Translate this page into:
Bioreduction of hexavalent chromium by chromium resistant alkalophilic bacteria isolated from tannery effluent
⁎Corresponding authors. malsalhi@ksu.edu.sa (Mohamad S. AlSalhi), rajasekargood@gmail.com (Aruliah Rajasekar)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Two chromium resistant alkalophilic bacterial strains (CRAB), namely Bacillus cohnii SR2 and Bacillus licheniformis SR3 were isolated and identified from tannery effluent. Bioreduction of chromium (VI) into chromium (III) by CRAB was investigated in the present study. Optimization of process parameters with respect to bioreduction percentage was studied by Response Surface Methodology (RSM) software, v12. Both the strains were found to achieve highest chromium (VI) bioreduction of 94 and 95% in 100 mg/L at the end of 25 h at pH 9.0. At an optimized concentration of 550 mg/L, a maximum bioreduction of 82 and 90% by SR2 and SR3 was noted within 25 h. In addition, both the strains showed tolerance to chromium until 1000 mg/L. Scale-up studies using Air lift bioreactor system by CRAB with real tannery effluent showed 100% bioreduction within 8 h. Therefore, this study signified the astonishing bioreduction capability of CRAB isolates that was quite evident in scale-up studies too.
Keywords
Alkalophilic
Bioreduction
Chromium resistant
Response surface methodology
Tannery effluent
1 Introduction
Hexavalent chromium Cr(VI) is one of the major environmental pollutant discharged from various industrial effluents, such as, pigment, paint, textile dyes, leather tanning, metal processing and electroplating industries, etc. (Adki et al., 2013; Dhandapani et al., 2020). Among all, tannery industries are considered to be the major contributors of environmental chromium discharges. Approximately, an estimate of 40 million litres of tannery wastewater is released per year, worldwide (Saranraj and Sujitha, 2013). Than the recommended permissible limits of 2 mg/L, chromium concentrations of 2000–5000 mg/L are estimated to be present in the aqueous matrices in India (Manivasagam et al., 1987). These tannery wastes also contribute to severe chromium contamination of nearly 50,000 ha of productive agricultural lands in Vellore district, Tamil Nadu, where more than 60% of the Indian tanneries are situated. This is due to improper industrial disposal practices and failure of implementation of strict regulatory standards for tannery effluent releasing industries. Though chromium exists in many valencies, Cr(VI) is reported as highly toxic and mutagenic, which is due to its mobility and solubility in the environmental matrices (Kotas and Stasicka, 2000). When consumed by animals, they induce respiratory problems, low immunity, infertility, birth defects, increased mortality rates and tumour formation (Thiele et al., 1995; Costa, 2003). Cr(VI), the most hazardous form of chromium may cause various human health problems such as, skin rashes, allergic reactions, nasal irritations, nose bleeding, ulcers, suppressed immunity, molecular level alterations, liver, kidney damage (Thiele et al., 1995).
Bacteria, yeast, protozoa, and fungi are widely distributed in water and terrestrial environments, in which chromium containing tannery effluents are released. Many research efforts of employing biological organisms to convert chromium(VI) to chromium(III) by reduction are available in the literature (Cervantes et al., 2001; Sultan and Hasnain, 2007; Sarangi and Krishnan, 2008; Govarthanan et al., 2019). Despite these many research contributions, the challenge remains in isolating and identifying an indigenous metal tolerance microbe to with stand and remediate high concentration of chromium in less time (Wang et al., 2016). Traditional physico-chemical methods for metal removal includes, reverse osmosis, chemical precipitation, adsorption and membrane processes However, these methods have some limitations like expensiveness, demands intensive labor skills and turns ineffective when the metal concentration is high (Kocaoba and Akin, 2002; Sathishkumar et al., 2019; Narenkumar et al., 2019). Consequently, the remedy environmental hazards due to chromium in tannery replacement treatment employing of bacteria is well thought-out as low cost effective and eco friendly technique towards future prediction (de Aquim et al., 2019; Desai et al., 2008; Saxena, 2020).
Our earlier report study on bioelectrokinetic remediation was high removal percentage of hexavalent chromium to trivalent chromium in chromium contaminated soil (Sarankumar et al., 2019). Design experiment of response surface methodology (RSM) was used to investigate with number of factors are integrating four different approaches by optimizing different variable (pH, Bioreduction time, Bioreduction percentage, Cr(VI) concentration) chosen this experiment. The box-behnken design (BBD) used in mechanism models of our study and data be analyzed via of fitting near empirical model, which compare to selected variables response Selvi and Rajasekar (2018).
The present study is one such another attempt of bioreducing toxic chromium (VI) to nontoxic chromium (III) with the help of two indigenous CRAB namely, Bacillus cohnii SR2 and Bacillus licheniformis SR3, isolated from tannery effluent. They were successfully tested for bioreduction of chromium (VI). The outcomes of the present work would certainly serve an effective solution to address the metal removal from chromium contaminated wastewater environments.
2 Materials and methods
2.1 Sample collection
The effluent from tannery industrial area at Vaniyambadi, Tamil Nadu (longitude 78.6219°E, latitude 12.6950°N), was collected in a sterile screw capped bottle and placed in an icebox and immediately transferred to the laboratory. Sample was kept at 4 °C. The collected effluent was subjected to physico-chemical parameters analysis as tabulated in Table 1.
