Translate this page into:
Biosynthesis of gold nanoparticles using marine microbe (Vibrio alginolyticus) and its anticancer and antioxidant analysis
⁎Corresponding authors. ssrajeshkumar@hotmail.com (Rajeshkumar Shunmugam), harijai2004@gmail.com (Haribalan Perumalsamy)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
The applications of green synthesised gold nanoparticles for the biomedical field are the most prominent and developing area in this modern era. In this present investigation, marine bacteria, Vibrio alginolyticus was used for the biosynthesis of gold nanoparticles. The biosynthesized gold nanoparticles (AuNps) were characterized by various spectroscopic analysis. The morphological analysis of AuNps was done through scanning electron microscope (SEM) and transmission electron microscope (TEM) respectively. The obtained size was estimated around 100–150 nm. The synthesized AuNps confirmed by its surface plasmon resonance peak (SPR) range using UV–Vis spectrophotometer. The bio-metabolites from marine bacteria which was responsible for the nanoparticles synthesis were analyzed using fourier transform infrared spectrophotometer (FT-IR). The biomedical application of synthesized eco-friendly AuNps analysed by different method such as, the antioxidant property and free radical scavenging activity through DPPH (1, 1- diphenyl 2-picrylhyorazyl) and metal chelating assay. The anticancer activity of colon carcinoma was confirmed through MTT assay while the apoptotic mediated cell death observed through the fluorescence analysis. The biosynthesized AuNPs showed a dose dependent inhibitory effect on the growth of colon cancer cells. The IC50 was 15 µg/ mL, and the maximum inhibition of the cell death was (>75%) obtained when treating 25 µg/mL. The apoptotic mediated cell death observed through nuclear condensation of the treated cells. The green synthesis of AuNPs emphases - to be cost effective and environmental friendly with anti-inflammatory and anti-cancer effects against colon cancer.
Keywords
Marine bacteria
Antioxidant
DPPH
Metal chelating assay
Anti-cancer activities
Colon carcinoma cell lines
1 Introduction
Cancer is a pathological condition associated with an abnormal growth of cells or tissues. Cancer related mortality is the major problem across the globe, as we do not have effective drugs or procedure available for its treatment, especially in case of malignant cancer. Among the list of cancers present today, colon cancer secures the third position causing maximum deaths in the world (Sauraj Kumar et al., 2017). The traditional treatment of the disease is by surgery, chemotherapy, radiation and others, but all have been proved to be toxic to the humans (Rajeshkumar et al., 2016). These chemotherapeutic agents have shorter half- life and meagre solubility in the aqueous medium, due to which there was a poor therapeutic efficiency. Therefore, new strategies had to be employed for the improvement of these drugs against cancer (Xia et al., 2015). The accumulation of oxidative stress in our body, which leads to various diseases in our body. The reactive oxygen species (ROS) free radicals and nitic oxide through inflammatory action cause various health issues in our body due to body normal metabolic acidity.
Nowadays, researchers are working on nano-drugs, as they provide a platform for effective delivery of drug molecules due to their pharmacokinetics and biodistribution behaviours. The synthesis of nano-sized drugs through conventional approaches like physical and chemical methods has proved to be hazardous to the atmosphere because of the usage of toxic chemicals, or usage of extreme temperature or pressure conditions. The desire to accomplish a non-toxic, eco-friendly method for the nanoparticle synthesis has been increased, researchers focus more on biological approaches which includes plant and its parts, microorganisms like bacteria, algae, fungi and etc (Rajeshkumar et al., 2016; Krishnaraj et al., 2014; Yan et al., 2015; Wei et al., 2013; Fayaz et al., 2011; Sarkar et al., 2012).
The nanoparticle synthesis using microorganisms has been widely preferred due to the large- scale production, its ease of handling, low cost and its availability in the atmosphere. The microorganism mediated nanoparticle synthesis can be done, both by extracellular or intracellular systems (Kumar et al., 2014; Malarkodi et al., 2013; Rajeshkumar et al., 2019). The gold nanoparticles is the most widely used metal nanoparticle because of their morphology, dimensions (Suganya et al., 2015) or various shapes like spheres, triangles, rods, flats sheets, icosahedrons, nodous ribbons and so on. Shapes play a very important role in the various applications associated with it like the optical coating or as conductive tips in scanning tunnelling microscopes (Du and Wang, 2007). It can also be used as substrates for catalysis reactions (Cai et al., 2011), quantitative or qualitative analysis and also different bio-applications due to their excellent optical, electronic properties, high biocompatibility and stability (Wei et al., 2013; Huang and El-Sayed, 2010). Gold nanoparticles conjugated with antibiotics, can improve the antibacterial or antimicrobial effectiveness by many folds. Biosynthesis/biogenic method serves as a better substitute to chemical and physical methods. Biological or green synthesis method assists as an enhanced source towards chemical and physical methods (Aisida et al., 2019). The biosynthesis process involved the use of natural materials such as an extract from a plant or plant secondary metabolites to serve as a potential stabilizing and reducing agent. There are numerous techniques have been approached by many researches through physical and chemical methods. However biologically synthesized methods, such as microbial synthesis is an alternative method for biocompatibility, facile, shape, size and easy-to-scale nanostructures are expedient (Aisida et al., 2019).
