Translate this page into:
Biological control of Streptomyces sp. UT4A49 to suppress tomato bacterial wilt disease and its metabolite profiling
⁎Corresponding author. jerrine.jj@gmail.com (Jerrine Joseph)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objectives
Evaluation of Streptomyces isolated from Udhagamandalam, The Nilgiris, Tamilnadu, India for the biocontrol potential against the phytopathogen Ralstonia solanacearum and its metabolite profiling.
Methods
Streptomyces strains were screened for plant growth promotion and antagonistic activity against R. solanacearum in in-vitro. The metabolite profiling of strain UT4A49 was studied through gas chromatography coupled with mass spectroscopy (GC–MS) analysis. Further, the identified lead compound 2,4-Di-tert-butylphenol (2,4-DTBP) was analyzed for its mechanism of action through in-silico docking study. The bioorganic fertilizer (BOF) amended potential Streptomyces strain UT4A49 was studied for biocontrol activity against R. solanacearum on tomato seedlings through pot culture experiments.
Results
The Streptomyces strain UT4A49 showed the promising production of indole acetic acid (IAA) (76.0 ± 2.0 µg/ml), siderophore, ammonia, amylase, protease, cellulase as well as it solubilized the phosphate in-vitro. The strain UT4A49 showed strong in vitro antagonistic activity against R. solanacearum with the inhibition zone of 19.83 ± 0.44 mm in diameter. The isolated major compound, 2,4-Di-tert-butylphenol was showed strong inhibition against R. solanacearum. In silico docking study revealed that the 2,4-DTBP showed lower binding energy score with all targeted proteins which found better protein–ligand binding stability. In the pot experiment, biocontrol activity of BOF amended Streptomyces strain UT4A49 showed significant biocontrol efficacy was 78.5% against R. solanacearum in tomato seedlings. The strain UT4A49 was identified as Streptomyces sp. based on its morphological and molecular properties.
Conclusions
The detailed literature survey revealed that this is the first report on strong bioactive properties of 2,4-DTBP against R. solanacearum. This present study proved that bioorganic fertilizer amended with Streptomyces sp. UT4A49 is a promising biocontrol agent for tomato bacterial disease control.
Keywords
Streptomyces
2,4-Di-tert-butylphenol
Bioorganic fertilizer
Ralstonia solanacearum
Tomato
- BOF
-
bioorganic fertilizer
- GC–MS
-
gas chromatography mass spectroscopy analysis
- IAA
-
indole acetic acid
- 2,4-DTBP
-
2,4-Di-tert-butylphenol
Abbreviations
1 Introduction
Ralstonia solanacearum, a Gram-negative rod-shaped bacterium, considered as the second largest bacterial phytopathogen at the global level. R. solanacearum has a vast significance genetic diversity which affects more than 200 plant species comes under 54 families in most of the tropical, subtropical and warm temperate regions of the globe (Chen et al., 2020). Production of large amount of exopolysaccharides (EPS) by this bacterium remains as the reason behind its aggressive virulence (Saputra et al., 2020). The production of EPS could help the pathogen to block and disturb the transportation of water and nutrients in plant xylem vessels. In addition, this bacterium can also survive in the soil or in water as a saprophyte for several years. This pathogen is difficult to control due to its complication in biology, species complex, and their nature of infection and has broad host range (Paudel et al., 2020). Resistant varieties, field sanitation, crop rotation and chemical fertilizers are mostly applicable but not advisable for the management of R. solanacearum. In addition, the above-mentioned agricultural technologies and using chemical fertilizers are enhancing the plant growth and increases the yield. But they are not cost effective, create environmental pollution and also hazardous to human health as in case of using chemical fertilizers (Ling et al., 2020).
Soil microorganisms are known to be an essential source of agro-active natural compounds (Egamberdieva et al., 2018; Khanna et al., 2019; Sharma et al., 2020). Nowadays, agricultural researchers are focusing to produce bio-fertilizers/bio-control agents using beneficial microorganisms to minimize the use of chemical-based fertilizers, pesticides and associated agrochemicals without sacrificing on the yield aspect (Egamberdieva et al., 2018; Khanna et al., 2019; Sharma et al., 2020). Actinobacteria, one of the largest phyla under bacteria, are widely found in the plant rhizosphere. Through secreting many hydrolytic enzymes, they can degrade a large variety of biopolymers and survive in aggressive environments by developing spores. Due to its high antimicrobial ability and their dominance in soil environment as saprophytes, they have attracted much attention to agriculture (Mishra et al., 2020). Streptomyces is a well-known actinobacterial genus which produces plant growth promoting phytohormones such as IAA, ammonia and siderophore as well as solubilizes phosphate (Olanrewaju and Babalola, 2019). However, there are limited studies on actinobacteria with reference to their agricultural applications.
In this study, Streptomyces strains were isolated from tomato rhizosphere soil and screened for in-vitro PGP and antagonistic activity against R. solanacearum. The biocontrol efficacy of bioorganic fertilizer amended Streptomyces strain UT4A49 studied against R. solanacearum on tomato seedlings in pot culture experiment. The bioactive compound 2,4-DTBP produced by the strain UT4A49 was identified through GC–MS analysis.
2 Methods and materials
2.1 Isolation and characterization of Streptomyces strains
Streptomyces strains were isolated from tomato rhizosphere soil sample collected from Udhagamandalam (Lat. 11°48′N; Long. 76°77′E), The Nilgiris, Tamil Nadu, India through standard spread plate method using starch casein nitrate (SCN) agar. The antibiotic Nystatin (20 µg/ml) was added to the isolation medium to prevent the growth of fungal cultures. All the plates were incubated at 28 °C for 30 days. The morphologically distinct Streptomyces colonies were recovered and inoculated into International Streptomyces Project 2 (ISP2) agar medium and incubated at 28 °C for 7 days. Later the pure Streptomyces cultures were sub-cultured on ISP2 agar slants and stored at 4 °C. Also, the growth level, consistency, presence of aerial and substrate mycelium, and pigment of isolated Streptomyces cultures were recorded.
