Translate this page into:
Biopolymer from edible marine invertebrates: A potential functional food
⁎Corresponding authors. abirami.ganesan@fnu.ac.fj (Abirami R. Ganesan), bala.m.k@sejong.ac.kr (Balamuralikrishnan Balasubramanian)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Edible marine invertebrates from crustaceans, molluscs and echinodermata can be considered as a novel and organic-based biopolymers for various functional applications. These biopolymers are made of polysaccharide and protein, which are utilized by humans as food and nutraceutical ingredients for specific diseases in decades. In recent times the potential application of biopolymers such as of glycosaminoglycans, fucosylated chondroitin sulfate, chitin and chitosan are increasing in the biomedical industry for treating various ailments. These compounds showed specific function as an antimicrobial, anti-inflammatory, anticancer, wound healing, hypoglycemic action and also in animal feed. Therefore, this review emphasis the specific structural and functional property of polysaccharide derived from edible marine invertebrates and their by-products. The global market value of this biopolymer in recent progress also evaluated in detail.
Keywords
Marine invertebrates
Glycosaminoglycans
Fucosylated chondroitin sulfate
Chitin
Chitosan
Functional foods
1 Introduction
In the marine ecosystem, most of the edible invertebrates are derived from the phylum of Mollusca, Arthropoda, Echinodermata. Globally, molluscs its shell were widely consumed edible marine invertebrate considered as traditional medicine; which ranges between 10000 and 20000 species comes under eight taxonomic classes including caudoforeata, aplacophora, monoplactoophora, polyplacophora, scaphopoda, gastropoda, bivalvia, cephalopoda (Santhanam, 2018). More than 90% of edible molluscs species derived from gastropods, bivalves and cephalopods. The shell of molluscs and whole powder with different fraction and combination of other natural sources were common approaches during ancient Greek practices and in the early Byzantine period (Voultsiadou, 2010). In terms of echinoderms, commonly consumed species found in this category of sea cucumber, an ancient healthy food consumed originally from Asia-Pacific countries. Anthocidaris japonicus had more commercial value which covers from 2,180,927 ha with a yield of 16,438,105 tons holds 34.166 billion in global market share. Some of edible species from sea urchin such as Strongylocentrotus intermedius, S. nudus, Glyptocidaris crenularis, A. crassispina, Hemicentrotus pulcherrmus, Tripneutes gratilla, and S. intermedius, are widely cultivated in China. Crustacean family contains maximum edible species in the marine invertebrate kingdom including lobster, crayfish, crab, shrimp, and prawns that are consumed as healthy food rich in dietary value (Chakravarthy et al., 2016). Moreover, arthropods are economically important among invertebrates as compared to other phyla in marine kingdom, and their market value is comparatively higher than other species. Recently, numerous added products were developed from krill and shrimp which comprise high nutritional value for human consumption (Hamed et al., 2016).
This review focuses on the edible marine invertebrates as a functional food and its nutraceutical application were critically evaluated. Several research groups have identified macromolecules from marine invertebrates as published in last 5-years through PubMed search, almost 113 scientific research outcomes available since 2015–2019 which reported the biological activity of edible marine invertebrates, mainly 25% of research report has been published in the year of 2018, 23% in 2017, 16.96% in 2016, 8% in 2014, 13.39% during 2015 and 2019. All these publications are comprised of various biological action of edible marine invertebrates conducted on humans and animals. Therefore, the main objective of this review is to emphasis and discuss polysaccharide biopolymer derived from edible marine invertebrates, also highlights and validates the commercial application of those organisms as a potential ingredient as functional foods.