Bacterial mediated synthesis of gold nanoparticles has been propagated in marine bacteria including Staphylococcus lentus (Baker and Satish, 2015) Pseudomonas veronii (Apte et al., 2016) Magnetospirillum gryphiswaldense (Cai et al., 2011) Escherichia coli (Postle, 1990) Geobacillus stearothermophilus (Fayaz et al., 2011) Rhodopseudomonas capsulate (He et al., 2007) Pseudomonas aeruginosa (Moon et al., 2008) Bacillus stearothermophilus (Luo et al., 2014). Vibrio alginolyticus is mostly found in marine estuaries, coastline and aquatic environments with universal spreading. It may exist as free-living, a parasite or associated with surfaces of organisms such as marine vertebrates/invertebrates and flora, and even humans. This species is also highly abundant and usually dominates Vibrio communities (Schets et al., 2011; Narracci et al., 2014).
In present study, the green synthesized gold nanoparticles with the assistance of marine bacterium Vibrio alginolyticus, and was characterized using various spectroscopic analysis such as, SEM, TEM and FT-IR; the synthesized AuNps was showed significant antioxidant through DPPH analysis and anticancer properties towards human colon cancer cell lines (HCA-7). Further, the synthesized AuNps initiated apoptotic-mediated cell-death on cancer cell through nuclear condensation. The biosynthesized metal nanoparticles by bacterial microorganisms provides a promising method for large-scale production with anti- inflammatory and anti-cancer effects towards colon cancer.
2 Materials and methods
2.1 Materials
The chemicals like HAuCl, DPPH, FeSO4, 2, 2′-bipyridyl, Tris- HCl buffer (pH 7.4), hydroxyl amine was bought from Sigma Aldrich, and the media required for cell culture maintenance was bought from Hi-media Pvt. Ltd. All other chemicals and reagents were used for this study are available commercially with grade quality.
2.2 Bacterial strain isolation and identification
The marine microbial bacterial strain, Vibrio alginolyticus was collected 10 feet depth of Bay of Bengal sea at marina beach, Chennai Tamil Nadu, India. The latitude and longitude is 13.0500° N, 80.2824° E. The Zobell marine broth medium was used for initial isolation directly form marine samples. Later we have used transferred in to nutrient agar medium (since it has various growth ingredients as per many researchers previously reported) for better growth. Also for synthesis of gold nanoparticles, the nutrient agar cultured bacteria were used for better synthesis. The strain was identified using biochemical kit purchased from Himedia, Mumbai, India.
2.3 Cell culture
The human colon cancer cell lines (HAC-7) was used for this study. The cell lines were cultured with DMEM containing 10% FBS and 1% antibiotic–antimycotic solution under 5% CO2 at 37 °C.
2.4 Extracellular biosynthesis of gold nanoparticle
For the synthesis of AuNps from Vibrio alginolyticus, was collected from sea shores nears Chennai, Tamiladu and cultivated in the laboratory conditions. The culture medium was maintained at a pH of 7 or above in aerobic conditions. A loop full of freshly prepared well grown Vibrio alginolyticus culture was inoculated to 100 mL of nutrient broth and was incubated at 40 °C for 24 h in an orbital shaker maintained at 120 rpm (Sabir et al., 2013). After the incubation period, the culture medium was centrifuged at 8000 rpm for 15 min and the supernatant was collected. Now, a precursor of 1 mM aqueous chloroauric acid (HAuCl) was added to this supernatant and was again incubated in shaker in same conditions for the nanoparticle synthesis. The nanoparticle biosynthesis using microbial sources depends upon the secondary metabolites excreted by the microorganisms, which is present on the cell wall, and is involved in the reduction of the metal ions. All the samples were prepared in triplicates, and were further used for other proceedings and the samples were lyophilized and submitted in powder form for further analysis.
2.5 Characterization studies
The synthesized AuNps was characterized for their optical wavelength properties using UV– visible spectrophotometer and the maximum absorption peak was scanned in the range from 300 to 600 nm. The morphological characterization of synthesized AuNps such as size and shape of the AuNps was analyzed using SEM and TEM and the functional groups which are adsorbed on the gold nanoparticles were identified through Fourier transform infra-red spectrophotometer (FTIR).