Parameters
Unit
Values
pH
(1–14)
8.50.5
Cr(VI)
(mg/L)
12.94914
Turbidity
(NTU)
2521.2
TDS
(mg/L)
48621.1
TSS
(mg/L)
34131.5
BOD
(mg/L)
12100.8
COD
(mg/L)
17312.0
Chlorides
(mg/L)
21342.1
Sulphates
(mg/L)
9621.2
Nitrates
(mg/L)
7.90.4
Calcium
(mg/L)
13141.1
Magnesium
(mg/L)
5682.5
2.2 Isolation of CRAB
The CRAB was isolated by spread plate method with Luria-Bertani (LB) medium. The pH of then LB medium was adjusted to 9.0 and autoclaved at 120 °C for 15 min. Sampling of 0.1 ml of effluent was used for spread plate method to enumerate the bacteria (Shakoori et al., 2000; Zahoor and Rehman, 2009). An incubation condition of 37 °C was maintained for 48 h. After 48 h, the individual colonies were observed and selected based on the morphological characteristics and sub-streaked on sterile LB plates. The selected colonies were subjected to biochemical characterisation following standard methods as described earlier (Holt et al., 1994).
2.3 Molecular identification of CRAB by 16S rDNA
The isolated CRAB was subjected for Genomic DNA Extraction following Kit protocol (Hi Media). The purified genomic DNA was amplified by PCR followed by 16S rRNA sequencing using universal primers as follows, forward primer: (5′-GGATGAGCCCGCGGCCTA-3′) and reverse primer: (5′-CGGTGTGTACAAGGCCCGGGAACG-3ʹ). The amplification was carried out for 35 cycles with conditions of initial denaturation step at 95 °C for 5 min, that was followed by 35 cycles of denaturation at 95 °C for 30 s, primer annealing at 50 °C for 30 s and the extension step at 72 °C for 1.5 min. The amplicons were further subjected to sequencing in Applied Biosystems Terminator version 3.1, and analysed in automated sequencing system (Model:ABI 3130). Obtained assembled sequences were submitted to NCBI followed by BLAST and CLUSTAL OMEGA software analysis. The assembled sequencing were used to construct phylogenetic tree (PT) using MEGA software, version 5.05 to explain phylogenetic similarity with closely related sequence by neigbor-joining method (Saitou and Nei, 1987).
2.4 Acclimatization studies of CRAB
Acclimatization experiments of chromium resistant alkalophilic bacterium were conducted in minimal salt medium (MSM) consisting (g/L) of sodium chloride-0.5; potassium phosphate-3; magnesium sulphate anhydrous-0.12; calcium chloride dehydrate-0.015; sodium phosphate-6 and yeast extract-3.0. The medium was prepared with an added concentration of 100 mg/L of Cr(VI) as potassium dichromate (K2Cr2O7). The pH adjustments were done using 1 N NaOH. Isolated alkalophilic strains, SR2 and SR3 at a concentration of 23 × 104 CFU/ml were inoculated individually into MSM medium. The inoculated flasks were incubated at 37 °C in a rotary shaker at 150 rpm for 30 h. The medium was supplemented with gradual increasing concentration of Cr (VI) obtain acclimatized cultures and the cultures were stored for further experimental use.
2.5 Optimization of growth parameters for CRAB towards bioreduction of Cr(VI) to Cr(III) using response surface methodology (RSM)
Optimization and Bioreduction of Cr (VI) to Cr(III) by CRAB was studied in sterile LB broth containing flasks with 100 mg/L of K2Cr2O7. The flasks were adjusted to different pH ranging from 8.0 to 10.0. The individual acclimatized culture of CRAB (17 × 104 CFU/ml) was inoculated into each flask and incubated in 37 °C at 150 rpm for 25 h. Optimization of bioreduction chromium (VI) was examined using one-variable-at-a-time approach based experimental design using RSM, Design expert software, v12 using Box-behnken model (Selvi and Rajasekar 2018; Sathishkumar et al., 2017). The experimental design, their independent variables are initial Cr(VI) concentration (100–1000 mg/L), pH (8–10) and bioreduction time (5–25 h) were taken to obtain the response for hexavelant chromium to trivalent chromium bioreduction. The design consisted of 17 runs and were performed in triplicate to optimize the levels of selected variables. The experimental range of each variable and levels of independent variables were considered as demanded by the Box-Behnken design (Table 2). The non-coded and coded units of the design matrix are presented in (Table 3). Every 5 h, the samples were withdrawn for the estimation of Cr (VI) reduction till the end of the incubation period of 25th h.
Variables
Unit
Range and Level
−1
0
+1
pH
–
8
9
10
Bioreduction Time
h
5
15
25
Cr(VI) concentrations
mg/L
100
550
1000
Std
Run
Factor 1
A: pHFactor 2
B: Cr(VI) concentration (mg/L)Factor 3
C:Bioreduction Time
17
1
9
550
15
12
2
9
550
25
13
3
9
100
15
6
4
10
550
5
8
5
10
550
25
16
6
9
550
15
7
7
8
550
25
2
8
10
100
15
11
9
9
100
25
15
10
9
550
15
10
11
9
1000
5
5
12
8
550
5
3
13
8
1000
15
14
14
9
550
15
9
15
9
100
5
1
16
8
100
15
4
17
10
1000
15
2.6 Estimation of Cr(VI) bioreduction
The bioreduced samples were collected by centrifugation at 6000g for 20 min. Cr(VI) was analysed by established methodology described by (Sarankumar et al., 2019). Estimation of bioreduced Cr(VI) was done by mixing supernatant sample Cr(VI) − 200 µl, Distilled·H2O − 800 µl, 6 M Sulphuric acid − 330 µl and diphenylcarbazide solution − 400 µl. Then the estimation volume was made up to 10 ml using distilled water. The purple colour formed and measured by UV-V is Spectrophotometer at 540 nm.