2 Biopolymers-Polysaccharide from edible marine invertebrates
2.1 Chemical structure of glycosaminoglycans (GAG)
Polysaccharides are abundant bioactive compounds found in the marine invertebrates which displays various structural variety and diversity of interactions with monosaccharide. The GAG are considered as a complex polysaccharide with linear chain, negatively charged polysaccharides. Some of GAG groups of polysaccharides are fucosylated chondroitin sulfate (FCS), chondroitin sulfate, dermatan sulfate, sulfated fucans and sulfated galactans (Carvalhal et al., 2019). The FCS, exclusively found in sea cucumbers and compress of 3-linked β-N-acetylgalactosamine and 4-linked β-D-glucuronic acid as the backbone with 3-linked fucose at glucuronic acid and also had different sulphation based on different species (Pomin, 2014). Sometimes FCS have 4-sulfated with minor amounts of 2,4-di-sulfation (Pomin, 2014). Dermatan Sulfates has a backbone of [→4)-iduronic acid-(α1 → 3)-N-acetylgalactosamine-4sulphate-(β1 → ] and majorly have C4-sulphated N-acetylgalactosamine and minor unit of C4-sulphated N-acetylgalactosamine. Heparan sulfate from marine invertebrate has alternative units of glucuronic acid and glucosamine and 40–60% of N-sulfation in glucosamine units with substantial number of C2- and C3-positioned O-sulfation at glucuronic acid units. The mimic of GAG is sulfated fucan which has backbone of α (1 → 3) fucose units with sulfation at C2 or C4 positions. The average molecular weight of sulfated fucan is above 100 kDa consist of approximately 100 units. Another mimic of GAG was sulfated galactans which had 3-linked galactose with sulphation at C2 position (Vasconcelos and Pomin, 2017). The fucoidan and FCS from sea cucumber have been studied from last two decades. Originally, it was found in L. grisea species; later many subspecies were identified such as Isostichopus, Sichopus Japonicus, T. ananas, I. badionotus, A. molpadioides. All these fucoidans are linear polysaccharides consisting of tetra-saccharide repeating units with sulfated patterns (Li et al., 2017a,b).
2.2 Biological action of FCS
Many researchers reported that the biological activities like anti-inflammation, anti-coagulation and anti-cancer effect of FCS isolated from sea cucumbers (Qi and Yang, 2018); depends on their structure, molecular weight, degree and position of sulfate, monosaccharide composition (Mohan et al., 2019). Vasconcelos et al. (2018) reported that even small alteration in the structure of sulfate may alter the biological response, i.e. 2-sulfated fucan from Strongylocentrotus franciscanus showed poor biological activity whereas the 4-sulfated fucan from Lytechinus variegatus, with 2-sulfated galactan from Echinometra lucunter had high biological action of anticoagulation. The degraded product of FCS from Holothuria mexicana showed comparable anticoagulant action like low molecular weight heparin with less bleeding risks (Mou et al., 2017). All the biological action of this polysaccharide are given in Table 1.
Phylum
Invertebrate type
Species
Polysaccharide and adjunct compounds
Biological activity
Refs.
Mollusca
Squid
L. vulgaris
β-Chitin and chitosan
Antioxidant and antimicrobial
Abdelmalek et al.(2017)
Arthropoda
Shirmp
P. monodon
Chitin and chitosan
Antioxidant and anticancer
Srinivasan et al. (2018)
Mollusca
Cuttlefish
S. kobiensis
Chitosan and phosphorylated chitosan
Antibacterial
Shanmugam et al. (2016)
Mollusca
Cuttlefish
S. prashadi
Sulfated chitosan
Antioxidant and anticoagulant
Seedevi et al. (2017)
Arthropoda
Shirmp
T. unimaculatus
Chitosan
α-amylase and β-glucosidase enzyme inhibition effect and anticoagulant
Arasukumar et al.(2019)
Echinodermata
Sea cucumber
S. herrmanni
Fucosylated glycosaminoglycan
Anticoagulant
Li et al. (2017a,b)
Echinodermata
Sea cucumber
H. coluber
Glycosaminoglycan & Fucosylated chondroitin sulfate
Anticoagulant & anti-FXase activity
Yang et al. (2018a,b)
Echinodermata
Sea cucumber
H. albiventer
Fucan sulfate
Anticoagulant activity & anti-FXase activity
Cai et al.(2018)
Echinodermata
Sea cucumber
S. horrens
Fucosylated glycosaminoglycan
Anticoagulant, anti-FIIa, and anti-FXase activity
Shang et al.(2018)
Echinodermata
Sea cucumber
H. scabra
Fucosylated chondroitin sulfate
Anticoagulant activity
Yang et al. (2018a,b)
Echinodermata
Sea cucumber
H. mexicana
Fucosylated chondroitin sulfate
Anticoagulant
Mou et al.(2017)
