Translate this page into:
Biological studies of freshwater fishes, Cyprinion acinaces and Carasobarbus apoensis, from Wadi Khadrah, Saudi Arabia
⁎Corresponding author. zahmed@ksu.edu.sa (Z. Ahmad)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The present study deals with the analysis of different indices, length weight relationship and age-length relationship of Cyprinion acinaces and Carasobarbus apoensis. The condition factor (K1) for combined sexes ranges from 1.22 to 1.02 for C. acinaces, highest in the spring season and lowest in winter, respectively. The hepato-somatic index for this species was high (3.06) in summer and lowest value (1.67) was recorded in fall season. The K1 values ranges from 1.35 (Winter) to 0.74 (Fall) where as hepatosomatic index was high (2.53) in Fall and lowest value (1.46) was recorded in Winter for C. apoensis. The values of b registered were 2.587 and 2.305 for C. acinaces and C. apoensis, respectively. The relationship for age-length were recorded as Lt = 12.106{1 − e−0.80(t+(0.26))} for C. acinaces and Lt = 23.806{1 − e−0.25(t+(0.11))} for C. apoensis. It was also registered in both the species that the increment in the length was decreased with the increase in age but the yearly increase in weight was high with the increase in age. Length at previous years (back calculated length) were also calculated using year marks (annuli) formed on the scale. It was registered that the increment in length was more in the first and second year of life which decreased in subsequent years.
Keywords
Cyprinion acinaces
Carasobarbus apoensis
Biology
Indices
Wadi Khadra
1 Introduction
Cyprinion acinaces and Carasobarbus apoensis belong to the family cyprinidae and are endemic to South West Arabian Peninsula. There is paucity of knowledge on the biological aspect of these species. Determination of age and growth parameter of fish is an essential tool useful in formulating the means for rational exploitation of the fishery resources. The role of age and growth are the attributes of great importance in harvesting the fish population and responses exhibited by them in various aspect of managements (Bhatt et al., 2000). Annual production of fish depends on the growth rate. Segregation of age groups in the catch of fish generally helps in the use of gears to avoid the fishing of undesired age-groups of fish. Future stocks of fishes can also be predicted from the age groups composition of the catch. This information is also useful in estimating age at which fish matures, mortality rate in the population and maximum size and age of species for fishing (Khan and Khan, 2014). Growth models reveal age of fish to their length or weight and are considered as final product of growth analysis (Jones, 2000). A number of growth models are available in which von Bertalanffy growth model is widely used. Some of the important contribution on the aspect of age and growth are those of Chang et al. (2012); Ujjania et al. (2012); Mayank et al. (2015); Sapounidis et al. (2015); Uckun and Gokce (2015); Abu El-Nasr (2017); Ortego-Garcia et al. (2017) and Hakami et al. (2018).
The mathematical expression of the relationship between the total length and total weight of the fish provide important information which may be helpful in formulating the management means of a fishery for its judicious exploitation. This relationship also demonstrates the well-being (relative robustness) and suitability of the environment to the particular species of fish. Besides this, the mathematical relationship between the length and weight is used for interconversion (Lagler, 1982). This relationship allows the estimation of the fish weight by using the fish length and can be used to estimate the gonadal development, rate of feeding and level of gonads maturation (Jisr et al., 2000). Some important contribution in the field of length weight relationship was made by Ortego-Garcia et al. (2017), Jisr et al. (2000), Oliveira et al. (2020) and Salahi et al. (2020). The different indices like condition co-efficient (K) also provide information regarding well-being of fish and suitability of habitat for the species. Notable contribution in this field were made by Sapounidis et al. (2015), Uckun and Gokce (2015), Jisr et al. (2000) and Oliveira et al. (2020). Hepato-somatic index is very important factor and provide information regarding the availability of food and its assimilation in the body tissue especially liver. Al-Harthi (2019) has described that freshwater fishes of Saudi Arabia are threatened by the anthropogenic activities and suggested about their conservation. As the fishes selected for this study are threatened in their natural habitat hence, an attempt was made to study the different biological parameters of two species of fish, Cyrinion acinaces and Carasobarbus apoensis endemic to Arabian peninsula, from the stream of Wadi Khadra, Madina region for their population management and conservation.