2.2 In-vitro screening for plant growth and enzyme production
The production of IAA from Streptomyces strains was estimated spectrophotometrically using Salkowski reagent (Gordon and Weber, 1950). Streptomyces strains were inoculated into ISP2 broth containing 0.2% of l-tryptophan and incubated in a rotary shaker with 120 rpm at 28 °C for 7 days. The culture supernatant (1 ml) was collected after incubation and 2 ml of Salkowski’s reagent added. The pink color formation was observed and the absorbance was taken at 530 nm. The amount of IAA production was measured in μg/ml (Gordon and Weber, 1950).
For the production of ammonia, all the Streptomyces strains were grown in peptone water broth and incubated in a rotary shaker at 120 rpm at 28 °C for 7 days. Nesseler’s reagent (0.5 ml) was added to 1 ml of culture supernatant and if brown to yellow color development confirms ammonia production. Then the absorbance was taken at 530 nm using a spectrophotometer. The amount of ammonia production was expressed in mg/ml (Cappuccino and Sherman, 1992).
The phosphate solubilization properties of Streptomyces strains were determined by culturing them on Pikovskaya (PVK) agar medium which contain tricalcium phosphate as the sole source of phosphorus (Pikovskaya 1948). The production of siderophore was evaluated on chrome azurol agar (CAS) plates.
Amylase and protease activity of Streptomyces were screened by inoculating them on starch agar and skim milk agar, respectively (Hankin and Anagnostakis, 1975). The cellulolytic activity was screened on mineral salt agar containing carboxy methyl cellulose (CMC) as sole carbon source (Gupta et al., 2012).
2.3 In-vitro screening of antagonistic activity
The antagonistic activity of the Streptomyces strains was screened by using agar plug method. Briefly, the Streptomyces strains were inoculated into ISP2 agar medium and incubated at 28 °C for 7 days. The bacterial phytopathogen Ralstonia solanacearum used in this study was inoculated into nutrient broth and incubated at 37 °C for 24 h. The phytopathogen R. solanacearum (107 CFU/ml) was spread on Mueller Hinton Agar (MHA) plates and a 6 mm diameter agar plugs were taken from Streptomyces plates using sterile cork borer. Then the agar plugs were aseptically placed over the pathogen inoculated MHA plates and incubated at 28 °C for 24 h. The pathogenic bacterial growth suppression was measured and expressed as zone of inhibition diameter in millimeter (Radhakrishnan et al., 2016).
2.4 Production, separation and identification of active molecule
The potential Streptomyces strain was inoculated into 50 ml of ISP2 broth to prepare the seed culture and incubated in a rotary shaker at 28 °C with 120 rpm speed for 72 h. For the production of antibacterial secondary metabolites, 500 ml of soybean medium (soybean meal (22 g/l), yeast extract (3 g/l), soluble starch (47 g/l), CaCO3 (2.7 g/l), (NH4)2 SO4 (2.7 g/l) and NaCl (2.7 g/l)) was used. The production medium was inoculated with 5% (v/v) of seed culture and incubated in rotary shaker at 28 °C with 120 rpm speed for 96 h. Later the supernatant was separated by centrifugation at 10,000 rpm for 10 min and extracted twice using ethyl acetate. The equal volume of solvent (v/v) was added to the supernatant and vigorously shaken for 15 min and incubated overnight. The solvent phase containing secondary metabolites were collected using separating funnel and concentrated in the rotary evaporator.
The secondary metabolites present in the crude ethyl acetate extract was separated by thin-layer chromatography (TLC) (pore size 60 Å, mesh size: 230–400, Merck) using organic solvents in different ratio. The separated secondary metabolites were scrapped from the preparative TLC and screened for the antagonistic activity against R. solanacearum by disc diffusion method. The phytopathogen R. solanacearum (107 CFU/ml) was spread on MHA plates and the metabolites impregnated discs (20 µl/disc) were placed over the agar plate, aseptically. Then the plates were incubated at 28 °C for 24 h. The clear zone of inhibition around the separated active compound was noted and calculated the retention factor (Rf). Finally, the active fraction alone was separated from the large quantity of crude extract by adopting preparative TLC.
The partially purified active compound was further subjected to GC–MS (Agilent Technologies 6890–5973 N) analysis. The capillary column TG-5 ms Phenyl Methyl Siloxane (30 m × 250 µm × 0.25 µm) system was used. In split mode, a mass detector is utilized. As a carrier, helium gas at a flow rate of 1.0 ml/min was used. The injector was set to 230 °C, and the oven temperature was set at 60 °C for 2 min before ramping to 280 °C for 8 min.
2.5 Docking study
2.5.1 Target and ligand preparation
The different proteins were selected as protein target based on the literature survey (Mishra et al., 2012; Kostlanova et al., 2005). Its crystal structures were retrieved from Protein Data Bank (PDB ID: 2CHH, 3P0W, 1UQX). Target preparation was done using Cresset Flare software in which the co-crystallized ligand was removed. The 3D structure of the ligand 2,4 DTBP were retrieved from the PubChem database, and saved in SDF format. The structures of these ligands will be imported to Cresset Flare software for docking study.
2.5.2 Docking analysis
The grid box for docking was defined according to the co-crystallized ligand of enzyme, and the docking calculations were carried out using Cresset Flare software in normal mode and default settings (Cheeseright et al., 2006). The obtained results consisted of Lipinski’s rule-of-five violations, Rank score, Virtual Screening (VS) score and dG (binding energy) which were generated and ranked based on the poses and binding energy scores.