Echinodermata
Sea cucumber
I. badionotus P. graeffei.
Fucosylated chondroitin sulfate
Anticoagulant
Li et al. (2017a,b)
Echinodermata
Sea cucumber
H. mexicana
Fucosylated chondroitin sulfate
Anticoagulant and anti-angiogenesis activity
Li et al.(2018)
Echinodermata
Sea cucumber
A. japonicus, S. chloronotus and A. molpadioidea
Fucosylated chondroitin sulfate
Antioxidant and anti-inflammatory activity
Mou et al. (2018a)
Echinodermata
Sea cucumber
H. mexicana
Glycosaminoglycan
Antioxidant
Mou et al. (2018b)
Echinodermata
Sea cucumber
M.magnum
Fucosylated chondroitin sulfate
Anticoagulant
Ustyuzhanina et al. (2017)
Echinodermata
Sea cucumber
T. ananas,
H. edulis,
H. nobilis,
H. fuscopunctata S. herrmanni
Fucosylated chondroitin sulfate
Anticoagulant
Xu et al.(2018)
Echinodermata
Sea cucumber
H. polii
Fucosylated chondroitin sulfate
Procoaculant and anticoagualant
Ben Mansour et al.(2017)
2.3 Anticoagulant activity of FCS
The most studied biological activity of polysaccharides from marine invertebrate is anticoagulant nature (Fig. 1). As many reports stated the anticoagulant action of FCS, Pomin (2014) showed this action in various species and genus of edible marine invertebrates. The FCS sulfated polysaccharides extracted and purified using DEAE-52 cellulose and Sepharose CL-6B which exhibits high anticoagulant activity [activated partial thromboplastin time (APTT) doubling time = 5 µg/mL] (Zhao et al., 2016). However, Cai et al. (2018) reported the activity of sulfated fucan from H.albiventer found at higher concentration i.e. 25.79 μg/mL and 115.47 μg/mL to double APTT and thrombin time (TT), respectively. Further, FCS from Massinium magnum had greater anticoagulant activity than enoxaparin. This anticoagulant activity of FCS was mediated by inhibition of factors which are involved in blood coagulation cascade. According to Li et al. (2017a,b) reported that fucosylated GAG from sea cucumber Stichopus herrmanni had potentially inhibiting thrombin and intrinsic factor Xase, and its action depends on sulfation pattern and molecular size. As compared to GAG and FCS extracted from H. coluber displayed strong APTT prolonging activities and intrinsic factor Xase inhibitory activities; but this action decrease with different molecular weight and predominant in FCS (Yang et al.. 2018a,b). The FCS from S. horrens showed strong anticoagulant action against APTT, TT, anti-factor IIa and anti-factor-Xa activity (Shang et al., 2018). Interestingly, FCS from H. polii exhibited procoagulant action at lower and higher concentration which acts as anticoagulant. Also, FCS mediated anticoagulant activity by antithrombin and heparin cofactor II (Ben Mansour et al., 2017). The structural backbone of FCS from H. scabra had → 4)GlcUAβ(1 → 3)GalNAcβ(1 → )and sulfated fucose branches were composed of β-d-glucuronic acid, N-acetyl-β-d- galactosamine, α-l-fucose and sulfate groups by molar ratio of 1:1.72:2.34:3.29 (Yang et al.. 2018a,b). The degree of fucosylation in FCS and its derivatives increase inhibition of thrombin by stimulating HCII (Xu et al., 2018).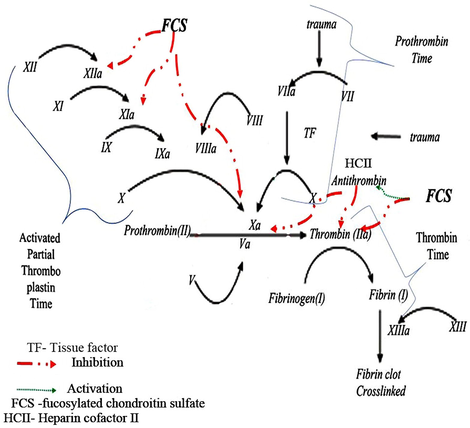
Mechanism of action of sulfated polysaccharide-FCS on initiation of pro-coagulant and anticoagulant reaction.