2.7 Scaling up studies using real tannery effluent
Air Uplift bioreactor (AUBR 5-LWinpact Major Science) was used for the scale-up studies in a batch process for 25 h of continuous operation. Real tannery effluent of volume 4.5 L with inoculation of 50 ml of individual CRAB stains at a concentration of 3 × 104 CFU/ml. Optimal pH and temperature of 9.0 and 37 °C were maintained during the study Morales and Cristiani-Urbina (2006). A constant pH of 7.0 was maintained by adding Conc. H2SO4. Oxygen supply of 150 L/h was supplied for the study. The sample was withdrawn regularly at the end of every 4 h to measure Cr (VI) bioreduction.
3 Results
3.1 Molecular identification of CRAB
Two different CRAB isolates from tannery effluent sample were initially subjected to biochemical characterization (Table 4), which was followed by 16S rRNA identification studies. The assembled DNA sequences of the isolates were submitted to NCBI database. Based on the relatedness of ten closely related bacterial species, the first isolate showed (99%) identity with Bacillus cohnii (Acc. No. MH 973722). Similarly, the second isolate showed (100%) identity with Bacillus licheniformis (Acc. No. MH 973723) both strains. Therefore, the isolates were designated as Bacillus cohnii SR2 (Fig. 1) and Bacillus licheniformis SR3 (Fig. 2) respectively.
Characteristics
SR2
SR3
Gram stain
+
+
Colony shape
Rod
Rod
Motility
Motile
+
Catalase
+
+
Methyl red
+
–
Indole
+
–
Voges-Proskauer
–
–
Citrate
–
–
Urease
–
–
Oxidase
+
–
Nitrate
+
+
Catalase
+
+
Starch hydrolysis
+
–
Glucose
+
–
Sucrose
+
–
Starch
+
–
Phenogram showing the phylogenic position of strain SR2 Genus Bacillus cohnii based on 16S rRNA Gene Sequence Analysis.
Phenogram showing the phylogenic position of strain SR3 Genus Bacillus licheniformis based on 16S rRNA Gene Sequence Analysis.
3.2 Acclimation studies of CRAB strains
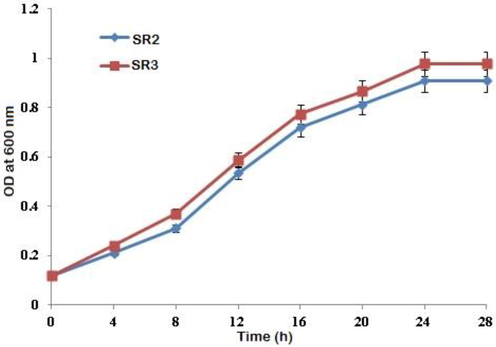
The growth trend of two CRAB strains namely, SR2 and SR3 with an initial concentration of 100 mg/Lof Cr(VI) was studied with respect to time at 600 nm. Fig. 4 shows the results of growth curve. The growth of both SR2 and SR3 showed a proper bacterial curve with a short lag phase of 3 h. This was followed by a long and steep increase in the growth curve indicating log phase, which extended till 23 h. At the end of 23 h, the bacterial cells have entered into stationary phase till 25 h, followed by decline phase. Similarly, SR3 showed a slightly improved growth trend than the SR2 strain.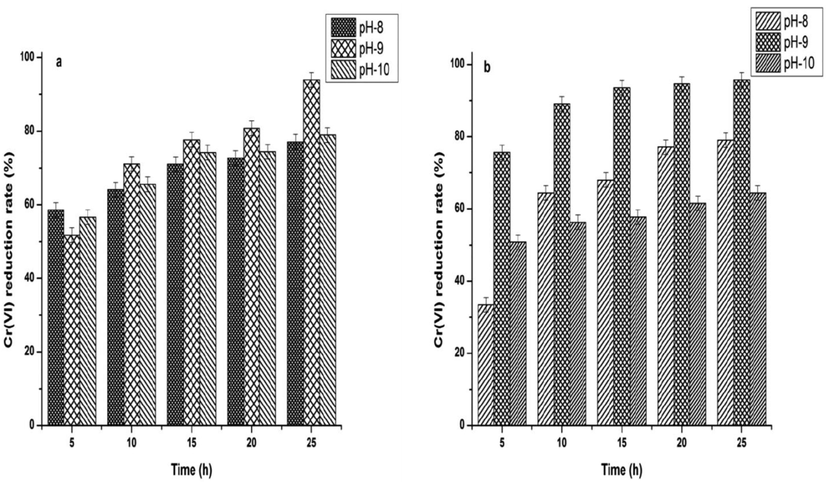
Effect of pH on the growth of chromium resistant isolates (a) Bacillus cohnii SR2 and (b) Bacillus licheniformis SR3, Error bars indicate the standard deviation of 3 measurements.