2 Materials and methods
2.1 Collection of fish
Cyprinion acinaces and Carasobarbus apoensis fish samples were collected from Wadi Khadra, Medina in each month. A total of 103 samples of C. acinaces were collected, with a length ranging between 6.5 and 13.0 cm and the weight ranged between 2.62 and 24.54 g. Specimens of Carasobarbus apoensis collected were 53 and their length ranges between 10.5 and 24.5 cm and weight were from 14.05 to 167.4 g. In the laboratory, length measurements were taken. Weights were recorded in gram using the RADWAG balance.
2.2 Condition factor (K)
The Fulton coefficient (K1) and Clark coefficient (K2) were calculated from the equation used by Aday et al. (2002): where: -
K: Condition factor (K1 = with total weight) and (K2 = weight without viscera).
W: The weight of the fish, with or without viscera, in grams.
L: The total length of the fish in centimeters.
2.3 Hepatosomatic index (HSI)
Hepatosomatic index was calculated from the equation given by Lagler (1978): whereas: -
HSI: Hepato-Somatic index.
LW: Weigh the liver in grams.
BW: Fish weight in grams.
2.4 Mathematical description of growth (Van Bertalanffy)
To calculate the growth in length the Von Bertalanffy's equation as used by Sapounidis et al. (2015) was followed: where:
TLt: Fish length at time t (centimeters).
K: Growth factor.
to: Theoretical age when length equals zero.
TL∞: Asymptotic or theoretical maximum length (in centimeters).
2.5 The relationship between weight and length
The relationship between weight and length was calculated by using the following formula:
W (weight) in grams, L (length) in centimeters, and a, b are constants. This relationship can be expressed logarithmically as follows (Lagler (1978)): where:
L: Total length of fish (cm).
W: Total weight of fish (grams).
a, b: Two linear regression constants.
2.6 Determination of the length of fish at previous ages:
To estimate the age of the fish, the scales are cleaned and dyed with alizarin red dye, placed between two glass slides, and images are taken with a digital camera fitted to an optical microscope (Olympus Db72) and connected to a computer. The following equation presented by Sapounidis et al, 2015 was used to calculate the length of fish at previous year (back calculated lengths): where:
TLi: Total length at age i.
TL: The total length of the fish at time of catch.
Ri: Length of the scale radius of the first year.
Rtot: The total radius of the scale.
b: The straight line constant.
3 Results and discussion
3.1 Different indices
The seasonal variations in the values of condition coefficient of C. acinaces and C. apoensis are presented in Tables 1 and 2, respectively. The condition coefficient (K1) and the condition factor (K2) for combined sexes of C. apoensis reached the highest values (1.35 and 1.18, respectively) in the winter season and the lowest values were recorded in the fall season (0.74 and 0.67). Lower values of these factors (K1 and K2) were registered in winter season (1.02 and 0.83) and maximum was reported in spring (1.22 and 0.99) for C. acinaces. The high values of the condition factor in certain period of the year are attributed to the sufficient abundance of food in the environment and high assimilation of food in the bodies of the fish. Where the high concentration of nutrients in the warm months increases the production of food items for the fish (Al-Kahem et al., 1997). The low values of the condition factor obtained in the winter season indicated the effect of the fish spawning cycle, the lower abundance of food in the environment, and the frequency rate of immature individuals in the samples (Al-Ghanim, 2005). In general, the seasonal changes in condition coefficient is affected by feeding activity; size of the fish; development of gonads, reproduction periods; parasitic loads and diseases (Welcome, 2001; Sapounidis et al., 2015; Uckun and Gokce, 2015). a-bMeans values within a row for each variable with clarification of the significant difference in the form of superscripts (P < 0.05). 1SEM = Standard error of means for seasons effect. a-bMeans values within a row for each variable with clarification of the significant difference in the form of superscripts (P < 0.05). 1SEM = Standard error of means for seasons effect.
