Translate this page into:
Biomedical and histological evidence of Boswellia sp. Burseraceae on kidney and liver function in mice
⁎Corresponding author. s.alosaimi@tu.edu.sa (Saad H. Alotaibi)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
Boswellia sp. is a species of Boswellia. Burseraceae is an essential conventional natural product that has received a lot of attention in the pharmaceutical industry in recent years. The aim of this study was to role of Boswellia sp. Burseraceae on kidney and liver of Balb/c mice. Different concentrations of Boswellia sp. Burseraceae solution was given to 5–7 weeks old wild-type Balb/c mice of both sexes (n = 24) for 21 days, with a control group given normal water to determine the physiological, biochemical, haematological, (0.5%, 1%), and histopathological effect of (0.5%, 1% and 5%) Boswellia sp..The results of this study showed that physiological parameters were changed with the increasing concentration of Boswellia sp. Burseraceae solution; like daily food consumption was reduced and fluid intake was more, decrease in body weight, urine output was low with less Na+ & K+ resulting into hypernatremia, hyperkalemia and hyperuricemia. Also elevated creatinine level in blood increase levels of bilirubin, Aspartate Aminotransferase (AST), Alanine Aminotransferase (ALT), Alkaline Phosphatase (ALP) and degeneration of cells, glomerular atrophy of kidney cells and degeneration of hepatocytes. In conclusion, all of the results support to dosage toxicity. Biochemical indicators such as white blood cells, glucose, and haemoglobin did not alter significantly. Based on the findings, it is concluded that the extract exhibits dosage toxicity, which is corroborated by histopathological indicators.
Keywords
Boswellia sp. Burseraceae
Physiological
Biochemical
Histopathological
Dose toxicity
1 Introduction
The pharmacological potential of plants is used and compared to synthetic drugs. Boswellia tree is well known for fragrant resin, a frankincense is the extract from the resin, resin reported to have many therapeutic uses, Boswellia has many species and native to tropical regions of Africa, Asia and the Americas. Frankincense is produced by 20 members of the genus Boswellia (Al-Harrasi et al., 2018), oleoresin is also called as “lubban” which means “white/cream”, Ethopia as “etan” in Greek as “libanos” in French as “Frankincense”. It has been used since ancient times for its antiseptic (Alotaibi, 2019), anti- arthritis and anti-inflammatory effects in folk medicine (Asif et al., 2014). Oleoresin has therefore been dedicated to better understanding their medical effects and describing the ingredients responsible for those effects throughout the last 20 years by researchers. A yellowish-brown oleogum resin with its essential oil was popular for its improving effects on human ailments (Perveen et al., 2018). Boswellia sacra containing the human cell metastatic cancer line MDA-MB-231, demonstrated cytotoxic efficacy (Michael et Al., 2019). Likewise, Boswellic acid triterpene pentacyclic in Boswellia serrata and B. carteri showed effective antidiabetic (Azemi et al., 2012), anti-arthritis, asthma, cancer, inflammatory bowels, Parkinson’s and Alzheimer's diseases (Beghelli et al., 2017). Boswellic acids, which are generated by the Boswellia carterii plant, exhibit immunomodulatory effects, inhibiting TH1 cytokines while encouraging TH2 cytokines in vitro (Bertocchi et al., 2018). The resin of Boswellia serrata exhibited diuretic action (Burlando et al., 2008). Three-dimensional NMR investigations and mass spectrometry techniques were utilized to identify Burseraceae based on chemical or spectral evidence for the atriterpenic lupan structure of Boswellia sp frankincense. The existence of several components by alkaloids, carbohydrate, and saponins was detected during phytochemical testing carried out by high-performance liquid chromatography (HPLC) and fingerprint of Boswellia sp. Burseraceous (Chevrier et al., 2005). In additional trials, of Boswellic Acid proved to be an important medication for chronic disease clinical management.
The current study evaluates the various pharmacological abilities of Boswellia sp. to crucial health aspects such as biochemistry, physiology, hematology, and histology. Boswellia has a fragrant resin known as Frankincense, which has been used as traditional medicine for many years for therapeutic ailments. Boswellia species have been known for many pharmacological effects, researchers have shown the medicinal properties of this plant, but few have shown the plant's toxicity. The current study found toxicity in Burseraceae species, with the extract having a negative effect on kidney and hepatocytes. As a result, this extract could not be employed in clinical studies.