2.6 Pot experiment
Tomato seeds were surface sterilized and germinated in a plastic tray on sterile wet tissue paper. Twenty-one days old tomato seedlings were transplanted into plastic pots which comprised as three different treatments: (1) CK (control treatment); (2) OF (Organic Fertilizer – red soil amended with coco peat and farm yard manure in the ratio of 2:1:1; (3) BOF-UT4A49, organic fertilizer amended with Streptomyces strain UT4A49.
The strain UT4A49 cultured for 7 days at 28 °C in YEME broth on a rotary shaker at 150 rpm and centrifuged at 10,000 rpm for 10 min. Subsequently, the supernatant of the culture UT4A49 (1 × 109 cfu/ml or absorbance at 600 nm = 0.7) used for the pot culture experiment as 20 ml/pot. After seven days, 20 ml of pathogenic suspension (1 × 108 cfu/ml or absorbance at 600 nm = 0.6) inoculated by soil drench method in each pot (Thilagam and Hemalatha, 2019). Three tomato plants were maintained in each pot, which were replicated five times for a totally randomized design. The progress of the wilt was monitored at regular intervals and the below mentioned formula used to determine the disease incidence and biocontrol effectiveness.
DI (%) = Number of wilted plants/Total number of plants × 100
BI (%) = [Total number of plants in treatment] − [Total number of plants in control] / Total number of plants in control] × 100
2.7 Characterization and taxonomy of potential Streptomyces strain
The phenotypic, microscopic and molecular identification of potential Streptomyces was characterized by standard protocol (Shirling and Gottlieb, 1996). The different growth pattern was studied in ISP1–ISP7 medium. The results were noted after incubation at 28 °C for 7–14 days. The micromorphology was examined using the scanning electron microscopy.
The genomic DNA was extracted using solute ready genomic DNA kit (Himedia). The primer pairs: 27F 5′AGAGTTTGATCMTGGCTCAG3′ (forward) and 1492R 5′TACGGYTACCTTGTTACGACTT3′ (reverse) were used to amplify the genomic DNA and sequencing was carried out at Eurofins Genomics India Pvt. Ltd., Bangalore. The phylogenetic neighbors and calculation of pair wise 16SrRNA gene sequence similarities were achieved using the MEGA version 6 and BLAST analysis (http://blast.ncbi.nlm.nihgov/Blast.cgi). The obtained 16SrRNA sequence was deposited in the NCBI-GenBank.
2.8 Statistical analysis
The collected data were subjected to an analysis of variance (ANOVA) using the SPSS were compared using the Least Significant Difference (LSD) method at probability level <0.05.
3 Results and discussion
3.1 Actinobacteria isolation
There are fifteen morphologically different actinobacterial strains were isolated from tomato rhizosphere soil. They showed good growth and produce powdery (9 strains) and leathery (6 strains) type of colonies in ISP2 agar medium. Also, all the isolated actinobacterial stains were able to produce aerial and substrate mycelium and were tentatively identified as Streptomyces. Among the fifteen strains, five strains were able to produce reverse side pigment and four strains were producing soluble pigment in YEME agar medium (Table 1). Similarly, Actinobacteria isolated from tomato and wheat rhizosphere soil samples showed morphological difference in color and pigment production (Anwar et al., 2016). Adegboye and Babalola (2013), isolated 341 Streptomyces strains based on the morphological character which were tough, leathery and often whitish to greyish colonies.
S. no
Strain no
Growth
Colony Consistency
Aerial Mass Color
Reverse Side Pigment
Soluble pigment
Suspected genera
1
UT4A42
Good
Powdery
Brown
Yellow
Yellow
Streptomyces
2
UT4A43
Good
Leathery
White
Yellow
Yellow
Streptomyces
3
UT4A44
Good
Powdery
Brown
–
–
Streptomyces
4
UT4A45
Good
Powdery
Ash White
–
–
Streptomyces
5
UT4A46
Good
Leathery
Pale White
–
–
Streptomyces
6
UT4A47
Good
Leathery
Brown
–
–
Streptomyces
7
UT4A48
Good
Powdery
White
Brown
–
Streptomyces
8
UT4A49
Good
Powdery
Ash White
–
–
Streptomyces
9
UT4A50
Good
Powdery
White
–
–
Streptomyces
10
UT5A51
Good
Powdery
Brown
–
–
Streptomyces
11
UT5A52
Good
Leathery
White
–
–
Streptomyces
12
UT5A53
Good
Leathery
Gray
–
–
Streptomyces
13
UT5A54
Good
Leathery
Gray
Blackish Green
Green
Streptomyces
14
UT5A55
Good
Powdery
Pale White
Pink
Pink
Streptomyces
15
UT5A56
Good
Powdery
Brown
–
–
Streptomyces
3.2 In-vitro screening of plant growth promoting and enzymatic traits
Among the fifteen actinobacteria strains, UT4A49 produced IAA, phosphate solubilization, siderophores, ammonia, amylase, protease and cellulase. It produced maximum range of IAA (76.0 ± 2.0 µg/ml) followed by the other strains UT5A52 (25.8 ± 0.7 µg/ml), UT5A54 (22.7 ± 1.0 µg/ml), UT4A45 (14.7 ± 0.9 µg/ml) and UT4A44 (6.5 ± 1.8 µg/ml). Totally five strains were able to produce IAA with the range between 6.5 and 76.0 µg/ml. None of strains were able to solubilize phosphate except UT4A49 in tricalcium-phosphate agar plate. Among fifteen strains six actinobacterial strains were able to produce siderophore in CAS agar plate with clear orange or yellow color zone. The level of ammonia production was estimated by spectrophotometer and showed the highest amount of ammonia (61.8 ± 1.5 mg/ml) was produced by UT4A42 and followed by UT4A49 (52.3 ± 1.3 mg/ml), UT5A52 (29.3 ± 0.9 mg/ml) and UT5A56 (19.3 ± 2.4 mg/ml). Totally six strains were able to produce ammonia with the range of 12.3–61.8 mg/ml. There are two strains (UT4A49 and UT5A52) were able to produce cellulase, amylase and protease enzymes (Table 2). + (positive) and − (negative). Mean values given with SEs in parentheses (n = 3). Different alpha characters in superscripts indicate statistically significant difference in measurements (P ≤ 0.05). Similar alpha characters in superscripts indicate no statistically significant difference.