2.4 Antioxidant activity of FCS
The FCS exhibit wonderful antioxidant activity. Polysaccharide, GAG from H.mexicana showed 1,1-diphenyl-2-picrylhydrazyl (DPPH), superoxide and hydroxyl radicals in a dose-dependent manner (Mou et al., 2018a,b). Some species in Echinodermata exhibit tetrafucose repeating unit fucoidan (Ta-FUC) from egg jelly of sea urchin (Strongylocentrotus pallidus) and sea cucumber Lytechinus variegatus, Thelenata ananas possess excellent antioxidant activity against superoxide radicals. Additionally, T.ananas is one of the most popular edible sea cucumber species consumed in China and Southeast-Asian countries (Yu et al., 2014b). The antioxidant activity of FCS also depend on the degree and position of sulphation and also its action, due to presence of numerous hydroxyl and reducing groups in the polysaccharides (Guru et al., 2015). Mou et al. (2018a,b) reported that the FCS from three edible sea cucumbers Apostichopus japonicus, Stichopus chloronotus and Acaudina molpadioidea showed better antioxidant action (Fig. 2) due to the difference in sulphation pattern and demonstrated significant reduction in carrageenan-induced oedema as a dose depended manner. They showed that FCS from these species exhibited nitrate radical scavenging activity in the range of 26–39% at 4 mg/mL concentration.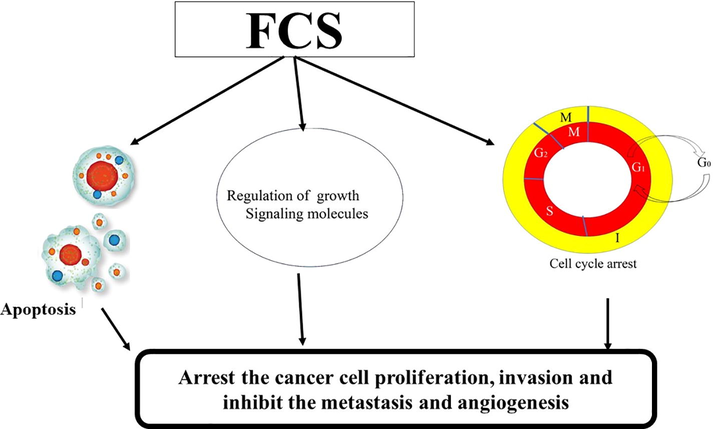
Mechanism of action of fucoslyated Chondroitin sulfate (FCS) in prevention of cell proliferation.
2.5 Chemical composition of chitin and chitosan and its application
Chitin and chitosan are natural polymers well known as mucopolysaccharide, abundant in the shell wall of marine invertebrates. Chitin is a neutral, white-coloured solid compound with a structure of n-acetyl-glucosamine monomers (Philibert et al., 2017). The α type is widely derived from shrimps and crabs, whereas β forms are found in squid which is more sensitive as compared to α forms (Cai et al., 2018). Thus, degree of deacetylation (40–98%) is key factor to dictate solubility of chitosan in aqueous medium; this unique feature makes chitosan in diversified application compared to polysaccharide such as cellulose, dextran, pectin, carrageenan which are neutral or acidic (El Knidri et al., 2018). According to Dima et al. (2017), Crustacean is primary source for production of chitosan; shrimp cuticle reveals 30–40% of chitin followed by 15–30% crab; rest is composed of proteins and minerals. Major functional groups are amino group, primary and secondary hydroxyl groups at C-2, C-3 and C-6 positions, respectively. Modifying the structure of these groups will create several chitosan oligomers suitable for various applications. It is second abundant polysaccharide next to cellulose and found to be more than 1000 tons/year among that about 70% from marine species such as shrimp, crab and lobster, and is also be found in exoskeletons of mollusks. Chitin and chitosan are biocompatible and biodegradable and have plenty of biological activities like antimicrobial activity, wound healing properties, antioxidant, anticholesterolemic, anticoagulation and homeostatic activity (Islam et al., 2017).