2 Methods
2.1 Experimental methods
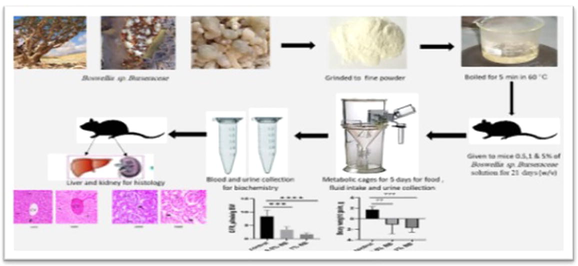
The current study was carried out with seven weeks old wild-type mice’s (n = 24), pursued from King Fahd Medical Research from Jeddah city. The animals were transferred under controlled environmental condition (22–24 °C; 50–70% humidity with a 12-hour of both light and dark cycles). Mice were provided with normal pelleted food and tap water, or drinking water with Boswellia extract of 0.5%, 1%, and 5% for 21 days. In all experiment’s animals were treated humanely and in accordance with regulations pertaining to their well-being and their rights.
2.2 Burseraceae solution preparation Boswellia Sp.
The crude form of Boswellia sp. Burseraceae Omani species were purchased from a local market in Taif, Saudi Arabia, and approved by plant specialist. The crude form of Boswellia sp. Burseraceae, was grained and mixed with normal water then boiled for 5 min, boiled for 5 min in 60 °C, the supernatant was taken and given to mice as follows, 0.5%, 1% and 5% of for 21 days, with control group given normal water for the same period.
2.3 Collection of urine and feces
All animals have been individually put in metabolic cages (Tecniplast, Hohenpeissenberg, China), for five days after 16 days of treatment with Boswellia sp Burseraceae solution. The daily food, fluid intake and 24-hour urine collection were measured during metabolic cages experiment and all collections were performed with control and treated group of Boswellia sp. Burseraceae solution.
2.4 Collection of blood
On the final day of experiment blood sample was obtained from all animals, extracting the blood sample for several biochemical analyses by punching the retro-orbital plexus, withdrawing it into the blood collection tube, replacing blood losses with 400 μl 0.9% of NaCl subcutaneously.
2.5 Biochemistry analysis
Liver and kidney function tests were performed with plasma and urine concentrations of both sodium and potassium were measured with flame photometry (Eppendorf, Germany). Biochemical tests such as plasma and urinary samples for creatine and urea was measured and with blood samples such as Alkaline Phosphatase (AP), Alanine Aminotransferase (ALT), Aspartate Aminotransferase (AST), Bilirubin (BOL), Cholesterol (CHL), High Density Lipoprotein (HDL), Triglycerides (TRI), was measured with Biolabo kits (France). Fasting blood glucose (8 h) was measured with glucometer (Roche, Germany). Using this method, a complete blood image and a photometric hemoglobin unit were calculated (MD-140a 140 from Medical Diagnostics Marx). All measurements were carried according to manufacturing requirements.
2.6 Histopathology analysis
For histological analysis mice were sacrificed on the last day of experiment by cervical dislocation under ether anesthesia. Saline water was poured on the kidney and liver organs and then fixed in formalin, after which they were cut into small sections and preserved in 10% formalin for three days. In ascender grades of ethyl alcohol, which ranges from 70, 80, 90 and to 95% for 60 mins each and finally for two adjustments of absolute ethyl alcohol (100%) for 60 mins per tissue washed with buffered saline. Two xylene changes for 60 min followed. Tissue is impregnated at 60 °C for 60 mins with melted paraffin wax (3 changes) and incorporated into paraffin wax. The tissue processor machine (Tissue-Tek VIP 5 Jr) from Sakura, Japan was used to perform all previous processing steps automatically. The Rotary Microtome, RM 2245, was used to cut 4 m parts (Leica, Germany). Dewaxed, hydrated, and stained for 5 min in Harris hematoxylin solution. The sections were stained in eosin for 3 min, washed in tap water, and then held in petri dishes that were each placed in ascending ethanol at 68 °C for different periods. Hematoxylin-and-and-eosin stained sections were viewed (Cordatos, 2002), for pathological alterations under a microscope (Nikon, Eclipse i80). The pictures were captured at various enlargements using a digital camera, which ranged from low-to-extreme zoom (OXM 1200C, Nikon, Japan).