S. No
Strain
IAA (µg/ml)
PO4 solubilization
Siderophore
Ammonia (mg/ml)
Amylase
Protease
Cellulase
1.
UT4A42
0.0
–
–
61.8 ± 1.5a
+
–
+
2.
UT4A43
0.0
–
+
0.0
+
–
+
3.
UT4A44
6.5 ± 1.8d
–
+
0.0
+
+
–
4.
UT4A45
14.7 ± 0.9c
–
–
14.3 ± 1.0e
+
+
–
5.
UT4A46
0.0
–
–
0.0
+
–
+
6.
UT4A47
0.0
–
–
0.0
–
+
+
7.
UT4A48
0.0
–
–
0.0
+
+
–
8.
UT4A49
76.0 ± 2.0a
+
+
52.3 ± 1.3b
+
+
+
9.
UT4A50
0.0
–
+
0.0
–
+
–
10.
UT5A51
0.0
–
+
0.0
–
–
+
11.
UT5A52
25.8 ± 0.7b
–
–
29.3 ± 0.9c
+
+
+
12.
UT5A53
0.0
–
–
0.0
–
+
+
13.
UT5A54
22.7 ± 1.0b
–
–
0.0
–
–
–
14.
UT5A55
0.0
–
–
12.3 ± 1.2e
+
–
–
15.
UT5A56
0.0
–
+
19.3 ± 2.4 d
–
+
+
The present findings are exactly correlated with other studies which evidenced that Streptomyces strains isolated from tomato and wheat rhizosphere soil samples produced IAA with presence of l-tryptophan ranges between 10 and 79.5 μg/ml (Anwar et al., 2016). The maximum amount of IAA (79.5 µg/ml) produced by S. nobilis WA-3 is also compared with present finding showed similar range of IAA (76.0 ± 2.0 µg/ml) produced by the strain UT4A49. Phosphorus is one of the most important elements which is required for plant growth promotion. Our present study is in concurrence with Passari et al., 2017 who reported that 21 out of 81 Streptomyces strains were able to solubilize the phosphate between the ranges from 3.8 mm to 9.7 mm. Similarly, Thilagam and Hemalatha (2019) and Anwar et al., 2016 were also reported that Streptomyces showed positive phosphate solubilization in-vitro which could be one of the important reasons to enhance the plant growth development. Siderophore production is another important source to control the plant disease. Furthermore, Tan et al. (2009) reported that the production of siderophore is not only the important factor for disease control and also it serves as plant growth developing element. Our present study is in concordance with others reported that 87% of Streptomyces strains were able to produce siderophore in in-vitro (Anwar et al., 2016). A production of ammonia is one of the important indirect mechanisms for plant growth and disease control (Minaxi et al., 2012). In this present study, six Streptomyces strains produced ammonia in peptone water which is also in concurrence with Passari et al. (2017) and Thilagam and Hemalatha (2019) reported that ammonia production by Streptomyces is important mechanism for increasing the shoot and root length. Passari et al. (2017) isolated endophytic Streptomyces strains isolated from Rhynchotoechum ellipticum plant showed ammonia production with ranges between 8.6 and 82.3 mg/ml. Similarly, Nimnoi et al. (2010) also reported that S. hainanensis S4303 could produce ammonia about 60.0 mg/ml. In addition, Anwar et al. (2016) reported that six Streptomyces strains were able to produce ammonia which promote the production of hydrogen cyanide and decrease the plant disease suppression.
Enzyme production by Streptomyces plays an important role in disease control against phytopathogens. Streptomyces strain 7.1 (Suárez-Moreno et al. 2019) isolated from rhizosphere soil samples showed both prominent cellulase and protease activity with 17.43 mm and 21.43 mm zone in agar plate which is concordance with our present study reported that UT4A49 and UT5A52 showed cellulase, protease and amylase activity. In present study 60% of Streptomyces strains showed amylase, protease and cellulase activity. Similarly, Passari et al. (2017) reported that Streptomyces strains produce 29.6% and 48.1% of cellulase and amylase among 81 actinobacteria strains tested.
3.3 In-vitro antagonistic activity of actinobacteria
Out of fifteen actinobacterial strains subjected to antagonistic activity, five strains were exhibited zone of inhibition against the phytopathogen R. solanacearum. The strain UT4A49 (19.83 ± 0.44 mm) showed the maximum zone of inhibition against R. solanacearum followed by UT5A53 (17.0 ± 0.7 mm), UT4A43 (15.67 ± 1.2 mm), UT5A54 (12.83 ± 0.6 mm) and UT5A56 (12.5 + 0.5 mm) (Fig. 1). This present antagonistic activity against R. solanacearum correlated with Ling et al. (2020) isolated Streptomyces sp. NEAU-HV9 from soil sample which showed antagonistic activity against R. solanacearum with the inhibition zone of 32.8 mm. Similarly, Tan et al. (2006) isolated 619 Streptomyces strains from tomato and screened antagonistic activity against R. solanacearum. Actinobacteria isolated from soil samples have proved successful antimicrobial activity against phytopathogens. Perez-Rojas et al. (2015) isolated 85 actinobacteria from compost pile and screened against R. solanacearum and 23.5% of actinobacteria strains showed inhibition. Similarly, in the present study 33% actinobacteria strains exhibited such zone of inhibition against R. solanacearum. And the maximum zone of inhibition revealed by UT4A49 which has been slightly lower inhibition than that obtained by Perez-Rojas et al. (2015) who isolated Streptomyces ACZII35 showed 32 mm zone of inhibition against R. solanacearum. But our present finding is significantly higher than the contemporary searcher reports who isolated Streptomyces strain LD120T showed zone of inhibition of about 12.3 mm in diameter against R. solanacearum (Zhuang et al., 2020).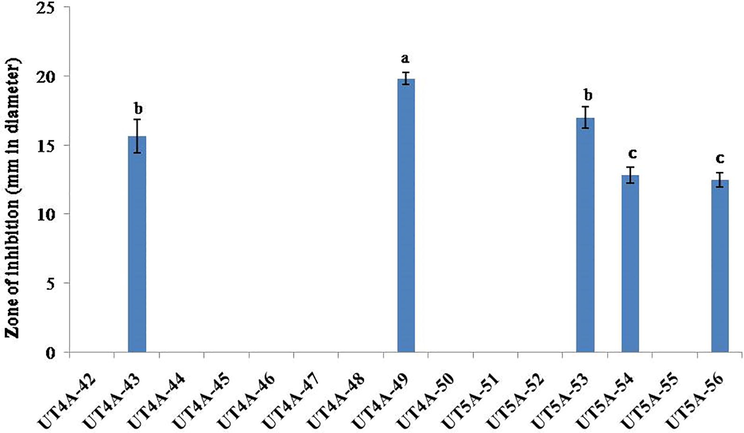
In-vitro antagonistic activity of Streptomyces strains against R. solanacearum.