2.6 Antimicrobial activity of chitin and chitosan
The antibodies in warm blooded animals are produced due to chitin, thus chitin serves as strong antimicrobial agents. Many reports are available in this context, two types of chitin (Chitosan M and Chitosan C) extracted from Metapenaeus monoceros showed the wide range of antimicrobial activity on E.coli, K.pneumoniae, S.typhi, S.aureus, B.cereus, M.luteus, F.oxysporum, F.solani and Fusarium sp (Budd, 1998). Among, chitosan-C had MW 5.8 kDa displayed action against bladder carcinoma cells-RT112 (IC50 of 140 μM); whereas, chitosan M had MW 19.78 kDa had lower antioxidant, antimicrobial and anticancer activity, which was less effective than other sulfated polysaccharide (Ganesan, 2018). The possible mechanism of action of chitin, and chitosan is due to interaction of negatively charged membrane molecules N-acetylmuramic acid, sialic acid and neuraminic acid of microbes with positive charged groups of chitosan. High MW chitosan alters cell permeability and arrests nutrient transport; whereas, low MW chitosan interferes transcription process and kills the microorganism (Younes and Rinaudo, 2015). On the other hand, deacetylated chitosan also showed equally active reaction against microbes such as S.aureus, E.coli, P.aeruginosa, K.pneumonia, C.albicans and C.parapsilosis which was found in the deacetylation of chitin, classical deacetylated chitosan (CDC) and ultrasound-assisted deacetylated chitosan (UDC) from Parapenaeus Longirostris with the degree of deacetylation (33.64%, 73.68% and 83.55%). Moreover, different acetylated chitosan displayed varying antioxidant activity. The chitin, CDC and UDC showed DPPH radical scavenging activity of 21.25%, 32.78% and 44.17%, respectively at 1 (mg/mL). Therefore, antioxidant and antimicrobial activity of chitosan depends on the degree of deacetylation (Hafsa et al., 2016).
2.7 Anticancer and antioxidant activity of polysaccharide
Chitosan and its derivatives exhibited potent anticancer activities under in vitro and in vivo method. The antioxidant nature of any natural products is directly correlated with its anticancer activity. Chitin and chitosan extracted from shrimp shells Penaeus monodon displayed 100% cytotoxic activity against ovarian cancer-cells (PA-1) at concentration of 50 μg/mL and 10 μg/mL and also exhibited DPPH radical scavenging activity of 59.02% and 68.25% at 1000 mg/mL, respectively (Srinivasan et al., 2018). However, Sayari et al. (2016) reported anti-proliferative ability found to have IC50 of 4.6 mg/mL against HCT116 of chitosan from Nephrops norvegicus. Further, chitins from Penaeus kerathurus waste, Carcinus mediterraneus shells and Sepia officinalis showed more than 50% of anticancer action against bladder cancer RT112 cells at a concentration of 330 μM, 100 μM and 62 μM, respectively. Also, they found that degree of acetylation and MW plays vital role in anticancer action. Further, Bouhenna et al. (2015) reported chitin from shrimp P.longirostris displayed 50% cytotoxic activity at 400 μg/mL and 200 μg/mL against Human larynx carcinoma (Hep2) cells and Human embryo rhabdomyosarcoma (Rd) cells. However, this study shows that anticancer activity depends on concentration of chitin and chemical derivatives, for example: electrostatic interactions or hydrophobic reaction between chitin and tumor cells are important for this action. Similarly, chitosan from P.longirostris exhibits cytotoxic activity against Hep2 cells and Rd cells at a concentration of 300 μg/mL and 190 μg/mL, respectively (Sedaghat et al., 2016). Chitin and chitosan mediate anticancer activity by activating immunity which increase cytotoxic T-lymphocytes by the production of lymphokines. Chitosan also increase the levels of IL-1 and IL-2 which lead to production of cytolytic T-lymphocytes (Younes and Rinaudo, 2015). These biological activities of chitin and chitosan obtained from marine invertebrates gave attention to develop a drug in pharmaceutical industry. The chitosan extracted from Scylla sp. had DPPH and hydroxyl radical scavenging activity of 11.37 mg/mL and 1.79 mg/mL, respectively which is higher than commercial chitosan and seaweed polysaccharide (Abirami and Kowsalya, 2015). The sulfated chitosan from Sepia prashadi also exhibits the scavenging activity of 83.5% for superoxide radical and 65% Hydroxyl radical at the concentration of 0.5 mg/mL and 3.2 mg/mL, respectively (Seedevi et al., 2017). The antioxidative mechanism of chitosan was mediated by hydrogen donating ability of amino and hydroxyl groups (attached to C-2, C-3 and C-6 positions of the pyranose ring) to unstable free radicals, which form stable molecule (Younes and Rinaudo, 2015).