2.7 Statistical analysis
The statistical analysis was performed using GraphPad Prism (version-8.4.3.686). The data was framed as mean (±) standard deviation and n indicates the number of independent experiments. Data was analyzed for paired students and one-way Anova analysis. The statistical analysis was considered as p values less than 0.05 as significant association.
3 Results
Results of physiological, biochemical, hematological (0.5% and 1%) and histopathological (0.5%,1% and 5%) studies were done with Boswellia sp. Burseraceae solution after 21 days of compared with control group given normal tape water. The effect of Boswellia sp. Burseraceae solution showed that there was decrease in weight loss, more of fluid intake with less food intake and less urinary output hence less fecal weight in (Table 1) and (Figs. 1–3). ** indicates very significant (P ≤ 0.01), difference between control and treated animal. *** indicates highly significant (P ≤ 0.001), difference between control and treated animal.
Parameters
Control
B. sp. Burseraceae (0.5%)
B. sp. Burseraceae (1%)
Body Weight, baseline (g).
19.59 ± 0.55
20.50 ± 0.38
21.63 ± 1.05
Body Weight, end (g).
21.30 ± 0.44
19.39 ± 0.89
19.84 ± 0.79
Body weight gain (g).
1.72 ± 0.23
−1.11 ± 0.72**
−1.79 ± 0.33***
Food Intake (g/24 h).
1.34 ± 0.19
0.96 ± 0.13
0.85 ± 0.16
Fecal wet weight (g/24 h).
0.47 ± 0.03
0.41 ± 0.05
0.38 ± 0.03
Fecal dry weight (g/24 h).
0.38 ± 0.02
0.33 ± 0.04
0.31 ± 0.03
Fluid Intake (ml/24 h).
2.96 ± 0.37
3.72 ± 0.17
4.39 ± 0.81
Urine output (ml/24 h).
1.02 ± 0.09
0.38 ± 0.10***
0.36 ± 0.10***
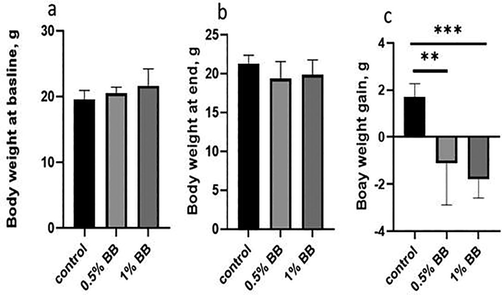
Effect of d Effect of (0.5% and 1%), Boswellia sp. Burseraceae solution on body weight at baseline (a), body weight at end (b) and body weight gain (c), as compared to control after 21 days of treatment. ** indicates very significant (P ≤ 0.01), difference between control and treated animal. *** indicates highly significant (P ≤ 0.001), difference between control and treated animal.
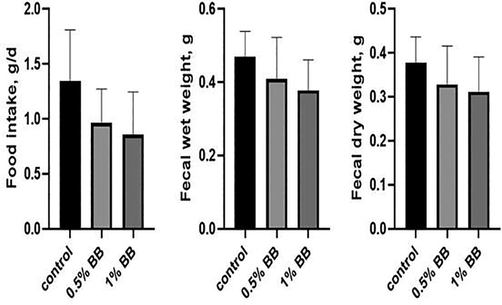
Effect of d Effect of (0.5% and 1%), Boswellia sp. Burseraceae solution on food intake (a), fecal wet weight (b) and fecal dry weight (c), as compared to control after 21 days of treatment.
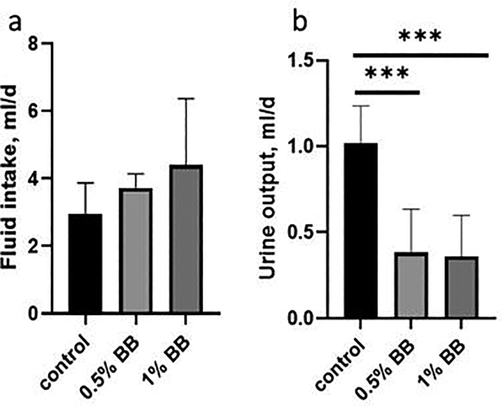
Effect of d Effect of (0.5% and 1%), Boswellia sp. Burseraceae solution on fluid intake (a), and urine output (b), as compared to control after 21 days of treatment, *** indicates highly significant (P ≤ 0.001), difference between control and treated animal.