2.6 Antioxidant activity
The antioxidant activity was assessed using DPPH (1, 1- diphenyl 2-picrylhyorazyl) assay, to determine free radical scavenging activity. 1 mL of 0.1 mM DPPH in methanol solution was mixed with 1 mL of AuNps solution at varying concentrations (10 µg/ mL to 60 µg/ mL). The solution was incubated for 30 min in dark place at room temperature. Ascorbic acid was used as standard (10–60 µg/mL). The activity was measured by confirmation of the violet colour solution turned pale yellow after addition of antioxidant agent. The decrease in absorbance was measured at 517 nm. The inhibition % was calculated with the help of the formula as shown in the eq. (1) (Bhuiyan et al., 2009; Sahu et al., 2013).
2.7 Metal chelating activity
The ferrous ion chelating property was measured for AuNps which was synthesised by marine bacteria Vibrio sp. 1 mL FeSO4 was mixed with synthesized the AuNPs at different concentrations (20 µg/ mL to 120 µg/ mL). 1 mL of 2, 2′-bipyridyl solution mixed with Tris- HCl buffer (pH 7.4) and finally added to the mixture containing hydroxyl amine – HCl and ethanol, maintained at Room Temperature for 10 min. The mixture was adjusted to a final volume of 5 mL. The absorbance of the solution mixture was measured at wavelength of 522 nm. The metal ion chelating activity was measured using the formula as shown in the eq.(2) (Mohan et al., 2012)
here,
A0 = absorbance of control A1 = absorbance of sample
2.8 Cell viability assay
The cytotoxicity of AuNPs was assessed towards human colon carcinoma cell line (HCA-7) through of Methyl Thiazolyl Tetrazolium (MTT) assay. The 2 × 104 cells per well were seeded in a 100 µL medium in 96- well plates and were incubated overnight. After the incubation, the cells were treated with different concentrations of AuNPs (5–25 µg) and incubated for next 24 h. The plate was washed with PBS and add 100 μL freshly prepared MTT solution (5 mg/mL) with medium into 96- well and incubated for 3–5 h. After removal of MTT solution add 200 μL of DMSO solution to all the well and the absorbance was measure at 570 nm. The experiment was performed and repeated in triplicates. The calculation was followed
The value of IC50 was calculated by plotting a graph of concentration of AuNPs with standard.
2.9 Apoptotic cell detection by Hoechst staining
Hoechst staining analysis was performed to detect apoptotic mediated cell death. The cells 5 × 104/ well of HCA-7 cells were seeded and apoptosis induction was measured. AuNps was prepared from two different concentrations (15 and 25 μg/mL) based on MTT analysis. Control wells were treated only 0.1% DMSO. Fluorescent image of HCA-7 cells which was involved in apoptotic cell death analysis was identified by Leica DMLB fluorescence microscope (Wetzlar, Germany).
3 Results and discussion
3.1 Visual observation
On the addition of aqueous chloroauric acid (HAuCl) to the bacterial culture solution, there was a change in the colour of the mixture which indicates the synthesis of AuNps. The color change was observed when the culture was incubated for 24 h. The successful conversion to gold nanoparticles by marine bacteria from light to dark yellow to final reddish-brown solution as shown in (Fig. 1A-C) is an indication of the formation of AuNps. Further confirmation of the size, shape being characterized with the help of electron microscopy of SEM and TEM imaging. Similar results were observed with Stenotrophomonas maltophilia, (Nangia et al., 2009), Streptomyces fulvissimus (Soltani Nejad et al., 2015) and Pseudomonas aeruginosa (Husseiny et al., 2007).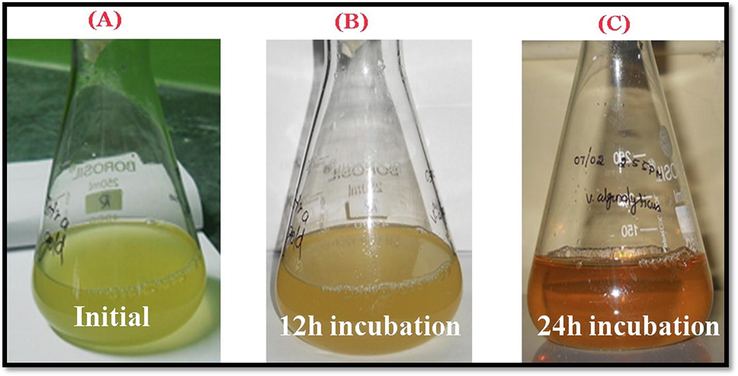
Visual observation of AuNPs synthesis using marine bacteria Vibrio alginolyticus(A) initial observation after incubation of culture with 1 mM of gold chloride (B) 12 h incubation and (C) visually observed brownish red colourization at 24 h incubation.
