 2022, 1088(1), 012005. 10.1088/1755-1315/1088/1/012005.
- Cultivation of blue green algae (Arthrospira platensis Gomont, 1892) in wastewater for biodiesel production. Chemosphere. 2023;139107
- [CrossRef] [Google Scholar]
- Green synthesis of silver nanoparticles using fresh water green alga Pithophoraoedogonia (Mont.) Wittrock and evaluation of their antibacterial activity. Appl. Nanosci.. 2015;5:703-709.
- [CrossRef] [Google Scholar]
- The effect of silver nanoparticles size, produced using plant extract from Arbutus unedo, on their antibacterial efficacy. Nanomaterials. 2017;10(7):7.
- [CrossRef] [Google Scholar]
- Silver nanoparticles: Synthesis, medical applications and biosafety. Theranostics.. 2020;10(20):8996.
- [CrossRef] [Google Scholar]
- Biosynthesis of silver nanoparticles by bamboo leaves extract and their antimicrobial activity. J Fiber Bioeng Inform.. 2013;6(6):77-84.
- [CrossRef] [Google Scholar]
- Green synthesis of nanoparticles: Current developments and limitations. Environ. Technol. Innov.. 2022;26:102336
- [CrossRef] [Google Scholar]