The symptoms of hyperkalemia high levels of K+ blood, and less levels of K+ in urine. As kidneys cannot remove the extra potassium from the blood, there is less excretion of potassium in urine and high levels of potassium in blood causing hyperkalemia, this condition can lead to cardiac arrest and death, (Table 2). Thus, the kidney is the major long-term regulator of Na+ and K+ homeostasis. Hence the histopathological studies of kidney with control sample shows the normal glomerulus and renal tubules (Fig. 4a, b), whereas the kidney after treatment of (0.5%) Boswellia sp. Burseraceae solution, for 21 days, showing, degeneration and cell swelling of tubular (Fig. 4c, d), (1%) Boswellia sp. Burseraceae solution showing glomerular atrophy and proximal tubules dilation (Fig. 4e,f), disorganized tubular appearance and degeneration with (5 %) Boswellia sp. Burseraceae solution, after 21 days (Fig. 4g, h). * indicates significant (P ≤ 0.05), difference between control and treated animal. ** indicates very significant (P ≤ 0.01), difference between control and treated animal. *** indicates highly significant (P ≤ 0.001), difference between control and treated animal. **** indicates extremely significant (P ≤ 0.0001), difference between control and treated animal.
Parameters
Control
B. sp. Burseraceae (0.5%)
B. sp. Burseraceae (1%)
Urinary Na+ excretion, (mmol/24 h).
239.00 ± 13.44
145.00 ± 6.83
133.33 ± 9.55
Plasma Na+ conc., (mM).
141.58 ± 1.63
167.26 ± 1.36
169.55 ± 4.59
Urinary K+ excretion, (mmol/24 h).
664.45 ± 20.07
562.0 ± 27.01
560.00 ± 19.54
Plasma K+ conc,, (mM).
4.47 ± 0.17
7.26 ± 0.49
7.41 ± 0.42
Urinary Ca2+ excretion, (mmol/24 h).
35.39 ± 0.65
32.85 ± 2.66
33.89 ± 0.87
Plasma Ca2+conc., (mM).
2.28 ± 0.17
2.86 ± 0.16
3.03 ± 0.20
Urinary urea excretion, (mg/dl).
19.63 ± 1.64
16.50 ± 2.37
14.20 ± 1.92
Blood urea conc., (mg/dl).
27.68 ± 3.56
47.95 ± 9.62
70.68 ± 5.29*
Urinary creatinine excretion, (mg/dl).
27.02 ± 4.18
19.04 ± 2.02
18.65 ± 1.65
Plasma creatinine conc. (mg/dl).
0.36 ± 0.04
0.56 ± 0.06*
0.69 ± 0.06**
Glomerular Filtration Rate (GFR), (µl/min/g BW).
8.40 ± 0.98
3.40 ± 0.45***
1.67 ± 0.23****
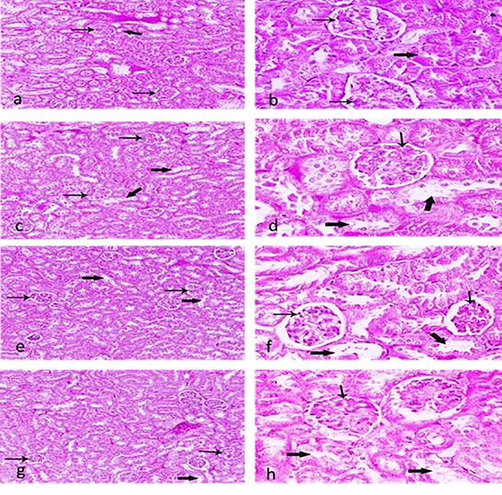
Section of kidney showing Glomerulus (thin arrow), Renal tubules (thick arrow) (a,b) control group, (c,d), (0.5%) of Boswellia sp. Burseraceae solution, showing degeneration, cell swelling of tubular, (e,f), (1%) of Boswellia sp. Burseraceae solution , showing glomerular atrophy and proximal tubules dilation, (g,h), (5%) of Boswellia sp. Burseraceae solution showing disorganized tubular appearance and degeneration. (H & E stain). (Magnification, C.100 X and D. 400X).