3.2 UV–Vis analysis
The high intensity surface plasmon resonance peaks by optical absorption spectra exhibited in the UV–Vis region by metallic nanoparticles is due to the band of electrons oscillating around the nanoparticle surface. Absorption of light on the surface of nanoparticles by the band of electrons vibrating at characteristic modes is known as the SPR phenomenon (Soltani Nejad et al., 2015). The SPR band was observed at 24 h at a wavelength of 530 nm, which indicates the Vibrio sp. synthesizing gold nanoparticles after the 24 h incubation (Fig. 2). This is a slow and steady process that may have a superior quality of stability. The bacteria may contain enzyme or biochemical which is responsible for stabilizing or reducing properties so, without any addition of external reducing or stabilization agents, the synthesis of gold nanoparticles is possible. When compared with soil bacteria the marine microbes were taking more time to synthesize the gold nanoparticles (Baker and Satish, 2015). Similar results were observed at a peak of 520–550 nm (Baker and Satish, 2015; Nangia et al., 2009; Soltani Nejad et al., 2015; Srinath and Ravishankar Rai, 2015).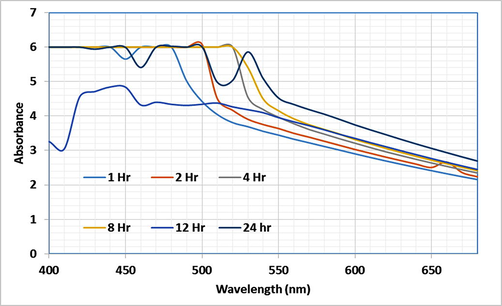
UV spectrum of the Vibrio sp. synthesized AuNPs at an incubation period at 24 h.
3.3 Electron Microscopic analysis (TEM)
The TEM analysis helped for the identification of the morphology of AuNps such as, size and shape of AuNPs synthesized by marine bacteria. The samples were prepared on copper grid which was carbon coated for SEM analysis and the yield of nanoparticles in given sample was analysed to be 1,000 nanoparticles per sample (Fig. 3A). The SEM images revealed that the presence of nanoparticles with irregular shape, while the TEM images indicated the synthesis of monodispersed, irregular shaped gold nanoparticles with an average size of nearly 50–100 nm (Fig. 3B). In these study, Both SEM and TEM images clearly indicated shape and size of the nanoparticle were almost similar with previous study characterization (Fayaz et al., 2011; Honary et al., 2012).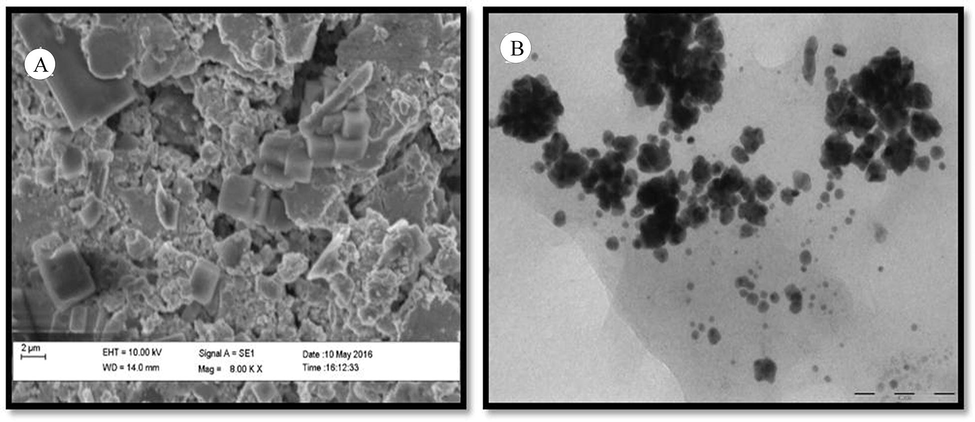
Electron microscopic observations. (A) SEM and (B) TEM of AuNPs synthesized using Vibrio sp.
3.4 FTIR analysis
The FTIR results were compared against biosynthesized AuNps and the marine Vibrio sp. Extract as represented in Fig. 4. The peaks were almost same when both were compared with each other. The FTIR measures the biomolecules responsible for gold nanoparticles synthesis. The different vibrational streches corresponds to following peaks like:1644 cm−1 for alkenes (strong), 1399 cm−1 for CH3 bend, 3542 cm−1 for alcohol or carboxylic acid (OH stretch), 2926 cm−1 for C–H stretch, 1066 cm−1 for C-F bonds and 557 cm−1 for C-Br (Fig. 4). These functional groups helped in the reducing or stabilizing of the formulated gold nanoparticles, which are produced by the bacteria in the form of enzymes or proteins.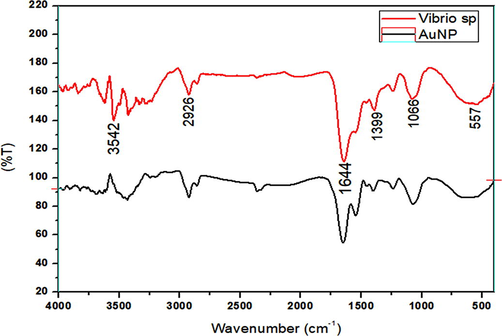
FT-IR spectrum of AuNPs synthesized using the marine bacteria and Vibrio sp.