Parameters
Seasons
SEM1
P value
Winter
Spring
Summer
Fall
Male * Female
Condition factor with viscera (K1, %)
1.02c
1.22a
1.10bc
1.17ab
0.032
0.0008
Condition factor without viscera (K2, %)
0.83b
0.99a
0.85b
0.98a
0.027
<0.0001
Hepatosomatic index (HIS, %)
1.36b
1.75b
3.06a
1.67b
0.192
<0.0001
Male
Condition factor with viscera (K1, %)
0.98b
1.20a
1.15a
1.15a
0.039
0.006
Condition factor without viscera (K2, %)
0.78b
0.98a
0.90a
0.97a
0.033
0.001
Hepatosomatic index (HIS, %)
2.07b
1.24c
3.03a
1.62bc
0.242
<0.0001
Female
Condition factor with viscera (K1, %)
1.08b
1.32a
1.04b
1.20ab
0.053
0.018
Condition factor without viscera (K2, %)
0.89ab
1.03a
0.80b
1.00a
0.043
0.007
Hepatosomatic index (HIS, %)
1.32b
1.84b
3.10a
1.75b
0.315
0.003
Parameters
Seasons
SEM1
P value
Winter
Spring
Summer
Fall
Male * Female
Condition factor with viscera (K1, %)
1.35a
1.26a
1.28a
0.74b
0.108
0.0008
Condition factor without viscera (K2, %)
1.18a
1.12a
1.14a
0.67b
0.062
0.001
Hepatosomatic index (HIS, %)
1.46b
2.13ab
2.10ab
2.53a
0.253
0.049
Male
Condition factor with viscera (K1, %)
1.20
1.23
1.44
1.05
0.073
0.096
Condition factor without viscera (K2, %)
1.07
1.10
1.30
0.97
0.093
0.084
Hepatosomatic index (HIS, %)
1.29
2.20
2.01
2.37
0.355
0.306
Female
Condition factor with viscera (K1, %)
1.53a
1.27a
1.11a
0.49b
0.166
0.0009
Condition factor without viscera (K2, %)
1.33a
1.12a
0.99a
0.43b
0.145
0.001
Hepatosomatic index (HIS, %)
1.70
2.08
2.19
2.66
0.332
0.250
The averages of the hepatosomatic index were monitored monthly and seasonally, and in the summer season the highest value was 2.53 and the lowest value was in the winter season 1.46 for C. apoensis whereas the values were maximum (3.06) and minimum (1.36) in summer and winter, respectively for C. acinaces. Inactivation of the gonads leads to an increase in the liver index values. The value of the liver index decreases during the maturation of the gonads and prevailing unfavourable physical and biological condition of the environment specially food abundance and temperature (Al-Ghanim, 2005; Ahmad, et al., 2013, Hakami et al., 2013) (see Table 3).
Number of specimens
Age of fish in year
1
2
3
4
7
69.0
24
70.9
92.0
29
72.1
93.2
103.0
12
73.0
94.6
104.2
121.0
Average length
71.25 ± 0.749
93.26 ± 0.613
103.6 ± 0.424
121.0 ± 0.0
Increase in length
71.25
22.1
10.34
17.4
Percent increase
100
23.697
9.981
14.380
3.2 Length weight relationship
The relationship between weight and length is from biological studies that indicate the health status of fish and their ability to benefit from their food and other conditions in their natural environment (da Costa and Araojo, 2003). This relationship is presented in Fig. 1 for Carasobarbus apoensis and Fig. 2 for Cyprinion acinaces. The importance of these factors lies in providing information about the time of maturation of the ovaries, the extent to which the fish benefit from their food and thus the stages of their growth, which information is necessary to lay the general foundations for the use of