Changes in plasma sodium may also affect blood pressure directly. This condition is called Hypernatremia (high level of sodium in the blood causes elevated blood pressure results in cardiovascular disease i.e. stroke, low levels of sodium in urine may causes diarrhea, indicate kidney problems, glomerulonephritis, hepatorenal syndrome or kidney failure, cirrhosis, elevated aldosterone levels, heart failure. Urinary urea decreases, as the number of Boswellia sp. Burseraceae solution in (Table 2), increases, low urinary urea concentrations may mean malnutrition, or low protein in the diet. The highest serum urea levels would minimize the urinary removal of urea because of advanced renal diseases and related significant decrease of the glomerular filtration rate (GFR) High serum uric acid disorder is called kidney disease hyperuricemia, which causes high blood pressure. Decrease in urinary creatinine was observed when treated with Boswellia sp. Burseraceae solution compared to control this can lead to coronary kidney diseases. Higher blood creatinine also means compromised kidney or kidney failure. As the result the creatinine abnormally increases its level in blood warn the possible malfunction of failure of the kidneys, decrease in creatinine clearance indicates decreased glomerular filtration rate (GFR) and impaired renal function (Table 2) and (Fig. 5a,c).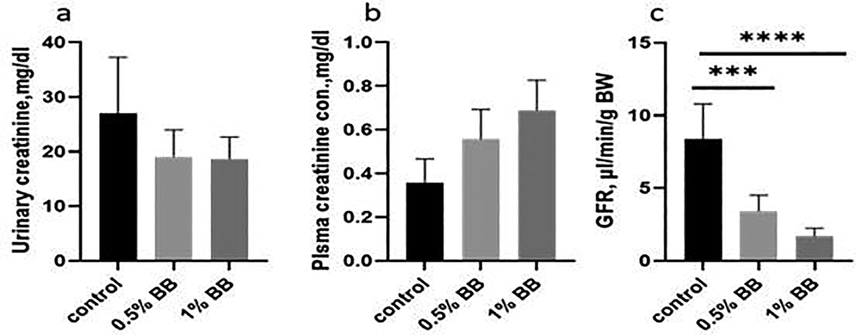
Effect of d Effect of (0.5% and 1%), Boswellia sp. Burseraceae solution on urinary creatinine (a), plasma creatinine (b) and glomerular filtration rate (GFR) (c), as compared to control after 21 days of treatment, difference between control and (0.5% and 1%), Boswellia sp. Burseraceae after 21 days of treatment. *** indicates highly significant (P ≤ 0.001), difference between control and treated animal. **** indicates extremely significant (P ≤ 0.0001), difference between control and treated animal.
The results showed the increasing hypoglycemic effect of Boswellia sp. Burseraceae solution in (Table 3), on fasting blood sugar levels, increasing hyperglycemic effect of Boswellia sp. Burseraceae solution on Non-fasting blood glucose suggest that fluid intake is caloric, later leads to diabetes. Triglycerides were relatively increased with the increasing concentration of Boswellia sp. Burseraceae solution, triglycerides in your blood can raise the risk of heart diseases such as elevated hypertension and diabetes. This extract suggests the presence of carbohydrate or sugary based. * indicates significant (P ≤ 0.05), difference between control and treated animal. ** indicates very significant (P ≤ 0.01), difference between control and treated animal. *** indicates highly significant (P ≤ 0.001), difference between control and treated animal. **** indicates extremely significant (P ≤ 0.0001), difference between control and treated animal.
Parameters
Control
B. sp. Burseraceae (0.5%)
B. sp. Burseraceae (1%)
Alkaline Phosphatase (ALP), U/L.
101.78 ± 1.07
200.90 ± 18.26***
208.03 ± 17.34***
Alanine Aminotransferase (ALT), U/L.
42.14 ± 5.65
51.86 ± 3.53
70.53. ± 2.91***#
Aspartate Aminotransferase (AST), U/L.
57.56 ± 2.75
93.10 ± 2.31*
109.20 ± 12.95**
Bilirubin (BOL), mg/dl.
0.28 ± 0.03
0.33 ± 0.09
0.40. ± 0.01
Cholesterol (CHO), mg/dl.