3.5 Antioxidant activity of gold nanoparticles
The DPPH (2,2-diphenyl-1-picryl-hydrazyl-hydrate) assay with reagent produces violet solution when assorted with ethanol solvent due to transfer of free electrons. When an antioxidant agent reacts with DPPH solution, the violet changes to a colourless solution, proving that the free radicals have been taken up by the antioxidant agent which has the hydrogen donating ability. The assay is an easy and swift way to calculate the antioxidants produced, by reading the absorbance with the help of spectrophotometry [33]. Fig. 5A depicts antioxidant activity by the AuNPs against the standard (ascorbic acid). The maximum antioxidant activity was at a concentration of 10 µg/mL and the activity decreased with the increase in concentration of the antioxidant agent and the % inhibition was calculated as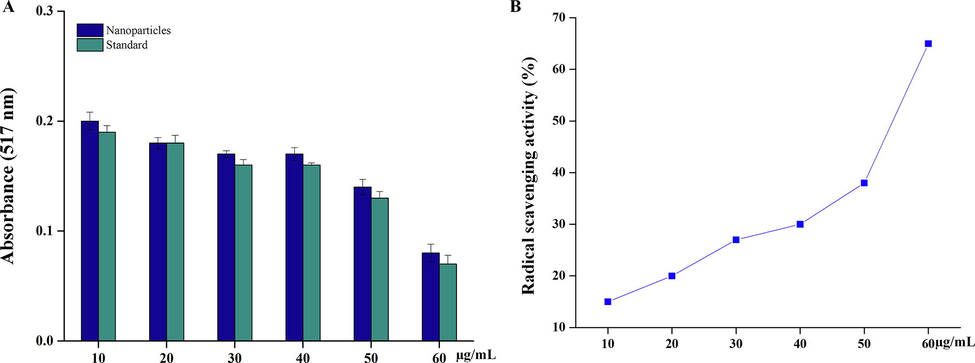
Antioxidant activity of the synthesised AuNPs DPPH assay. (A) Absorption of AuNPs activity. (B). % of radical scavenging activity by the treatment of AuNPs.
3.6 Metal ion chelating assay
Transition metal ions, especially iron can stimulate lipid per oxidation by Fenton reaction (H2O2 + Fe2+ - --> Fe3 + OH– +OH–) and can also accelerate lipid per oxidation by decomposing lipid hydroperoxides into peroxyl and alkoxyl radicals that can perpetuate the chain reaction (Fig. 6A). According to the graph, Fig. 6B, there was an increase in the metal chelating activity 22.06% by the treatment of AuNps.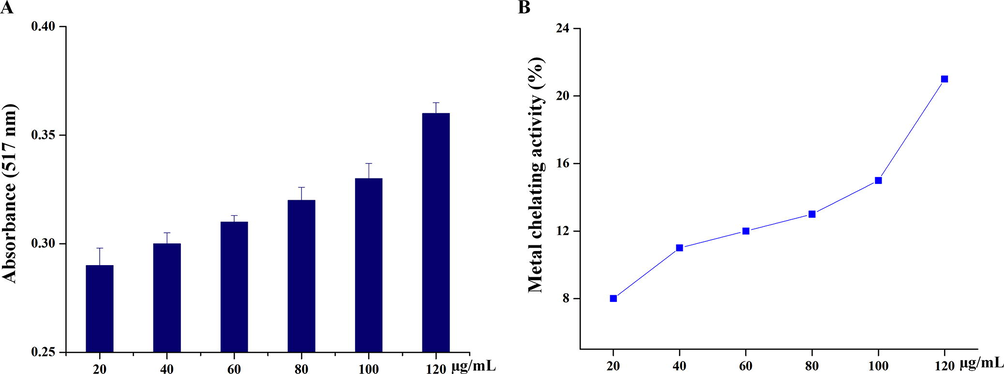
Metal chelating assay. (A) Absorption of AuNPs activity. (B) % of metal chelating activity by the treatment of AuNPs.