these fish and to develop a general concept for their management on sound scientific foundations, especially when growth is homogeneous (Hakami et al., 2018). Among the factors affecting the value of the regression coefficient of fish are embryonic changes, fish nutrition, sexual maturity, age difference, diseases and parasite infection (Yildirim et al., 2001). When looking at the length and weight data for this study it was found that the value of “b” recorded in C. acinaces was 2.58 and for C. apoensis it was 2.30 which are less than the isometric value “3”. Values below the ideal value indicate various obstacles that affect fish growth, including lack of food and fluctuations in temperature, which negatively affects the growth of Fish (Uckun and Gokce, 2015; Abu El-Nasr, 2017). The b values ranges from 2.173 to 3.472 for the freshwater fishes reported by Tah et al. (2012). Zhu et al. (2021) have reported b value as 3.015 for freshwater fish. There are certain factors which affect the fish growth, such as fish size and quantity and quality of food (Handeland et al., 2008). When considering the length and weight data for this study, it was found that the value of “b” is 2.30 which is less than the hypothetical value 3, indicates that the growth was heterogeneous or negatively allometric. The results of this study are in agreement with the results of many studies that have shown that the law of cube did not fully apply to the fish that they studied as the regression coefficient was clearly less than the value of 3, and that these low values for parameter (b) may be attributed to the influence of various environmental factors that affected the growth of fish as previously mentioned (Sivakami, 1987) (see Table 4).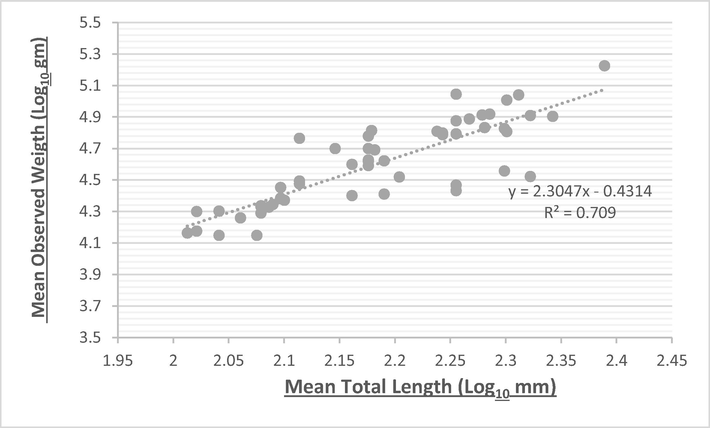
Length to weight relationship of Carasobarbus apoensis.
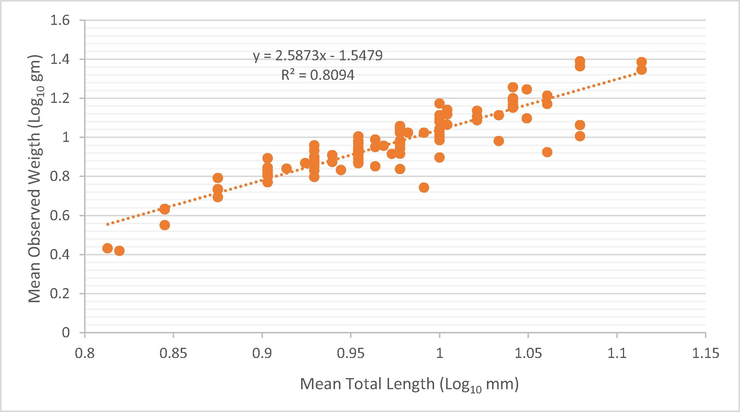
Length to weight relationship of Cyprinion acinaces.
Number of specimens
Age of fish in years
1
2
3
4
5
6
7
7
67.0
7
67.8
94.0
6
67.4
94.4
115.0
5
66.9
95.2
116.1
136.0
7
68.3
95.7
115.9
137.8
159.0
8
67.7
93.8
114.7
135.4
161.0
185.0
9
68.2
95.5
115.3
137.1
160.3
186.5
203.0
Average length
67.61 ± 0.545
94.77 ± 0.806
115.4 ± 0.591
136.57 ± 1.078
160.1 ± 1.015
185.75 ± 1.061
203.0 ± 0.0
Increase in length
67.61
27.16
20.63
21.17
23.53
25.65
17.25
Percent Increase
100
40.17
21.10
18.19
17.23
16.02
9.28
3.3 Age and growth
The age and growth parameters of fish have been most commonly estimated from the annual growth mark on the scale or otolith (Sapounidis et al., 2015; Abu El-Nasr, 2017; Ortego-Garcia et al., 2017; Hakami et al., 2018). The scale examination of Cyprinion acinaces and Carasobarbus apoensis have revealed that both species have a clear annuli on them. “Numerous studies on the scale structure of fishes have been undertaken in the world, and the results have been successfully used for growth studies, calculation of minimum harvestable size, and determination of age in fishes” (Rifflart et al., 2006; Abedi et al. 2011).