Growth curve of Bacillus cohnii SR2 and Bacillus licheniformis SR3 with concentration of hexavalent chromium solution.
3.3 Optimization of growth parameters by RSM
At an optimized pH of 9.0, SR2 strain showed a maximum Cr(VI) bioreduction efficiency of 94% at the end of 25 h. Whereas, at pH 8.0 and 10.0. The bioreduction of chromium (VI) was observed as decreased up to 77% and 79% (Fig. 3a). Similarly, at an optimum pH of 9.0, SR3 too showed a maximum Cr(VI) bioreduction of 95% at the end of 25 h. Whereas, at pH 8.0 and 10.0, SR3 strain showed 79% and 65%, Cr(VI) bioreduction (Fig. 3b). No change in pH was observed in the medium pH at the end of the experiment.
Bioreduction ability of CRAB was tested at various concentrations (100–1000 mg/L) and the results are shown in (Fig. 5a and b). The trend of Cr(VI) bioreduction was found to be decreased with increasing Cr(VI) concentration. On analysis, SR2 strain showed a maximum Cr(VI) bioreduction efficiency of 94, 90, 82, 71 and 51% at 100, 300, 500, 700 and 1000 mg/L respectively at the end of 25 h (Fig. 5a). As shown in (Fig. 5b), SR3 strain showed a better Cr(VI) bioreduction efficiency of 95, 93, 90, 83 and 54% at 100, 300, 500, 700 and 1000 mg/L respectively than the other strain.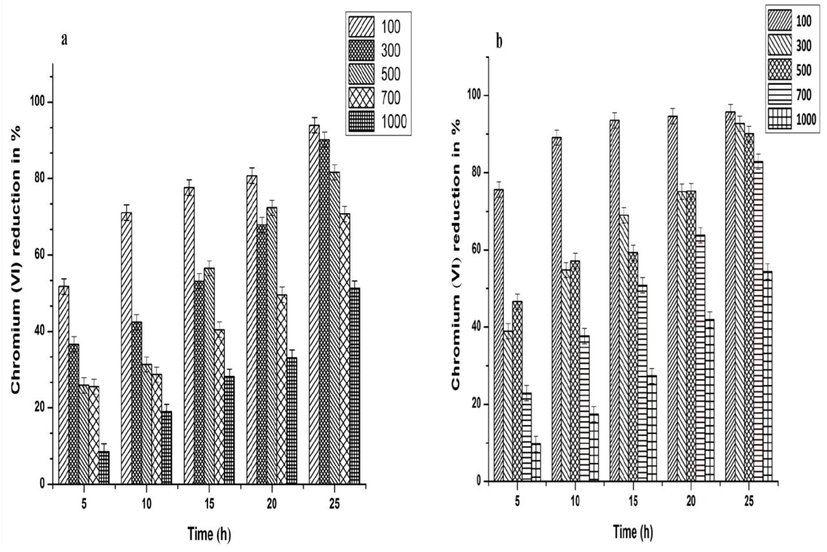