3.4 TLC based purification, bioactivity and GC–MS analysis
Among various solvent systems used for TLC, ethyl acetate and methanol with the ratio of 9:1 (v/v) was found to be the better solvent system for the separation of antibacterial compounds. Bioassay of TLC extract reveals that the totally eight compounds were separated with the Rf value of a-0.90, b-0.82, c-0.78, d-0.70, e-0.64, f-0.58, g-0.53 and h-0.41. The partially purified bioactive compound with Rf value of d-0.70 showed clear zone of inhibition against R. solanacearum with the inhibition zone of 23.5 ± 0.86 mm (Fig. 2). The GC–MS analysis of TLC based purified compound which has maximum antibacterial activity (Rf value- 0.70) was confirmed the presence of 2,4-Di-tert-butylphenol (2,4-DTBP) with the retention time 17.004 min as illustrated in Fig. 2. Totally six compounds were identified and were found to be the derivatives of aromatic compounds. 1-Dodecanol (C12H26O), 2,4-Di-tert-butylphenol (C14H22O), 1-Tetradecanol (C14H30O), Diisobutyl phthalate (C16H22O4), Methyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate (C18H28O3), 2-Hydroxy-1-(hydroxymethyl)ethyl icosanoate (C23H46O4) were found in GC–MS analysis and 2,4-DTBP was shown as antibacterial activity possessing compound in this study with highest peak number (peak area- 86.81%) (Fig. 2). 2,4-DTBP is a common natural product that exhibits potent toxicity against various pathogens (Zhao et al, 2020). Streptomyces mutabilis G61 isolated from Sahara soil sample which produced 2,4-DTBP having strong antimicrobial activity against various pathogens including the fungal phytopathogen Fusarium culmorum and F. graminearum (Belghit et al., 2016). Also, the endophytic actinobacteria S. globosus JQ926176 produced 2,4-DTBP which has strong antioxidant activity (Akshatha et al., 2016). Likewise, 2,4-DTBT produced by Flavobacterium johnsoniae having antimicrobial activity against the phytopathogen Phytophthora capsici in pepper (Sang and Kim, 2012). Similar to this present study, 2,4-DTBT purified by TLC based purification and antimicrobial activity was evidenced against Aspergillus (Maria Teresa et al., 2014). In addition, Zhou et al. (2013) reported that the study of 2,4-DTBT on tomato seed germination and seedling growth was increased than the control treatment. This has further corroborated our findings.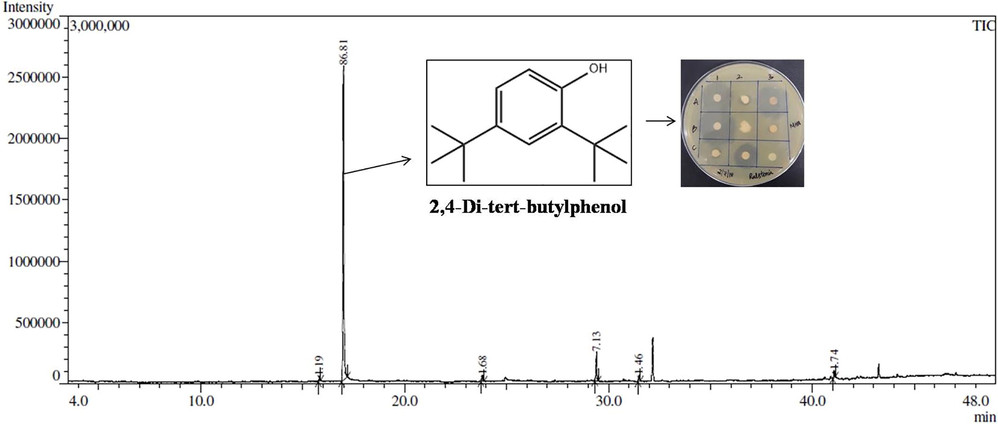
Antibacterial bioactive compound 2,4-Di-tert-butylphenol purified from UT4A49. GC chromatogram has showing one highest peak with a retention time of 17.004 min as the major component of the inhibition zone. Complementary mass spectrometry analysis showed the identification of the compound as 2,4-Di-tert-butylphenol. Bioactive compound 2,4-DTBP showing zone of inhibition against R. solanacearum.