2.8 Wound healing action of polysaccharide
Wound healing is a complex physiological process which involves inflammation mediated by necrosis of wounded tissue, granulation and restoration of tissue. The inflammation which response of wound healing migrate fibroblasts and synthesize extra cellular matrix like collagen. The FCS from H.mexicana has high affinity towards fibroblast growth factor 1 and 2, growth factors involved in neovascularization and also inhibited thrombin and factor Xa activation by antithrombin-III (Li et al., 2018). Thus, shell of mullosk extracts used for skin repair systems.
2.9 Antidiabetic action of sulfated polysaccharide
Chitinous materials obtained from demineralized crab shell powder, shrimp head powder, and squid pen powder was fermented using Paenibacillus sp. for production of α-glucosidase inhibition which found to be efficient than Ulvan polysaccharide (Abirami and Kowsalya, 2013). This bacterium used carbon–nitrogen from these chitin, exhibited highest inhibition with IC50 value of 38 µg/mL, 108 µg/mL and 422 µg/mL, respectively. All these chitins were displayed potent action in rat intestinal α-glucosidase as compared to commercial antidiabetic drug fermented nutrient broth shows 81 µg/mL (Nguyen and Wang, 2017). The hypoglycemic mechanism of GAG from Urechis unicinctus was evaluated on diabetic mice. The GAG effectively reduces blood glucose level and increases liver enzymes such as SOD, GSH-Px GCK activity to normal in GAG administrated mice (Yuan et al., 2015a,b).
3 Commercial values of edible marine invertebrate macromolecules
The most important component of marine animal exoskeleton is chitin and chitosan. They are commercially manufactured from marine shell waste, such as squid, shrimp, krill and crabs. Chitosan have wide range of application in food, drug, nutraceutical, cosmetics and agriculture. The world wide utilization of chitin is increasing by 6.82% mainly in Europe and China are potential users because of the greater interest of downstream applications with world share of 63.30% in 2015 (Newswire, 2019). Mainly these chitins are derived in two forms food and industrial grade. As of now the world share of chitin from marine invertebrates was US$ 45 million in 2019, the chitin market will enlist a 4.4% CAGR regarding income, the worldwide market size will reach US$ 59 million by 2024 (Newswire, 2019). Marine derived fucoidan expected to grow at annual growth rate of 3.8% for period of 2018–2023 and will reach $37 million from $30 million. The overall market value of marine polysaccharides expected to grow in the annual growth rate 4.2% over the period of 2017–2026 and polysaccharide market expected to reach more than $18 billion at 2026 (Newswire, 2019).
4 Conclusion and future recommendation
Biopolymer from waste by-products of invertebrates were in high demand rather than using whole organism. Although, numerous researches is being undertaken in this area, the development of novel biopolymers at commercial scale found limited. Sufficient experimental trials are needed to utilize these waste into valorization required to meet global demand. However, administration of these biopolymer in healthcare and food practices leads to the safe selection of raw material, as the chemical constituents might cause adverse effects to the consumer as well as environment. In conclusion, marine invertebrate based biopolymers seem to be promising and futuristic ingredient to utilize as a functional element in food applications and disease avoidance.
Acknowledgements
The authors were grateful to provided necessary facilities at the Fiji National University, Far Eastern Federal University, Sejong University-Republic of Korea and Periyar University. The authors extend their appreciation to National Research Foundation grant (2018R1C1B5086232) funded by Korean Government (MEST) and Talent Research Start-up Project of Guangdong Ocean University (R18007) of their support.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- β-Chitin and chitosan from squid gladius: biological activities of chitosan and its application as clarifying agent for apple juice. Int. J. Biol. Macromol.. 2017;104:953-962.