Effect of various Cr(VI) concentration (100–1000 mg/L) towards bioreduction by (a) Bacillus cohnii SR2 and (b) Bacillus licheniformis SR3. Error bars indicate the Standard deviation of 3 measurements.
Optimization of growth parameters were validated using RSM and are expressed on terms of interaction results on effect of variables towards reduction Cr(VI) of by SR2 and SR3 strains are shown in (Fig. 6a and b). Central values of the factors were observed as pH 9.0, Cr(VI) 550 mg/L initial concentration, and bioreduction time of 25 h. The evaluated results showed good correlation with respect to bioreduction of Cr(VI) efficiency calculation as follows in terms of coded equation of the BBD can be given follow;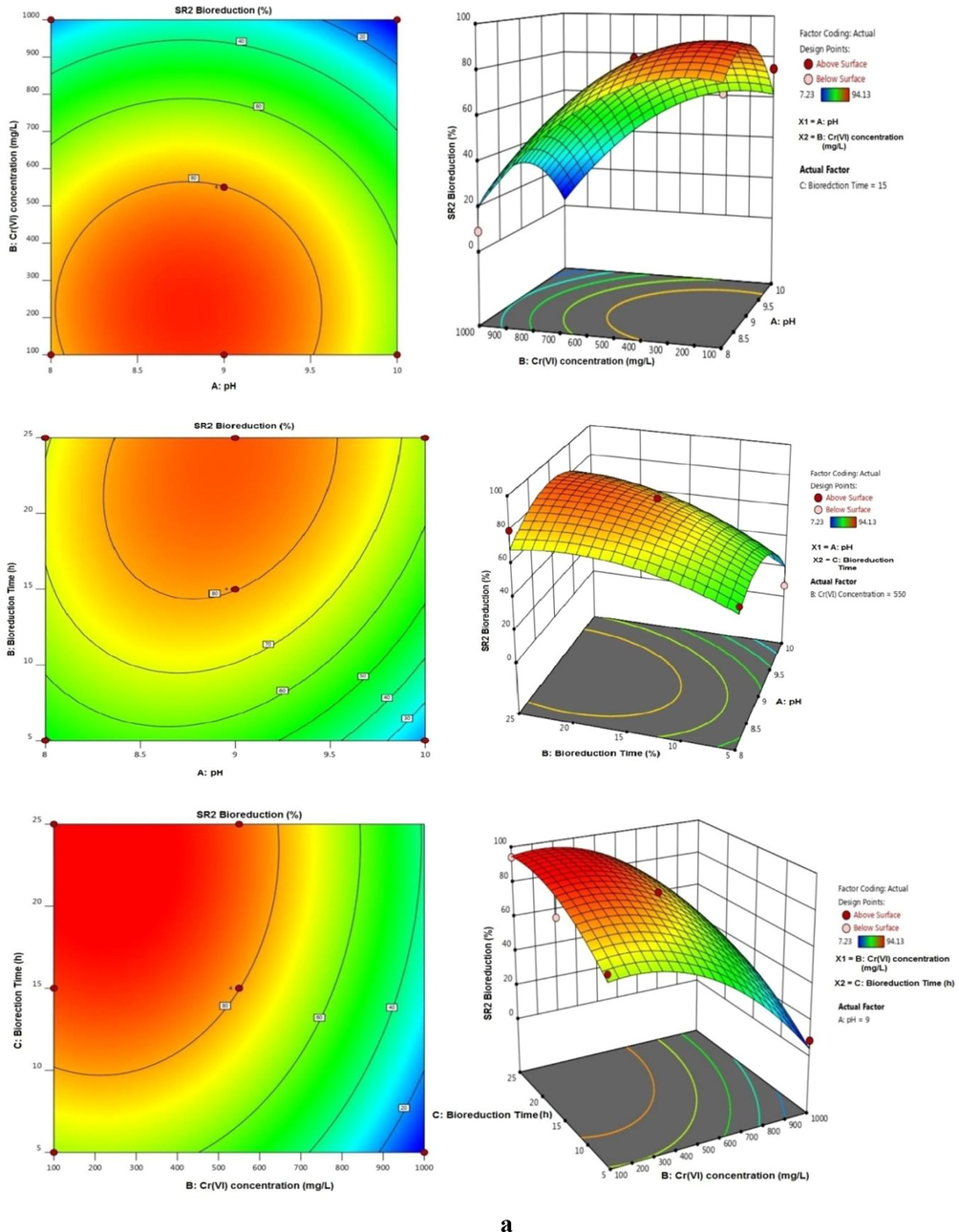
Response Surface methodology plot showing the optimized parameters of (a) Bacillus cohnii SR2 and (b) Bacillus licheniformis SR3 for Cr(VI) bioreduction. (Factors: Time − 15 h, pH − 9.0, concentration of Cr(VI) − 550 mg/L).
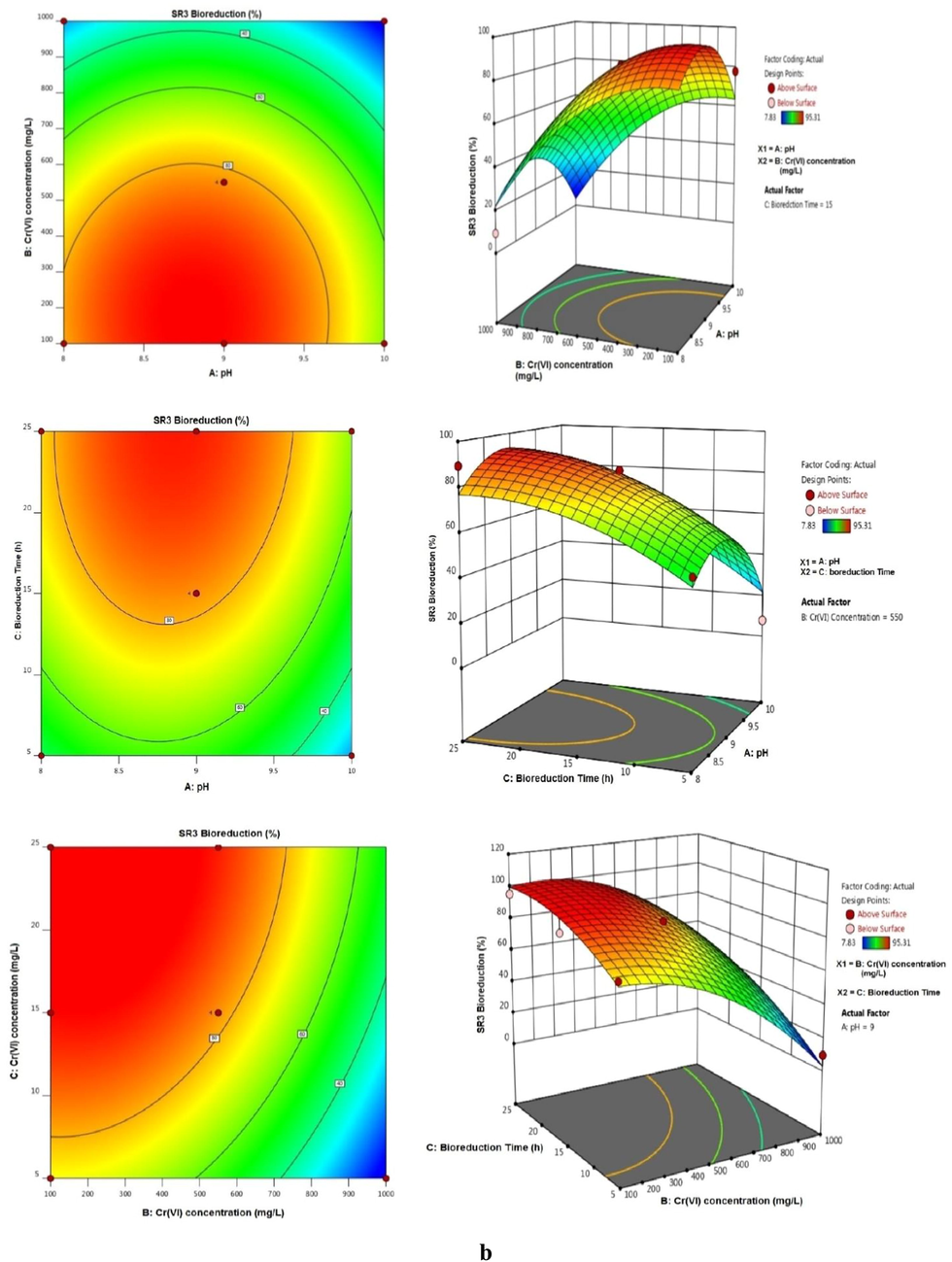
Response Surface methodology plot showing the optimized parameters of (a) Bacillus cohnii SR2 and (b) Bacillus licheniformis SR3 for Cr(VI) bioreduction. (Factors: Time − 15 h, pH − 9.0, concentration of Cr(VI) − 550 mg/L).