3.7 Cytotoxicity assay of the biosynthesised AuNPs
In the present study, the anticancer activity of gold nanoparticles was evaluated by a dose dependant inhibition activity against colon cancer cell lines. There are many drugs which are toxic to the body with side effects. Therefore, an alternate green synthesized AuNps was developed as a chemotherapeutic agent. The IC50 value was 15 µg/mL observed, when compared with the standard (Fig. 7A). The morphological observation of HCA-7 cell line showed cytotoxicity effect by the treatment of AuNps on dose-dependent manner. Untreated HCA-7 cell line showed very clear cell morphology with undisturbed cell organelles (Fig. 7B). Whereas AuNps treated with dose-dependents were showed significant cell damage with undistinguished cell debris, which indicate that biosynthesized AuNps showed very significant effects towards human colon cancer cells.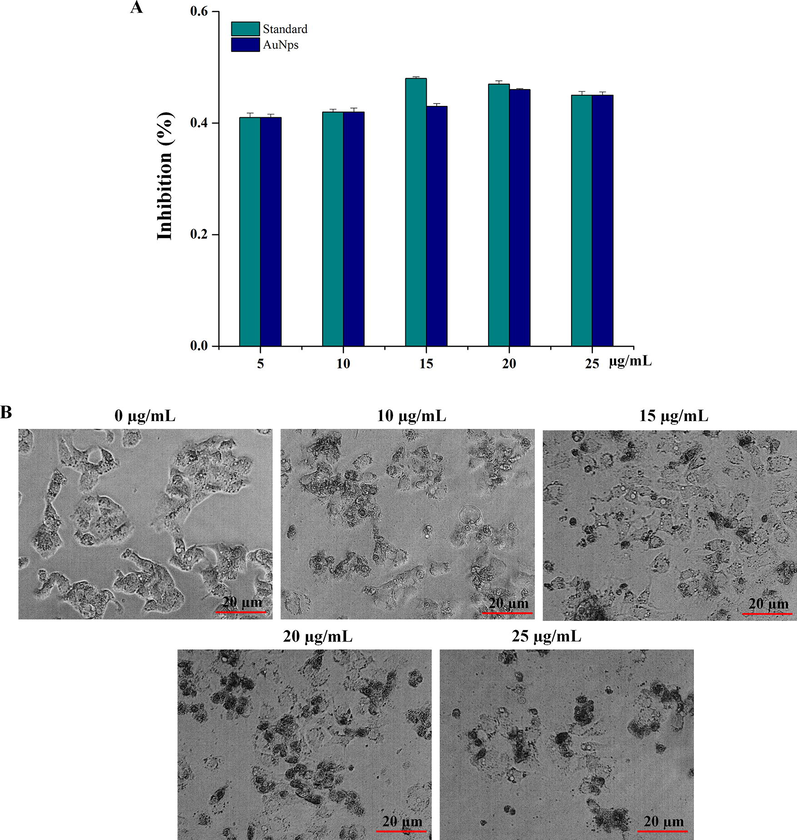
Antiproliferative activity of AuNPs against colon cancer cell lines. (A) Cytotoxicity observation of AuNps. (B) Morphological observation of HCA-7 cell line by the treatment of AuNPs.
3.8 Apoptotic cell death detection
The apoptotic cell death of detection on HCA-7 cell line was observed by Hoechst staining analysis. AuNps treated (15 and 25 µg/ml) with HCA-7 cell line showed the prominent nucleus with fluorescence accretion through damaged of cell wall after the treatment. Whereas untreated cell was less stained and its indicated that no cell wall was damaged (Fig. 8). The mechanism behind anticancer effect of AuNPs was proposed as, AuNPs might have entered in to the cytoplasm through damaged cell membranes and induced apoptotic mediated cell death via the intracellular proteins or enzymes (Rajeshkumar et al., 2016; Krishnaraj et al., 2014; Sathyabama and Sankaranarayanan, 2015). Similar results were observed, once silver nanoparticles were treated against the lung and liver cancer (Du and Wang, 2007), but the IC50 values were better when compared with AuNPs synthesized using leaf extracts of Bauhinia tomentosa (Mukundan et al., 2015).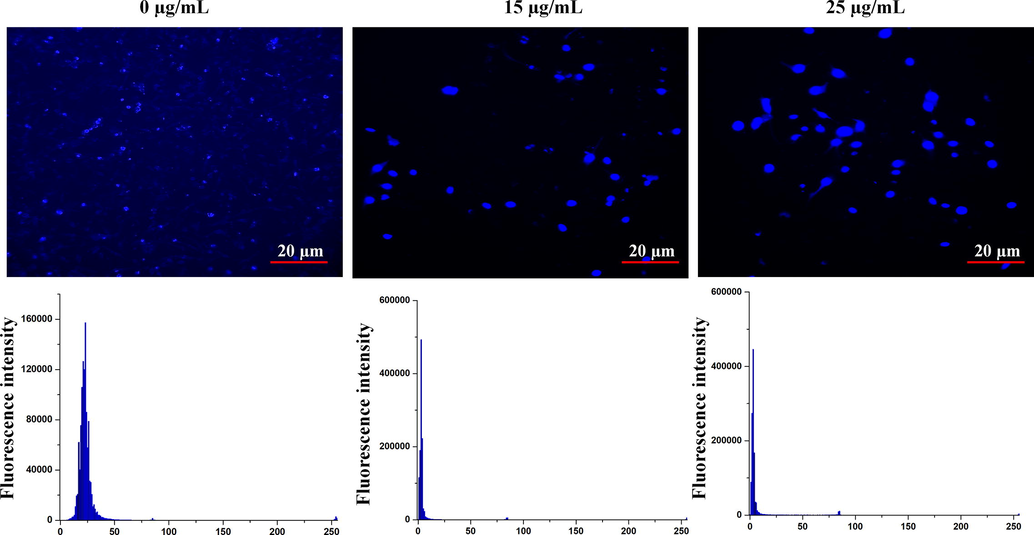
Apoptotic cell death detection by Hoechst staining
4 Conclusion
The microbe mediated synthesis of gold nanoparticles is comparatively a non-toxic method due to non-addition of chemicals externally, the process can be easy for scale-up and is economically stable. The bacteria produce proteins or enzymes once they are in anxious circumstances which helps to reduce the toxic chemical such as, gold chloride in gold nanoparticles. In the present investigation, a reddish-brown solution was produced at a maximum absorbance peak of 530 nm. The electron microscope of SEM and TEM analysis proved the synthesis of irregular shaped gold nanoparticles with the size ranging from of 100–150 nm and the FT-IR analysis depicted common functional groups like aldehydes, ketones, flavonoid responsible for the capping and the stabilizing of the gold nanoparticles. The antioxidant and anticancer activity of the gold nanoparticles proved that it has potential adverse effects. It could be used as an alternative therapeutic agent for the treatment of many diseases.