3.5 Docking study
Analysis of the docking results showed the potential strength of binding affinity of 2,4-DTBT into the binding sites of target proteins with minimum binding energies (ranging from −4.06 to −4.259 kcal/mol), ligand efficiency −0.271 to −0.284 kcal/mol), virtual screening score (−4.838 to −5.136) and rank score (−5.222 to −5.698) (Table 3). In this present study the better protein–ligand binding stability was identified by 2,4-DTBT into d-glucaratedehydratase (−4.259 kcal/mol) which showed least binding energy score followed by mannose-binding lectin (−4.116 kcal/mol) and alpha-methylmannoside (−4.06 kcal/mol). This finding is also correlated with others reports showed that the lower binding energy score was found better the protein–ligand binding stability was identified (Konappa et al., 2020). This 2,4-DTBT compound formed the best possible binding pose with the residues of target proteins such as mannose-binding lectin, d-glucaratedehydratase and alpha-methylmannoside, respectively as shown by their corresponding 3D interaction models (Fig. 3). Thus, the docking study results also substantiated that 2,4-DTBP is the lead compound against R. solanacearum by recorded better affinity with low binding energy against each target proteins.
S. No
Ligand
Protein
Rank score
Binding energy (kcal/mol)
Virtual screening score
Ligand efficiency (kcal/mol)
1
Mannose-binding lectin
2CHH
−5.698
−4.116
−4.838
−0.274
2
d-glucaratedehydratase
3POW
−5.222
−4.259
−5.136
−0.284
3
Alpha-methylmannoside
1UQX
−5.44
−4.06
−4.919
−0.271
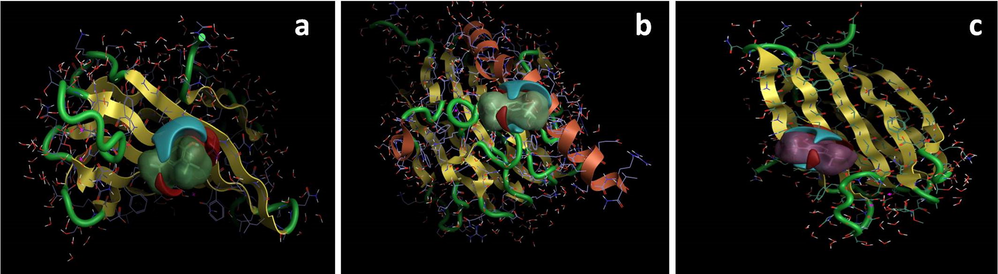
In-silico molecular docking shows the binding interaction of compound 2,4-DTBP with R. solanacearum target proteins (a) mannose-binding lectin (2CHH), (b) d-glucaratedehydratase (3P0W) and (c) alpha-methylmannoside (1UQX) based on the binding energy generated by AutoDock program.
3.6 Pot experiment
In pot experiment, BOF-UT4A49 effectively suppressed the development of bacterial wilt caused by R. solanacearum in tomato seedlings. In the CK treatment, wilt incidence was 96.3% and visible symptoms appeared first when compared to other treatments. The treatment of BOF-UT4A49 significantly decreased the severity of disease incidence, which was 21.5%, whereas the disease incidence in the OF treatment was 53.3% (Fig. 4). The treatment BOF-UT4A49 increased the biocontrol efficiency, which reached 78.5%, whereas OF treatment showed 46.6% biocontrol efficacy. These results confirm that the BOF-UT4A49 significantly enhanced the bacterial wilt suppression on tomato plants when compared to organic fertilizer used alone (Fig. 5). This finding concurs with previous works that confirmed the successful application of Streptomyces against R. solanacearum in tomato plants under pot culture study (Ling et al., 2020; Zhuang et al., 2020; Saputra et al., 2020). Streptomyces microflavus have been applied as effective biocontrol agent against tomato bacterial wilt control in pot culture experiment (Shen et al., 2021). Finding from present study the results suggest that BOF-UT4A49 has the promising potential to be developed as a biocontrol agent to control bacterial wilt disease on tomato.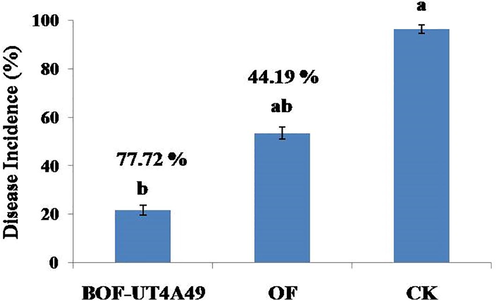
Disease incidence of three different treatments such as BOF-UT4A49 represents bio-organic fertilizer amended with Streptomyces strain UT4A49, OF represents organic fertilizer, CK represents control treatment.

The bacterial wilt symptoms on tomato in three treatments (CK and OF treatment showed wilting and the treatment BOF-UT4A49 showed no wilt).