The formation of year-marks on the scale or other hard organs occurs mainly during the period of low feeding and may happen at different times in different fishes. The growth of fish may be affected by a number of factors prevailing in the environment such as temperature, availability of food and spawning cycle. These factors are supposed to affect the formation of year-mark specially feeding regime. Formation of annulus in rock hinder and red hinder occurred from March to May (Potts and Manooch, 1995) and in December in Barbus sharpyi (Mohamed et al., 2012).
The assessment of growth rates showed that the growth in length was higher during the first year of life. The data embodied in Table 5 for Cyprinion acinaces and Table 6 for Carasobarbus apoensis indicate that back calculated length gradually decrease in subsequent years in both the species. Same pattern of growth in length of Siganus rivulatus was reported by El-Gammal (1988). The growth pattern in the length registered in the present study for Cyprinion acinaces and Carasobarbus apoensis is similar to the previously published results.
Species
Number
Growth Factors
a
b
r2
L∞
K
Cyprinion acinaces acinaces
53
−1.54
2.587
0.80
121.06
0.80
0.26
Carasobarbus apoensis
103
0.681
2.305
0.71
238.06
0.25
0.11
Year class
Mean length (cm)
Calculated length(cm)
Yearly increment (cm)
Calculated mass (gm)
Yearly increment (gm)
1
6.9
7.4
_
11
_
2
9.2
10
2.3
12
1
3
10.3
11.2
1.1
14
2
4
12.1
11.7
1.8
18
4
3.4 Van-Bertalanfy growth function
The current study indicated a hypothetical mathematical description of growth in C. acinaces and C. apoensis, as their ages ranged between (1–4) and (1–7) years, respectively (Tables 6 and 7). The growth rate during the first year was the largest and with age, a decrease in the growth rate was observed, which is consistent with the study of Mohamed et al. (2012) performed on Barbus sharpyi fish. Since the value of asymptotic (L∞) length is inversely related to the growth factor K, so fish that take a long life to reach the value of L∞ have a lower value of growth constant (K) in them compared to fish of short life that often reach the value of L∞ in one or two years. This relationship was clearly observed when comparing the values of L∞ and K in the current study. The value of the final length L of fish is also affected by changes in the amount of available food and fish population density, while the K value is affected by genetic and physiological factors (Beverton and Holt, 1957). The value of asymptotic length (L∞) or weight (W∞) may be more or less than the observed length and weight values of fish. In present study the observed length values in both the species are more than the asymptotic values. Similar to present study Gonçalves et al. (2003) registered asymptotic (L∞) value smaller than the maximum length of fish and stated that this may be because of the faster growth in the first two years which is slows down afterwards. They also mentioned that slow attainable of largest size, being a characteristic of longevity. Mouine et al. (2010) and Gómez-Márquez et al. (2008) reported asymptotic values of fish more than the observed values and mentioned that mainly young individuals were the main part of the catch. Ogle and Isermann (2017) found that the asymptotic length was more in females than males, hence, the males take more time to attain maximum length than female. In present study it is clear that the C. apoensis taking more time than C. acinaces to attain asymptotic length as the growth constant (K) values are higher in acinaces than apoensis (Table 7).
Year class
Mean length (cm)
Calculated length(cm)
Yearly increment (cm)
Calculated mass (gm)
Yearly increment (gm)
1
6.7
6.1
_
17
_
2
9.4
10
2.7
20
3
3
11.5
13.1
2.1
25
5
4
13.6
15.5
2.1
33
8
5
15.9
17.3
2.3
41
8
6
18.1
18.8
2.2
51
10
7
19.8
19.9
1.7
61
10
4 Conclusion
The Wadi Khadrah is suitable for normal growth of both the species. An average growth was performed by both the species. The values of different indices were found similar to commonly registered for other species of fish in various environment. The values of “b” registered were little <3, but the values were near to 3. Growth in length and weight follow the general trends in both the species.