- [Google Scholar]
- Antidiabetic activity of Ulva fasciata and its impact on carbohydrate metabolism enzymes in alloxan induced diabetic rats. Int. J. Res. Phytochem. Pharmacol.. 2013;3(3):136-141. 20153122040
- [Google Scholar]
- Ulva fasciata nanoparticles characterization and its anticancer activity. World J. Pharm. Pharm. Sci.. 2015;4:1164-1175.
- [Google Scholar]
- Chemical composition, structural features, surface morphology and bioactivities of chitosan derivatives from lobster (Thenus unimaculatus) shells. Int. J. Biol. Macromol.. 2019;135:1237-1245.
- [Google Scholar]
- Characterization and anticoagulant activity of a fucosylated chondroitin sulfate with unusually procoagulant effect from sea cucumber. Carbohydr. Polym.. 2017;174:760-771.
- [Google Scholar]
- Effects of chitin and its derivatives on human cancer cells lines. Environ. Sc. Poll. Res.. 2015;22(20):15579-15586.
- [Google Scholar]
- Arthropod body-plan evolution in the Cambrian with an example from anomalocaridid muscle. Lethaia. 1998;31(3):197-210.
- [Google Scholar]
- An anticoagulant fucan sulfate with hexasaccharide repeating units from the sea cucumber Holothuria albiventer. Carbohydr. Polym.. 2018;464:12-18.
- [Google Scholar]
- Antithrombotics from the Sea: Polysaccharides and Beyond. Mar. Drugs. 2019;17(3):170.
- [Google Scholar]
- Chakravarthy, A., Kammar, V., Shashank, P., 2016. Arthropods: Evolution and Ecology. In Economic and Ecological Significance of Arthropods in Diversified Ecosystems (1-16): Springer.
- Dima, J. B., Sequeiros, C., Zaritzky, N., 2017. Chitosan from Marine Crustaceans: Production, Characterization and Applications: InTech.
- Extraction, chemical modification and characterization of chitin and chitosan. Int. J. Biol. Macromol.. 2018;120:1181-1189.
- [Google Scholar]
- Development of edible film from Acanthophora spicifera: Structural, rheological and functional properties. Food Biosci.. 2018;23:121-128.
- [CrossRef] [Google Scholar]
- Antioxidant and free radical scavenging potential of crude sulphated polysaccharides from Turbinaria ornata. Biologia. 2015;70(1):27-33.
- [Google Scholar]
- Antioxidant and antimicrobial proprieties of chitin and chitosan extracted from Parapenaeus Longirostris shrimp shell waste. Ann. Pharm. Fr.. 2016;74(1):27-33.
- [Google Scholar]
- Industrial applications of crustacean by-products (chitin, chitosan, and chitooligosaccharides): a review. Trends Food Sci. Technol.. 2016;48:40-50.
- [Google Scholar]
- Chitin and chitosan: structure, properties and applications in biomedical engineering. J. Polym. Environ.. 2017;25(3):854-866.
- [Google Scholar]
- Fucosylated chondroitin sulfate oligosaccharides exert anticoagulant activity by targeting at intrinsic tenase complex with low FXII activation: Importance of sulfation pattern and molecular size. Eur. J. Med. Chem.. 2017;139:191-200.
- [Google Scholar]
- A novel structural fucosylated chondroitin sulfate from Holothuria Mexicana and its effects on growth factors binding and anticoagulation. Carbohydr. Polym.. 2018;181:1160-1168.
- [Google Scholar]
- Structural elucidation and biological activity of a highly regular fucosylated glycosaminoglycan from the edible sea cucumber stichopus herrmanni. J. Agric. Food Chem.. 2017;65(42):9315-9323.
- [Google Scholar]
- Purification and characterization of fucose-containing sulphated polysaccharides from Sargassum tenerrimum and their biological activity. J. Appl. Phycol. 2019:1-13.
- [Google Scholar]
- Structural comparison, antioxidant and anti-inflammatory properties of fucosylated chondroitin sulfate of three edible sea cucumbers. Carbohydr. Polym.. 2018;185:41-47.
- [Google Scholar]
- Preparation and antioxidant properties of low molecular holothurian glycosaminoglycans by H2O2/ascorbic acid degradation. Int. J. Biol. Macromol.. 2018;107:1339-1347.