The final equation in terms of actual factors can be given as,
3.4 Scaling up studies
Studies on Cr(VI) bioreduction by CRAB strains in real tannery wastewater was also demonstrated and presented in (Fig. 7). As the Fig. 7 represented, a phenomenal result of complete 100% bioreduction by the CRAB strains viz. SR2 and SR3 was observed within 8 h of time.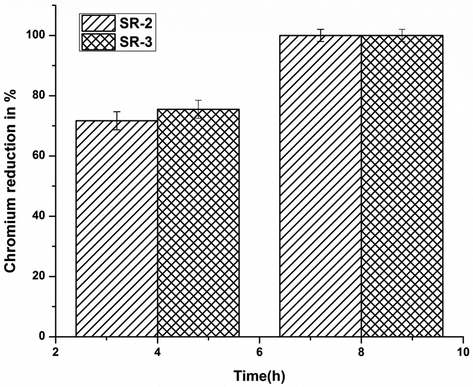
Cr(VI) bioreduction in tannery effluent by alkalophilic strains Bacillus cohnii SR2 and Bacillus licheniformis SR3, Error bars indicate the standard deviation of 3 measurements.
4 Discussion
Chromium (VI) is a frequent pollutant discharged in natural waters in the form of various industrial effluents (Pattanapipitpaisal et al., 2002; Koenig et al., 2016). Despite many methods reported so far, a wide range of biological processes are considered to show good and reliable results in terms of chromium removal. This is due to reports on the involvement of various biological existence of ciliates, fungi, bacteria, algae, mosses, higher plant and macrophytes in chromium contaminated environments towards removal of various heavy metals from aqueous solution (Rahman et al., 2008).
As reported earlier, Cr(VI) bioreduction by biological system is one of the possible alternative methods (Mukherjee et al., 2015). In the present work, Cr(VI) reduction ability of two CRAB strains, B. cohnii SR2 and B. licheniformis SR3 isolated from tannery effluent has been employed for Cr(VI) bioreduction as reported (Basu et al., 1997; Govarthanan et al., 2014). Although there are previous reports of bioreduction, the real challenge lies in isolating, identifying and employing an efficient organism for Cr(VI) bioreduction in very less time. So, we here in the present study have attempted to report on the maximum Cr(VI) bioreduction by our chromium resistant bacterial strains, SR2 and SR3 within 25 h of time, which is less time reported so far. Various environmental factors, viz., pH, temperature, concentration, bioreduction time of the pollutant play a major role bioremediation processes (Selvi et al., 2014; Sathishkumar et al., 2016). In the present study, the optimal conditions have shown to favour increased Cr(VI) bioreduction in less time. This was very evident with our results showing the maximum Cr(VI) bioreduction at an optimized pH 9.0 by both SR2 and SR3 strains respectively.
Since, pH played an important role in bioreduction Cr (VI), no change in the pH observed throughout the experiment inferred that, both the strains, SR2 and SR3 were able to grow well under alkaline condition and to perform Cr(VI) bioreduction. A similar observation on the influence of alkaline pH 9.0 towards bacterial Cr(VI) bioreduction (Sarangi and Krishnan, 2008; Kavita and Keharia, 2012; Emadzadeha et al., 2016). Earlier reports on Cr(VI) bioreduction using Bacillus sp. showing maximum reduction at an optimized of pH 9.0 was found to support our investigation (Mohapatra et al., 2017; Karthika et al., 2017).
The analysis of (ANOVA) for the quadratic model showed an F-values of 12.85 and 13.18 implies the model is significant in case of all response. The significant of the model was validated by the P values (0.0500 and 0.0500) indicate model terms are significant which were noted to be less than 0.5 in all case. The adequate precision was found be greater than 4 in all two causes. The correlation coefficient values were found to be quite close to 1.0 (0.9429 and 0.9443) there by further validating the model. Among several single factors, B (Cr(VI)), C (Bioreduction time) were found to be significant. The interaction factor BC and all quadratic factors A2, B2, C3 were found to be significant. The most significant interaction BC was presented as 3D mesh in Fig. 6 (a and b) showed a mesh in all the two cases viz., R1, R2. The region indicated the region of maximum activity (Selvi et al., 2014).