Also, the synthesized gold nanoparticles were biocompatible in nature, which clearly depicted by their non-cytotoxic properties, compared with other metallic nanoparticles.
Funding Information
This work was partially supported by National Research Foundation grant (Grant No. 2019R1I1A1A01063845), Republic of Korea.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Synthesis and bio-evaluation of xylan-5-fluorouracil-1-acetic acid conjugates as prodrugs for colon cancer treatment. Carbohydr. Polym.. 2017;157:1442-1450.
- [Google Scholar]
- Synthesis and enhanced anti-microbial activity of biosynthesized silver nanoparticles against clinical pathogens. J. Mol. Stru.. 2016;1116:165-173.
- [Google Scholar]
- Novel Functionalized Selenium Nanoparticles for Enhanced Anti-Hepatocarcinoma Activity In vitro. Nanoscale Res Lett. 2015;10(1)
- [CrossRef] [Google Scholar]
- Anticancer activity of eco-friendly gold nanoparticles against lung and liver cancer cells. Genet. Eng. Biotech.. 2016;14:195-202.
- [Google Scholar]
- Acalypha indica Linn: Biogenic synthesis of silver and gold nanoparticles and their cytotoxic effects against MDA-MB-231, human breast cancer cells. Biotechnology Reports. 2014;4:42-49.
- [Google Scholar]
- Green synthesis of biocompatible carboxylic curdlan-capped gold nanoparticles and its interaction with protein. Carbohyd. Polym.. 2015;117:771-777.
- [Google Scholar]
- Rapid and efficient sonochemical formation of gold nanoparticles under ambient conditions using functional alkoxysilane. Ultrasonics Sonochemistry. 2013;20(1):610-617.
- [Google Scholar]
- Biosynthesis of silver and gold nanoparticles using thermophilic bacterium Geobacillus stearothermophilus. Process Biochemistry. 2011;46(10):1958-1962.
- [Google Scholar]
- Mycogenesis of gold nanoparticles using a phytopathogen Alternaria alternata. Biopro. Biosys. Eng.. 2012;35:637-643.
- [Google Scholar]
- Exploitation of anaerobic enriched mixed bacteria (AEMB) for the silver and gold nanoparticles synthesis. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2014;462:264-270.
- [Google Scholar]
- Eco-friendly synthesis and characterization of gold nanoparticles using Klebsiella pneumoniae. J. Nanostruc. Chem.. 2013;3:30.
- [Google Scholar]
- Antibacterial and antioxidant potential of biosynthesized copper nanoparticles mediated through Cissus arnotiana plant extract. Journal of Photochemistry and Photobiology B: Biology. 2019;197:111531.
- [CrossRef] [Google Scholar]
- Suganya, K.S.U., Govindaraju, K., Kumar, V. G., Dhas, T. S. V., Karthick, G., (2015) Singaravelu, M. Elanchezhiyan, Blue green alga mediated synthesis of gold nanoparticles and its antibacterial efficacy against Gram positive organisms. Materi. Sci. Eng C. 47, 351–356.