53.00 ± 3.21
81.60 ± 13.28
103.50 ± 2.77**
High Density Lipoprotein (HDL), mg/dl.
30.00 ± 0.45
53.80 ± 2.30****
60.50. ± 1.23****#
Triglycerides (TRI), mg/dl.
54.60 ± 3.29
55.40 ± 1.76
58.33 ± 5.17
Fasting blood glucose (FBG), mg/dl.
80.83 ± 3.72
87.50 ± 2.62
93.50 ± 1.48*
Non-fasting blood glucose (N-FBG), mg/dl.
97.60 ± 5.47
117.20 ± 3.26*
128.00 ± 5.73**
There were no major effects on white blood cells (WBC), haemoglobin (Hb), mean corpuscular volume (MCV), granulocytes (GRA), lymphocytes (LYM), MIDC in data on haematology in mice with a growing concentration of Boswellia sp. Burseraceae solution (includes combine value of all types of blood cells such as monocytes, eosinophils, basophils and lymphocytes). With the above parameters, (Table 4) did not find significant higher levels. * indicates significant (P ≤ 0.05), difference between control and treated animal. ** indicates very significant (P ≤ 0.01), difference between control and treated animal.
Parameters
Control
B. sp. Burseraceae (0.5%)
B. sp. Burseraceae (1%)
White Blood Cells (WBC), 10^3/ml.
6.18 ± 0.52
7.22 ± 0.14
7.37 ± 0.63
Lymphocyte (LYM) , 10^3/ml.
4.22 ± 0.24
4.43 ± 0.47
4.57 ± 0.72
Lymphocyte (LYM), %.
91.48 ± 0.71
91.65 ± 0.60
91.97 ± 0.96
Granulocytes (GRA), 10^3/ml.
0.18 ± 0.02
0.17 ± 0.03
0.15 ± 0.02
Granulocytes (GRA), %.
2.58 ± 0.31
2.30 ± 0.37
2.27 ± 0.39
MID, %.
4.84 ± 0.29
5.53 ± 0.32
6.52 ± 0.83
Hemoglobulin (Hb), g/l.
14.24 ± 0.40
12.48 ± 0.54*
11.95 ± 0.44**
Mean Corpuscular Volume (MPV), fl.
7.22 ± 0.23
6.10 ± 0.09**
5.93. ± 0.29**
Cholesterol was relatively high with the increasing concentration of Boswellia sp. Burseraceae solution but high levels of cholesterol can increase your risk of heart disease. Elevated bilirubin was observed with the increasing concentration of Boswellia sp. Burseraceae solution showing inflammation in hepatocytes, this could be the Gilbert’s syndrome, high levels can lead to jaundice as compared to normal liver cells in (Fig. 6 a, b). Similarly Aspartate Aminotransferase (AST) was also elevated with increasing concentration of the extract suggesting that any damage in liver, increasing AST levels in blood is Alanine Aminotransferase (ALT) is an enzyme found in the liver and kidney cells, ALT is found to be high in any case of damage of liver and kidney cells, our results showed the increasing concentration of ALT with increasing concentration of Boswellia sp. Burseraceae solution also histopathological evidences showed degeneration of hepatocytes. It is a sign of a problem with the liver or the gallbladder if ALP increases above the normal level more likely than otherwise, this may include hepatitis, cirrhosis, gallstones, or the liver is having a blockage in the bile ducts. Our results coincide with the increase ALP with increasing concentration of Boswellia sp. Burseraceae solution supported by liver showing sever focal degeneration of hepatocytes, revealing inflammatory cells of hepatocytes, degenerative cells with massive congested blood vessels in (Table 3), (Fig. 6 c,h).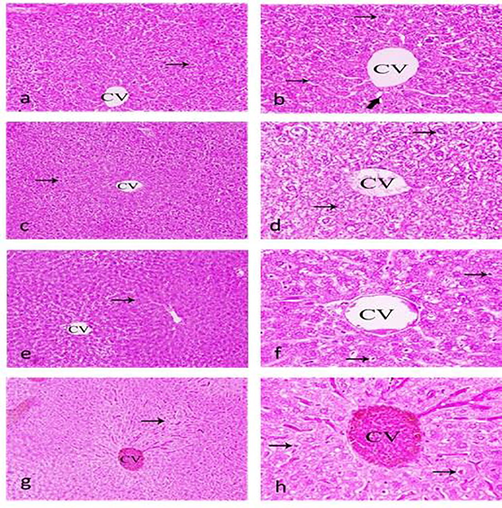
Sections of liver showing normal hepatic tissues. hepatocytes (thin arrow), Kupffer cells (thick arrow) central vein (CV), (a,b) control group, (c,d), (0.5%) of Boswellia sp. Burseraceae solution, showing sever focal degeneration of hepatocytes, central vein, (e,f), (1%) of Boswellia sp. Burseraceae solution, showing, hepatic cells, central vein, fatty degeneration with vacuolization and sinusoid dilation, (g,h), (5%) of Boswellia sp. Burseraceae solution showing, hepatocytes, central vein, this group revealed inflammatory cells, focal degeneration cells concomitant with massive congested blood vessels. (H & E stain), (Magnification, C.100 X and D. 400X).