Initial concentration of chromium (VI) is a crucial factor which will influence the rate of reduction (Selvi and Rajasekar, 2018). Our study showed the Cr(VI) reducing ability of B. cohnii SR2 (81%) and B. licheniformis SR3 (90%) at an optimal concentration of 550 mg/L within 25 h . However, a fair bacterial growth reflected in significant Cr(VI) reduction of 70% and 83% till 700 mg/L. This trend proved the astonishing chromium resistant behaviour of the isolated strains. According to other reports, Bacillus sp. reduced Cr(VI) to 54% of 100 mg/L at the end of 24 h incubation (Masood and Malik, 2011). Similarly, Bacillus sp. and S. capitis reduced 100 µg/ml of Cr(VI) to 40% and 29%, respectively (Shakoori et al., 2000). Similar Cr(VI) bioreduction study using A. baumannii and P. stutzeri bacterium reported a maximum of 40% with 1000 mg/L at 24 h. On comparing with other reports, the results of our study demonstrated a maximum Cr(VI) bioreduction in very less time of 25 h. In addition, complete bioreduction in less time taken in real tannery effluent too confirmed the astonishing efficiency of our isolates that can be used to treat chromium containing wastewater. This complete bioreduction in real effluent by our isolates is due to the low concentration of Cr (VI) in tannery effluent (12.44 mg/L). However, the outcome in real effluent proved the chromium resistant behaviour of the isolates, thus evidencing their application in treating chromium containing real industrial effluents. An another study reported on use of chromium resistant Bacillus and Staphylococcus sp. were reported to treat chromium containing industrial effluent with a maximum reduction percentage (Shakoori et al., 2000; Sanjay et al., 2018; Houri et al., 2020). On comparison with these existing reports, the alkalophilic bacterial isolates used in our study has proved its potentiality through spectacular outcomes with respect to Cr(VI) bioreduction and hence can be considered as potential biological candidates to remediate Cr(VI) contaminated wastewater.
5 Conclusion
Two potent Cr(VI) reducing alkalophilic bacterial strains B. cohnii SR2 and B. licheniformis SR3 were identified from tannery effluent. Optimization process parameters was carried out for pH, bioreduction time, and Cr(VI) initial concentration by RSM studies. The overall results confirm that, both B. Cohnii SR2 and B. licheniformis SR3 have the ability to reduce the Cr(VI) to Cr(III) as optimal pH of 9.0 and 550 mg/L of chromium. Under optimized conditions, both the isolates, SR2 and SR3 showed a maximum bioreduction of 82% and 90% within 25 h of time. Real effluent treatment studies in an air lift bioreactor too showed proved the potentiality of the CRAB isolates. Further research to elucidate the mechanism behind the bioreduction of Cr(VI) is under progress.
Acknowledgements
The authors are grateful to the Researchers Supporting Project Number (RSP-2019/68), King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Nopalea cochenillifera a potential chromium (VI) hyper accumulator plant. Environ. Sci. Pollut. Res.. 2013;20:1173-1180.
- [Google Scholar]
- Isolation and characterization of chromium-resistant bacteria from tannery effluents. Bull. Environ. Cont. Toxicol.. 1997;58:535-542.
- [Google Scholar]
- Interactions of chromium with microorganisms and plants. FEMS Microbiol. Rev.. 2001;25:335-347.
- [CrossRef] [Google Scholar]
- Potential hazards of hexavalent chromate in our drinking water. Toxicol. Appl. Pharmacol.. 2003;188:1-5.
- [Google Scholar]
- Water reuse: an alternative to minimize the environmental impact on the leather industry. J. Environ. Manage.. 2019;230:456-463.
- [Google Scholar]
- Evaluation of in vitro Cr(VI) reduction potential in cytosolic extracts of three indigenous Bacillus sp. isolated from Cr(VI) polluted industrial landfill. Bioresour. Technol.. 2008;99:6059-6069.
- [Google Scholar]
- Ureolytic bacteria mediated synthesis of hairy ZnO nanostructure as photocatalyst for decolorization of dyes. Mater. Chem. Phys. 2020:122619.
- [Google Scholar]
- Bioleaching characteristics, influencing factors of Cu solubilization and survival of Herbaspirillum sp. GW103 in Cu contaminated mine soil. Chemosphere. 2014;109:42-48.
- [Google Scholar]
- Biogreen remediation of chromium-contaminated soil using Pseudomonas sp. (RPT) and neem (Azadirachta indica) oil cake. Int. J. Environ. Sci. Technol.. 2019;16(8):4595-4600.
- [Google Scholar]
- Bergey's Manual of Determinative Bacteriology (ninth ed.). Baltimore: Williams & Wilkins Co; 1994.
- Heavy metals accumulation effects on the photosynthetic performance of geophytes in Mediterranean reserve. J. King Saud Univ.-Sci.. 2020;32(1):874-880.
- [Google Scholar]
- Characterization of multifarious plant growth promoting traits of rhizobacterial strain AR6 under Chromium (VI) Stress Microbiol.. 2017;20:465-471.