- [Google Scholar]
- Purification, structural characterization and anticoagulant properties of fucosylated chondroitin sulfate isolated from Holothuria mexicana. Int. J. Biol. Macromol.. 2017;98:208-215.
- [Google Scholar]
- Newswire, G., 2019. Chitosan Market Value To Reach Around US$ 22 Billion by 2026. Retrieved from https://www.globenewswire.com/news-release/2019/06/15/1869271/0/en/Chitosan-Market-Value-To-Reach-Around-US-22-Billion-by-2026.html.
- Reclamation of marine chitinous materials for the production of α-glucosidase inhibitors via microbial conversion. Mar. drugs. 2017;15(11):350.
- [Google Scholar]
- Current status and new perspectives on chitin and chitosan as functional biopolymers. Appl. Biochem. Biotechnol.. 2017;181(4):1314-1337.
- [Google Scholar]
- Biology and Ecology of Edible Marine Bivalve Molluscs. Apple Academic Press; 2018.
- Chitin and chitosan from the Norway lobster by-products: antimicrobial and anti-proliferative activities. Int. J. Biol. Macromol.. 2016;87:163-171.
- [Google Scholar]
- Chitin from Penaeus merguiensis via microbial fermentation processing and antioxidant activity. Int. J. Biol. Macromol.. 2016;82:279-283.
- [Google Scholar]
- Evaluation of antioxidant activities and chemical analysis of sulfated chitosan from Sepia prashadi. Int. J. Biol. Macromol.. 2017;99:519-529.
- [Google Scholar]
- Precise structures of fucosylated glycosaminoglycan and its oligosaccharides as novel intrinsic factor Xase inhibitors. Eur. J. Med. Chem.. 2018;148:423-435.
- [Google Scholar]
- Preparation, characterization and antibacterial activity of chitosan and phosphorylated chitosan from cuttlebone of Sepia kobiensis (Hoyle, 1885) Biotechnol. Rep.. 2016;9:25-30.
- [Google Scholar]
- Chitin and chitosan preparation from shrimp shells Penaeus monodon and its human ovarian cancer cell line, PA-1. Int. J. Biol. Macromol.. 2018;107:662-667.
- [Google Scholar]
- A highly regular fucosylated chondroitin sulfate from the sea cucumber Massinium magnum: structure and effects on coagulation. Carbohydr. Polym.. 2017;167:20-26.
- [Google Scholar]
- The sea as a rich source of structurally unique glycosaminoglycans and mimetics. Microorganisms. 2017;5(3):51.
- [Google Scholar]
- Anticoagulant and antithrombotic properties of three structurally correlated sea urchin sulfated glycans and their low-molecular-weight derivatives. Mar. Drugs. 2018;16(9):304.
- [Google Scholar]
- Therapeutic properties and uses of marine invertebrates in the ancient Greek world and early Byzantium. J. Ethnopharmacol.. 2010;130(2):237-247.
- [Google Scholar]
- Modulating the degree of fucosylation of fucosylated chondroitin sulfate enhances heparin cofactor II-dependent thrombin inhibition. Eur. J. Med. Chem.. 2018;154:133-143.
- [Google Scholar]
- Separation, purification, structures and anticoagulant activities of fucosylated chondroitin sulfates from Holothuria scabra. Int. J. Biol. Macromol.. 2018;108:710-718.
- [Google Scholar]
- Structural analysis and anticoagulant activities of two sulfated polysaccharides from the sea cucumber Holothuria coluber. Int. J. Biol. Macromol.. 2018;115:1055-1062.
- [Google Scholar]
- Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar. Drugs. 2015;13(3):1133-1174.
- [Google Scholar]
- Hypoglycemic effects of glycosaminoglycan from Urechis unicinctus in diabetic mice. J. Med. Food. 2015;18(2):190-194.
- [Google Scholar]
- Structural Characterization and Immunostimulatory Activity of a Homogeneous Polysaccharide from Sinonovacula constricta. J. Agric. Food Chem.. 2015;63(36):7986-7994.
- [Google Scholar]
- Anticoagulant Activity and Structural Characterization of Polysaccharide from Abalone (Haliotis discus hannai Ino) Gonad. Molecules. 2016;21(6):697.
- [Google Scholar]