4 Discussion
The use of natural compounds in the prevention and treatment of various chronic diseases has taken on increasing significance over recent years because they lack significant toxicities. Boswellia was used from ancient times to treat ailments (Hamidpour et al., 2013). Frankincense from different species of Boswellia is known for antiseptic (Hussain et al., 2013), anti-inflammatory, ant arthritis (Khan, 2012), antidiabetic (Kiela et al., 2005), immunomodulatory (Mantle et al., 2001), diuretic (Maraghehpour et al., 2016), although cytotoxicity or proliferative stimulation can occur (Nayeem and Quadri, 2015), Hepatotoxic effect of Boswellia with pronounce hepatomegaly and steatosis was observed (Roy et al., 2019). In vitro cytotoxicity studies have also been observed on human skin cell lines (Schmiech et al., 2019).
Recent diuretic activity in Boswellia serrata extracts has been associated with diarrhea, urticaria, nausea, and skin rashes (Siddiqui, 2011). The other skin disorders were also observed (Su et al., 2015). Previous studies from triterpene lupan structure of Boswellia sp. frankincense, Burseraceae concludes as there is a lack of studies dealt with Boswellia on toxicity, therefore, the current study indicates an assessment of the impact of Boswellia sp. Burseraceae as natural supplement on all parameters like physiological parameters, biochemical, haematological and histopathological.
These results clearly state that the extract of Boswellia sp. Burseraceae solution contains carbohydrate or sugary taste where the animal had more fluid intake compared to food intake, with increasing concentration of the fluid intake of Boswellia sp. Burseraceae solution with the animal, it was also shown that the Na+ and K+ was less excreted and found increasing level in blood (hypernatremia, hyperkalemia), decrease in urinary urea, highest levels of serum urea, hyperuricemia, decrease in urinary creatinine, elevated creatinine level in blood, increasing hypoglycaemic effect on fasting blood sugar levels, increasing hyperglycaemic effect on Non-fasting blood glucose. High cholesterol, elevated bilirubin, and similar elevated values for AST, ALT, and ALP in blood indicate that this drug is affecting liver and kidney cells, as evidenced by histological evidence of kidney and hepatocyte cell damage. The clinical study on Boswellia sp. Burseraceae extracts would be risky because it would compare the outcomes on mice.
5 Conclusion
The Boswellia sp., Burseraceae solution displayed dosage toxicity, considerable hyperglycemic, hyperlipidemic, hyperbilirubinemia, and this investigation revealed that Boswellia sp., Burseraceae frankincense had toxic effects on the kidneys and hepatocytes. As the results showed, there was a considerable increase in the levels of liver and kidney function tests. Because the animal was not susceptible to bacterial infection, hematological parameters yielded ineffective findings. Despite the fact that the entire extract has disadvantages, these findings do not support the use of this extract in traditional medicine
Acknowledgement
We acknowledge Taif University for Researchers Supporting Project Number (TURSP-2020/83), Taif University, Taif, Saudi Arabia.
Funding
This work was suported by Taif University for Researchers Supporting Project Number (TURSP-2020/83), Taif University, Taif, Saudi Arabia.
Declarations
Author(s) declare that all works are original and this manuscript has not been published in any other journal.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Chemical, molecular and structural studies of Boswellia species: β-Boswellic Aldehyde and 3-epi-11β-Dihydroxy BA as precursors in biosynthesis of boswellic acids. PLoS One. 2018;13(6):e0198666.