- [Google Scholar]
- Reduction of hexavalent chromium by Ochrobactrum intermedium BCR400 isolated from a chromium-contaminated soil. 3 Biotech.. 2012;2:79-87.
- [Google Scholar]
- Removal and recovery of chromium and chromium speciation with MINTEQA2. Talenta. 2002;57:23-30.
- [Google Scholar]
- Particles and enzymes:combining nanoscale zero valent iron and organochlorinerespiring bacteria for the detoxification of chloroethane mixtures. J. Hazard. Mater.. 2016;308:106e112.
- [Google Scholar]
- Chromium occurrence in the environment and methods of its speciation. Environ. Pollut.. 2000;107:263-283.
- [Google Scholar]
- Hexavalent chromium reduction by Bacillus sp. strain FM1 isolated from heavy-metal contaminated soil. Bull. Environ. Contam. Toxicol.. 2011;86:114-119.
- [Google Scholar]
- Bioconversion of fish solid waste into PHB using Bacillus subtilis based submerged fermentation process. Environ. Technol. 2017
- [Google Scholar]
- Removal of hexavalent chromium by Trichoderma viride in an airlift bioreactor. Enzyme Microb. Technol.. 2006;40(1):107-113.
- [Google Scholar]
- Surfactant-assisted bioremediation of hexavalent chromium from contaminated water. Desalin. Water Treat.. 2015;53:746-751.
- [Google Scholar]
- Industrial effluents origin; characteristics effects, analysis and treatment, Shakti publications. India: Coimbatore; 1987. p. :79-92.
- Impact and role of bacterial communities on biocorrosion of metals used in the processing industry. ACS Omega 2019
- [Google Scholar]
- Reduction of Cr(VI) and bioaccumulation of chromium by gram positive and gram negative microorganisms not previously exposed to Cr-stress. Environ. Technol.. 2002;23:731-745.
- [Google Scholar]
- Influence of EDTA and chemical species on arsenic accumulation in Spirodela polyrhiza L (duckweed) Ecotox. Environ. Saf.. 2008;70:311-318.
- [Google Scholar]
- The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Bio. Env.. 1987;4:406-425.
- [Google Scholar]
- Isolation and identification of chromium reducing bacteria from tannery effluent. J. King Saud Univ.-Sci.. 2018;32(1):265-271.
- [Google Scholar]
- Comparison of in vitro Cr(VI) reduction by CFEs of chromate resistant bacteria isolated from chromate contaminated soil. Bioresour. Technol.. 2008;99:4130-4137.
- [Google Scholar]
- Electrokinetic (EK) and bio-electrokinetic (BEK) remediation of hexavalent chromium in contaminated soil using alkalophilic bio-anolyte. Indian Geotech. J. 2019:1-9.
- [Google Scholar]
- Microbial bioremediation of chromium in tannery effluent: a review. Int. J. Microbiol. Res.. 2013;4:305-320.
- [Google Scholar]
- Sequential electrochemical oxidation and bio-treatment of the azo dye congo red and textile effluent. J. Photochem. Photobiol., B. 2019;200:111655
- [Google Scholar]
- Bioreduction of hexavalent chromium by Pseudomonas stutzeri L1 and Acinetobacter baumannii L2. Ann. Microbiol.. 2016;67:91-98.
- [Google Scholar]
- Electrochemical decolorization of methyl red by RuO2-IrO2-TiO2 electrode and biodegradation with Pseudomonas stutzeri MN1 and Acinetobacter baumannii MN3: An integrated approach. Chemosphere. 2017;183:204-211.
- [Google Scholar]
- Saxena, G., Purchase, D., Bharagava, R.N., 2020. Environmental hazards and toxicity profile of organic and inorganic pollutants of tannery wastewater and bioremediation approaches. In: Bioremediation of Industrial Waste for Environmental Safety, pp. 381–398.
- A statistical approach of zinc remediation using acidophilic bacterium via an integrated approach of bioleaching enhanced electrokinetic remediation (BEER) technology. Chemosphere. 2018;207:753-763.
- [Google Scholar]
- Biodegradation of cefdinir by a novel yeast strain, Ustilago sp. SMN03 isolated from pharmaceutical wastewater. World J. Microbiol. Biotechnol.. 2014;30:2839-2850.
- [Google Scholar]
- Hexavalent chromium reduction by a dichromate-resistant gram-positive bacterium isolated from effluents of tanneries. Appl. Microbiol. Biotechnol.. 2000;53:348-351.
- [Google Scholar]
- Reduction of toxic hexavalent chromium by Ochrobacterium intermedium strain SD Cr-5 stimulated by heavy metals. Bioresour. Technol.. 2007;98:340-344.
- [Google Scholar]
- Thiele DJ (1995) Metal detoxification in eukaryotic cells. Crisp. Data Base of National Institute of Health: Washington, DC.
- Chromium isotope fractionation during subduction-related metamorphism, black shale weathering, and hydrothermal alteration chemical. Geology. 2016;423:19-33.
- [Google Scholar]
- Isolation of Cr(VI) reducing bacteria from industrial effluents and their potential use in bioremediation of chromium containing wastewater. J. Environ. Sci.. 2009;21:814-820.
- [Google Scholar]