- Biosynthesis of gold nanoparticles assisted by Escherichia coli DH5 a and its application on direct electrochemistry of hemoglobin. Electrochem. Commun.. 2007;9:1165-1170.
- [Google Scholar]
- Biosynthesis of gold nanoparticles by biosorption using Magnetospirillum gryphiswaldense MSR-1. Chem. Eng. J.. 2011;175:70-75.
- [Google Scholar]
- Gold nanoparticles: Optical properties and implementations in cancer diagnosis and photothermal therapy. Journal of Advanced Research. 2010;1(1):13-28.
- [Google Scholar]
- Biosynthesis of gold nanoparticles by Pseudomonas veronii AS41G inhabiting Annona squamosa L. Spectrochim. Acta -A: Molecul. Biomol. Spec.. 2015;150:691-695.
- [Google Scholar]
- Application of nanoparticles derived from marine Staphylococcus lentus in sensing dichlorvos and mercury ions. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2016;501:1-8.
- [Google Scholar]
- Postle, K., (1990) Aerobic regulation of the Escherichia coli tonB gene by changes in iron availability and the fur locus. J. Bacterio. 172, 2287–2293.
- Biosynthesis of gold nanoparticles using the bacteria Rhodopseudomonas capsulata. Materl. Lett.. 2007;61:3984-3987.
- [Google Scholar]
- Genomic, genetic and structural analysis of pyoverdine-mediated iron acquisition in the plant growth-promoting bacterium Pseudomonas fluorescens SBW25. BMC Microbiol. 2008;8(1):7.
- [CrossRef] [Google Scholar]
- Aptamer biosensor for sensitive detection of toxin A of Clostridium difficile using gold nanoparticles synthesized by Bacillus stearothermophilus. Biosensors and Bioelectronics. 2014;54:217-221.
- [Google Scholar]
- Vibrio Alginolyticus : An Emerging Pathogen of Foodborne Diseases. Internat. J. Sci. Tech.. 2013;2:302-309.
- [Google Scholar]
- Free Radical Scavenging Activities of Zizyphus mauritiana. Evaluation.. 2009;5:318-322.
- [Google Scholar]
- DPPH Free Radical Scavenging Activity of Some Leafy Vegetables used by Tribals of Odisha, India. J. Med. Plants Stu.. 2013;1:21-27.
- [Google Scholar]
- Research Article Metal ion chelating activity and hydrogen peroxide scavenging activity of medicinal plant Kalanchoe pinnata. J. Chem. Pharm. Res.. 2012;4:197-202.
- [Google Scholar]
- An in-vitro biosynthesis of zinc oxide nanoparticles using rich flavonoid extract from the petals of Delonix regia and evaluation of their antioxidant and anticancer properties. Inter. J. Pharmacog. Phytochem. Res.. 2015;7:1112-1119.
- [Google Scholar]
- A novel bacterial isolate Stenotrophomonas maltophilia as living factory for synthesis of gold nanoparticles. Microb Cell Fact. 2009;8(1):39.
- [CrossRef] [Google Scholar]
- Biosynthesis of gold nanoparticles using streptomyces fulvissimus isolate Biosynthesis of gold nanoparticles by Streptomyces fulvissimus. Nanomed. J.. 2015;2:153-159.
- [Google Scholar]
- Biosynthesis of gold nanoparticles using Pseudomonas aeruginosa. Spec.chim.Acta A: Mol. Biomol. Spec.. 2007;67:1003-1006.
- [Google Scholar]
- Rapid biosynthesis of gold nanoparticles by Staphylococcus epidermidis: Its characterisation and catalytic activity. Materials Letters. 2015;146:23-25.
- [Google Scholar]
- A novel biological synthesis of gold nanoparticle by Enterobacteriaceae family. Trop. J. Pharmaceu. Res.. 2012;11:887-891.
- [Google Scholar]
- ScienceDirect Green Synthesis of Silver Nanoparticles using Leaves Extract of Bauhinia tomentosa Linn and its invitro Anticancer Potential. Mat. Today Proceed.. 2015;2:4309-4316.
- [Google Scholar]
- Incubation period induced biogenic synthesis of PEG enhanced Moringa oleifera silver nanocapsules and its antibacterial activity. J Polym Res. 2019;26(9)
- [CrossRef] [Google Scholar]
- Potentially human pathogenic vibrios in marine and fresh bathing waters related to environmental conditions and disease outcome. International Journal of Hygiene and Environmental Health. 2011;214(5):399-406.
- [Google Scholar]
- Mar Piccolo of Taranto: Vibrio biodiversity in ecotoxicology approach. Environ Sci Pollut Res. 2014;21(3):2378-2385.
- [Google Scholar]