- [Google Scholar]
- Biophysical properties and finger print of Boswellia Sp. Burseraceae. Saudi J. Biol. Sci.. 2019;26:1450-1457.
- [Google Scholar]
- Diuretic activity of Boswellia serrata Roxb. oleo gum extract in albino rats. Pak. J. Pharm. Sci.. 2014;27:1811-1817.
- [Google Scholar]
- The antioxidant capacity and anti-diabetic effect of Boswellia serrata triana and planch aqueous extract in fertile female diabetic rats and the possible effects on reproduction and histological changes in the liver and kidneys. Jundishapur J. Nat. Pharm. Products. 2012;7:168.
- [Google Scholar]
- Beghelli, D., Isani, G., Roncada, P., Andreani, G., Bistoni, O., Bertocchi, M., Lupidi, G., Alunno, A., 2017. Antioxidant and ex vivo immune system regulatory properties of Boswellia serrata extracts. Oxidative Med. Cell. Longevity, 2017.
- Bertocchi, M., Isani, G., Medici, F., Andreani, G., Tubon Usca, I., Roncada, P., Forni, M., Bernardini, C. 2018. Anti-inflammatory activity of Boswellia serrata extracts: an in vitro study on porcine aortic endothelial cells. Oxidative Med. Cell. Longevity (2018).
- Comparison of the irritation potentials of Boswellia serrata gum resin and of acetyl-11-keto-β-boswellic acid by in vitro cytotoxicity tests on human skin-derived cell lines. Toxicol. Lett.. 2008;177(2):144-149.
- [Google Scholar]
- Boswellia carterii extract inhibits TH1 cytokines and promotes TH2 cytokines in vitro. Clin. Diagn. Lab. Immunol.. 2005;12:575-580.
- [Google Scholar]
- (乳香 Rǔ Xiāng; Boswellia species): from the selection of traditional applications to the novel phytotherapy for the prevention and treatment of serious diseases. J. Tradit. Complem. Med.. 2013;3:221-226.
- [Google Scholar]
- Chemistry and biology of essential oils of genus boswellia. Evidence-Based Complem. Alternative Med.. 2013;2013:1-12.
- [Google Scholar]
- Medicinal properties of frankincense. Int. J. Nutrit., Pharmacol., Neurol. Dis.. 2012;2:79.
- [Google Scholar]
- Effects of Boswellia serrata in mouse models of chemically induced colitis. Am. J. Physiol.-Gastrointestinal Liver Physiol.. 2005;288:G798-G808.
- [Google Scholar]
- Adverse and beneficial effects of plant extracts on skin and skin disorders. Adverse Drug React. Toxicol. Rev.. 2001;20:89-103.
- [Google Scholar]
- Traditionally used herbal medicines with antibacterial effect on Aggegatibacter actinomycetemcomitans: Boswellia serrata and Nigella sativa. J. Indian Soc. Periodontol.. 2016;20:603.
- [Google Scholar]
- Comparative analysis of pentacyclic triterpenic acid compositions in oleogum resins of different boswellia species and their in vitro cytotoxicity against treatment-resistant human breast cancer cells. Molecules. 2019;24(11):2153.
- [CrossRef] [Google Scholar]
- Evaluation of boswellia serrata leaf extracts in albino mice. Int. J. Pharm. Pharm. Sci.. 2015;7:502-505.
- [Google Scholar]
- Antifungal activity of essential oil of Commiphora molmol Oleo Gum Resin. J. Essential Oil Bear. Plants. 2018;21:667-673.
- [Google Scholar]
- Roy, N.K., Parama, D., Banik, K., Bordoloi, D., Devi, A.K., Thakur, K.K., 2019. Padmavathi, G., Shakibaei, M., Fan, L., Sethi, G. An update on pharmacological potential of boswellic acids against chronic diseases. Int. J. Mol. Sci., 20, 4101.
- Boswellia serrata, a potential antiinflammatory agent: an overview. Indian J. Pharmaceut. Sci.. 2011;73:255.
- [Google Scholar]
- Frankincense and myrrh suppress inflammation via regulation of the metabolic profiling and the MAPK signaling pathway. Sci. Rep.. 2015;5:1-15.
- [Google Scholar]