3.7 Morphological, physiological and molecular characterization of the strain UT4A49
The micromorphological studies by SEM analysis revealed that Streptomyces strain UT4A49 formed branched substrate mycelium and aerial mycelium which carried smooth surface spores in rod shaped spore (1.5–3.0 μm length and 0.6–0.9 μm width) chains (Fig. 6a). Similarly, Zhuang et al. (2020) isolated Streptomyces strain LD120T from Physcomitrium sphaericum sample characterized the SEM analysis showed long spore chains with warty surfaced spores with size about 0.5–0.6 μm length and 0.7–0.8 μm width. It produces good growth in yeast extract malt extract agar (ISP2), oat meal agar (ISP3), inorganic salts - starch agar (ISP4) and peptone yeast extract iron agar (ISP6) medium and moderate growth in tryptone yeast extract agar (ISP1) and glycerol asparagine agar (ISP5) medium. Similarly, Ling et al. characterized the growth of Streptomyces sp. NEAU-HV9 in different media and it grown well in ISP 1, ISP 2, ISP 3, ISP 4, ISP 5, ISP 6, ISP 7, Bennett’s agar and nutrient agar. Streptomyces strain RS-25 showed variety of growth pattern in different media (Singh et al., 2018). An abundant growth was occurred in ISP3, ISP5 and ISP6 whereas moderate growth pattern in ISP2 and ISP4 medium (Singh et al., 2018).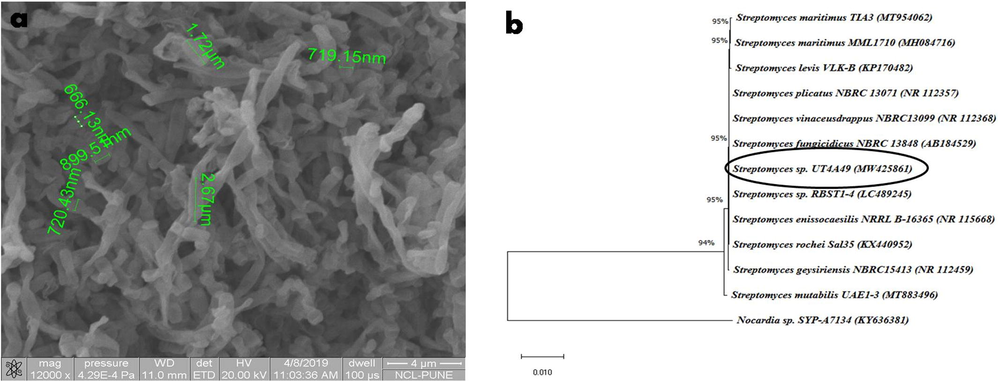
Identification of the strain UT4A49. (a) SEM analysis of strain UT4A49 and (b) Neighbor-joining phylogenetic tree based on 16SrRNA gene sequence comparing Streptomyces UT4A49 to other Streptomyces species collected from NCBI-BLAST. The numbers on the branches indicate the percentage bootstrap values of 1000 replicates; only values >50% are indicated.
Molecular identification of 16SrRNA gene sequencing is used as a reliable technique and it comprising of about 1500 bp with hyper variable and conserved regions, is universal in all bacterial microorganisms (Tsukuda et al., 2017). Further, the 16SrRNA gene sequence of strain UT4A42 produces 1458 nucleotide base pair length and 99.45% similarity with Streptomyces maritimus in BLAST search. The phylogenetic analysis of Streptomyces strain UT4A49 was closely clade with Streptomyces sp., S. rochei, S. enissocaesilis, S. fungicidicus and S. vinaceusdrappus by UPGMA clustering (Fig. 6b). The accession number for the strain UT4A49 is MW425861 received from NCBI-GenBank which was submitted as Streptomyces sp. Similarly, others also used the same type of primers to identify the Streptomyces and were produced more than 1450 nucleotide base pair in length showed more than 99% of similarity in BLAST search (Anwar et al., 2016; Akshatha et al., 2016; Belghit et al., 2016).
4 Conclusion
Through the present study it has been established that Streptomyces sp. UT4A49 produces significant plant growth promoting and extracellular enzymatic properties. GC–MS analysis showed that the major compound 2,4-Di-tert-butylphenol is a promising bioactive compound produced by Streptomyces sp. UT4A49 exhibited the strong antimicrobial activity against R. solanacearum. Also, the compound 2,4-DTBP showed promising binding affinity toward different proteins in docking experiments, and their antagonistic ability by drug-like features were demonstrated through the docking analysis. In addition, the biocontrol activity of bioorganic fertilizer amended Streptomyces sp. UT4A49 against R. solanacearum, suggesting that it has the potential as a biocontrol agent for controlling bacterial wilt on tomato seedlings. From this present study, it is obvious that Streptomyces sp. UT4A49 could be a promising microorganism for the development of bio-organic fertilizer (BOF-UT4A49) against the bacterial phytopathogen R. solanacearum.
Author contributions
Conceived and designed the experiments: JJ, RM. Performed the experiments: MK, AS, GV. Analysis/interpretation of data: MK, GV. Drafting of the manuscript: MK, AS. Supervising the work: JJ, RM, GV. Critical revision of the manuscript: RM, JJ.
Acknowledgements
Authors acknowledge the management of Sathyabama Institute of Science and Technology (SIST), Chennai Tamil Nadu, India for the research facilities provided.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Isolation, characterization and antibacterial activity of Streptomycetes from rhizosphere soils in North West Province, South Africa. Asia Life Sci.. 2013;9:403-421.
- [Google Scholar]
- Actinomycete endophytes from the ethno medicinal plants of southern India: Antioxidant activity and characterization studies. J. Biol. Active Products Nature. 2016;6(2):166-172.
- [Google Scholar]
- Screening of rhizospheric actinomycetes for various in-vitro and in-vivo plant growth promoting (PGP) traits and for agroactive compounds. Front. Microbiol.. 2016;7:1334.
- [Google Scholar]
- Activity of 2,4-Di-tert-butylphenol produced by a strain of Streptomyces mutabilis isolated from a Saharan soil against Candida albicans and other pathogenic fungi. J. Mycol. Med.. 2016;26(2):160-169.
- [Google Scholar]
- In: Microbiology: A Laboratory Manual (third ed.). New York: Benjamin/cummings Pub. Co.; 1992. p. :125-179.
- Molecular field extrema as descriptors of biological activity: definition and validation. J. Chem. Inf. Model.. 2006;46(2):665-676.
- [Google Scholar]
- Sustainable and ecofriendly approach of managing soil born bacterium Ralstonia solanacearum (Smith) using dried powder of Conyza canadensis. Pathogens. 2020;9(5):327.
- [CrossRef] [Google Scholar]
- Interactive effects of nutrients and Bradyrhizobium japonicum on the growth and root architecture of soybean (Glycine max L.) Front. Microbiol. 2018:9:1000.
- [Google Scholar]
- Isolation of cellulose-degrading bacteria and determination of their cellulolytic potential. Int. J. Med.. 2012;2012:1-5.
- [Google Scholar]
- The use of solid media for detection of enzyme production by fungi. Mycology. 1975;67(3):597-607.