Acknowledgements
The authors would like to thanks to the Deanship of scientific research, King saud university, Riyadh for providing the financial support to the present research through RSP research project number RSP 48/2021.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Reproductive biology and age determination of Garra rufa Heckel, 1843 (Actinopterygii: Cyprinidae) in central Iran. Turk. J. Zool.. 2011;35:317-323.
- [Google Scholar]
- Age and growth of the fish, Garres filamentosus (Cuvier, 1829) from Hurghada Red Sea, Egypt. Egyptian J. aquat. Res.. 2017;43:219-227.
- [Google Scholar]
- The influence of stunted body size on the reproductive ecology of bluegill, Lepomis macrochirus. Ecol. Freshwater Fish.. 2002;11:190-195.
- [Google Scholar]
- Studies on the feeding ecology of Cyprinion mhalensis dwellin in Wadi Bua, Taif, Saudi Arabia. Pak. J. Zool. 2013:351-358.
- [Google Scholar]
- Al-Ghanim, K.A.A., 2005. Ecology of sailfin molly, Poecilia latipinna (Lesuer,1821) in Wadi Haneefah stream, Riyadh, Saudi Arabia. Ph.D. Thesis, Department of Zoology, College of Science, King Saud University, pp-522.
- Al-Harthi, I.G.Z. 2019. Conservation of freshwater fishes in Saudi Arabia. Ph.D. thesis submitted in the University of Hull.
- Length-weight relationship, condition co-efficient (k) and relative condition co-efficient (kn) of Oreochromis niloticus from Wadi Haneefahstream, Saudi Arabia. Geobios. 1997;24:3-8.
- [Google Scholar]
- On the dynamics of exploited fish populations. Fish. Invest., London Ser.. 1957;11:583p.
- [Google Scholar]
- Population structure of Himalayan maha seer, a large cyprinid fish in the regulated foothill section of the river Ganga. Fish. Res.. 2000;44:267-271.
- [Google Scholar]
- Age validation, growth estimation and cohort dynamics of the bony flying fish Hirundichthys oxycephalus off eastern Taiwan. Aquat. Biol.. 2012;15:251-260.
- [Google Scholar]
- Length-weight relationship and condition factor of (Desmarest) Perciformes, Sciaenidae) in the Sepetiba Bay, Rio de Janeiro State, Brazil. Rev. Bras. de Zool.. 2003;20:685-690.
- [Google Scholar]
- Age, growth and mortality of the rabbit fish Siganus rivulatus (Forsskål, 1775), from the Red Sea. Bull. Inst. Oceanogr. Fish. ARE.. 1988;14:13-21.
- [Google Scholar]
- Age and growth of the tilapia, Oreochromis niloticus (Perciformes: Cichlidae) from a tropical shallow lake in Mexico. Rev. Biol. Trop. (Int. J. Trop. Biol.). 2008;56:1-10.
- [Google Scholar]
- Age and growth, maturity, mortality and yield-per-recruit for two banded bream (Diplodus vulgaris Geoffr.) from the south coast of Portugal. Fish. Res.. 2003;62:349-359.
- [Google Scholar]
- Studies on Feeding Ecology of fresh water fish (Barbus arabicus) Dwelling in “Beesh Dam”, Jazan, Saudi Arabia. Life Sci. J.. 2013;10:39-45.
- [Google Scholar]
- Age determination of Barbus arabicus (Trewavas, 1941) in Saudi Arabia using the vertebral bone. J. Aqua. Sci. Oceangraphy. 2018;1:103-108.
- [Google Scholar]
- The effect of temperature and fish size on growth, feed intake, food conversion efficiency and stomach evacuation rate of Atlantic salmon post-smolts. Aquaculture. 2008;283:36-42.
- [Google Scholar]
- Length-weight relationships and relative condition factor of fish inhabiting the marine area of the Eastern Mediterranean city, Tripoli-Lebanon. Egyptian J. Aquat. Res.. 2000;44:299-305.