- [Google Scholar]
- Role of plant growth promoting bacteria (PGPRs) as biocontrol agents of Meloidogyne incognita through improved plant defense of Lycopersicon esculentum. Plant Soil. 2019;436(1-2):325-345.
- [Google Scholar]
- GC-MS analysis of phytoconstituents from Amomum nilgiricum and molecular docking interactions of bioactive serverogenin acetate with target proteins. Sci. Rep.. 2020;10:16438.
- [Google Scholar]
- The fucose-binding lectin from Ralstonia solanacearum. A new type of beta-propeller architecture formed by oligomerization and interacting with fucoside, fucosyllactose, and plant xyloglucan. J. Biol. Chem.. 2005;280(30):27839-27849.
- [Google Scholar]
- A Streptomyces sp. NEAU-HV9: isolation, identification, and potential as a biocontrol agent against Ralstonia solanacearum of tomato plants. Microorganisms. 2020;8(3):351.
- [CrossRef] [Google Scholar]
- The avocado defense compound phenol-2,4-bis (1,1-dimethylethyl) is induced by arachidonic acid and acts via the inhibition of hydrogen peroxide production by pathogens. Physiol. Mol. Plant Pathol.. 2014;87:32-41.
- [Google Scholar]
- Characterization of multifaceted Bacillus sp. RM-2 for its use as plant growth promoting bioinoculant for crops grown in semi arid deserts. Appl. Soil Ecol.. 2012;59:124-135.
- [Google Scholar]
- In-silico mutagenesis and docking study of Ralstonia solanacearum RSL lectin: performance of docking software to predict saccharide binding. J. Chem. Info Modeling. 2012;52(5):1250-1261.
- [Google Scholar]
- Microbial enzymes in biocontrol of phytopathogens. In Springer Nature 2020:259-285.
- [Google Scholar]
- Endophytic actinobacteria isolated from Aquilaria crassna Pierre ex Lec and screening of plant growth promoter’s production. World J. Microbiol. Biotechnol.. 2010;26:193-203.
- [Google Scholar]
- Streptomyces: implications and interactions in plant growth promotion. Appl. Microbiol. Biotechnol.. 2019;103(3):1179-1188.
- [Google Scholar]
- Insights into the functionality of endophytic actinobacteria with a focus on their biosynthetic potential and secondary metabolites production. Sci. Rep.. 2017;7:11809.
- [Google Scholar]
- Taxonomy and phylogenetic research on Ralstonia solanacearum species complex: A complex pathogen with extraordinary economic consequences. Pathogens. 2020;9(11):886.
- [CrossRef] [Google Scholar]
- Actinomicetos aislados del compost y su actividad antagonista a fitopatógenos de la papa (Solanum tuberosum spp. andigena Hawkes) Revista Mexicana de Fitopatología. 2015;33:116-139.
- [Google Scholar]
- Mobilization of phosphorus in soil connection with the vital activity of some microbial species. Microbiol.. 1948;17:362-370.
- [Google Scholar]
- Bioactive potential of actinobacteria isolated from certain under-studied regions in India. J. Appl. Pharma. Sci.. 2016;6:151-155.
- [Google Scholar]
- The volatile-producing Flavobacterium johnsoniae strain GSE09 shows biocontrol activity against Phytophthora capsici in pepper. J. Appl. Microbiol.. 2012;113(2):383-398.
- [Google Scholar]
- Biological control of Ralstonia solanacearum causes of bacterial wilt disease with Pseudomonas putida and Streptomyces spp. on some tomato varieties. IOP Conf Ser Earth. Environ. Sci.. 2020;515:012007.
- [CrossRef] [Google Scholar]
- Insights into the role of Streptomyces hydrogenans as the plant growth promoter, photosynthetic pigment enhancer and biocontrol agent against Meloidogyne incognita in Solanum lycopersicum seedlings. Plants. 2020;9(9):1109.
- [CrossRef] [Google Scholar]
- Identification and application of Streptomyces microflavus G33 in compost to suppress tomato bacterial wilt disease. Appl. Soil Ecol.. 2021;157:103724.
- [CrossRef] [Google Scholar]
- Methods of characterization of Streptomyces species. Int. J. Syst. Bacteriol.. 1996;61:313-340.
- [Google Scholar]
- Isolation and purification of antibacterial compound from Streptomyces levis collected from soil sample of north India. PLoS ONE. 2018;13(7):e0200500.
- [Google Scholar]
- Plant-growth promotion and biocontrol properties of three Streptomyces spp. isolates to control bacterial rice pathogens. Front. Microbiol.. 2019;10:290.
- [Google Scholar]
- Tan H, Deng Z, Cao L, 2009. Isolation and characterization of actinomycetes from healthy goat faeces. Lett. Appl. Microbiol. 49: 248–253.
- Isolation of endophytic actinomycetes from different cultivars of tomato and their activities against Ralstonia solanacearum in vitro. World J. Microbiol. Biotech.. 2006;22(12):1275-1280.
- [Google Scholar]
- Plant growth promotion and chilli anthracnose disease suppression ability of rhizosphere soil actinobacteria. J. Appl. Microbiol.. 2019;126(6):1835-1849.
- [Google Scholar]
- Comparative RNA function analysis reveals high functional similarity between distantly related bacterial 16 S rRNAs. Sci Rep. 2017;7:9993.
- [Google Scholar]
- Effects of 2,4-di-tert-butylphenol on tomato leaf mould and seedling growth. Shengtaixue Zazhi. 2013;32:1203-1207.
- [Google Scholar]
- Natural sources and bioactivities of 2,4-di-tert-butylphenol and its analogs. Toxins. 2020;12:35.
- [Google Scholar]
- Characterization of a novel endophytic actinomycete, Streptomyces physcomitrii sp. nov., and its biocontrol potential against Ralstonia solanacearum on tomato. Microorganisms. 2020;8:2025.
- [Google Scholar]