- [Google Scholar]
- Fitting growth curves to retrospective size-at-age data. Fish. Res.. 2000;46:123-129.
- [Google Scholar]
- Khan, S., Khan, M.A., 2014. Importance of age and growth studies in fisheries management. Reviewed Proc. Nat. Seminar (NCSV), 8 March, 2014. 194-201.
- Length-weight relationship and condition factor. In: Bagenal T.B., ed. Freshwater Fishery Biology. Oxford: Blackwell; 1982. p. :159-166.
- [Google Scholar]
- Lagler, K.F., 1978. Capture, sampling and examination of fishes In: Methods for the assessment of fish production in freshwater, T.B. Bagenal (eds.), Blackwell, Oxford, pp-7-47.
- Studies on age, growth and age composition of commercially important fish species, Cirrhinus mrigala (Hamilton, 1822) from the tributary of the Ganga river, India. Eur. J. Exp. Biol.. 2015;5:16-21.
- [Google Scholar]
- Age and growth of Bunni, Barbus sharpyi Gunther, 1874 in Swab marsh, south Iraq. Iraqi J. Aquacult.. 2012;9:63-82.
- [Google Scholar]
- Age and growth of Diplodus vulgaris (Sparidae) in the Gulf of Tunis. Cybium. 2010;34:37-45.
- [Google Scholar]
- Estimating age at a specified length the Von Bertalanffy growth function. North Am. J. Fish. Manage.. 2017;37:1176-1180.
- [Google Scholar]
- Length-weight relationship and condition factor for twelve fish species from the Igarape Fortaleza basin, a small tributary of the Amazonas River estuary. Acta Amaz.. 2020;50:8-11.
- [Google Scholar]
- Age, growth, and length-weight relationship of roosterfish (Nematistius pectoralis) in the eastern Pacific Ocean. Fish. Bull.. 2017;115:117-124.
- [Google Scholar]
- Age and growth of red hind and rock hind collected from North Carolina through the Dry Tortugas, Florida. Bull. Mar. Sci.. 1995;56:784-794.
- [Google Scholar]
- Scale reading validation for estimating age from tagged fish recapture in a brown trout (Salmo trutta) population. Fish. Res.. 2006;78:380-384.
- [Google Scholar]
- Length-weight relationships of four fish species associated to shrimp trawl fishery as by catch in the Persian Gulf, Iran. Thalassas: Int J. Mar. Sci.. 2020;36:33-35.
- [Google Scholar]
- Life histry trait, growth and feeding ecology of a native species (Barbus strumicae Karaman, 1955) in Nestos River, a flow regulated river in northern Greece. North-Western J. Zool.. 2015;11:331-341.
- [Google Scholar]
- Tah, L., Goore Bi, G., Da Costa, K.S., 2012. Length-weight relationships for 36 freshwater fish species from two tropical reservoirs: Ayame I and Buyo, Cote d’Ivoire. Revista de Biol. Trop. 60.
- Uckun, A.A., Gokce, D., 2015. Growth and reproduction of Cyprinion macrostomum (Heckel, 1843) and Cyprinion kais (Heckle, 1843) populations in Karakaya Dam Lake (Euphrates River) Turkey.
- Length-Weight Relationship and Condition Factors of Indian Major Carps (C. catla, L. rohita and C. mrigala) in Mahi Bajaj Sagar, India. Res. J. Biol.. 2012;2:50-56.
- [Google Scholar]
- Welcome, R.L., 2001. Inland Fisheries, Ecology and Management. Fishing news books, London, UK, Blackwell Scientific Publications.
- On the age, growth and reproduction of the barbel, Barbus plebejus escherichi (Steindachner, 1897) in the Oltu Stream of Coruh River (Artvin-Turkey) Turk. J. Zool.. 2001;25:163-168.
- [Google Scholar]
- Age, growth, and mortality of silver carp Hypophthalmichthys molitrix (Valenciennes, 1844) in the middle and lower reaches of the Pearl River, and implications for management and conservation. Int. J. Lim.. 2021;57:1-8.
- [Google Scholar]







