Translate this page into:
Biological change of western Saudi Arabia: Alien plants diversity and their relationship with edaphic variables
⁎Corresponding author. stalharthi@outlook.com (Saud T. Alharthi)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objectives
In the current study, the diversity of invasive alien species in the diverse region of western Saudi Arabia and their existence in different habitats were examined. Methods: A total of 58 stands in different habitats (i.e., mountains, protected areas, wadis, coastal areas, farmlands, and ruderal areas) along the western region of Saudi Arabia were examined to record the different native and alien plant species in the study area. Results: The results revealed the existence of 146 plant species including 52 alien species. Alien plant species were characterized as casual (4 species), naturalized (45 species), and invasive (3 species). Invasive and casual species were mainly trees and shrubs while naturalized species were mainly annual herbs and subshrubs. Moreover, most of the invasive and casual alien species belonged to the American region while the naturalized species were mainly from the Saharo-Arabian region. Opuntia dillenii (Ker Gawl.) Haw. and Prosopis juliflora (Sw.) DC. showed the highest frequencies among the identified alien plant species as each was found in 4 out of the 6 examined habitats. Interestingly, P. juliflora was the only invasive species in the ruderal areas. O. dillenii dominated mountain habitats, protected areas, and farmlands. Canonical correspondence analysis showed that the distribution of O. dillenii was correlated with soil pH and organic matter content in the soil, while the distribution of P. juliflora showed a correlation with the soil contents of K, Ca, and Mn. Conclusions: The results of the current study could help in enhancing the strategic conservation of plants and controlling the spread of these invasive alien species in Saudi Arabia.
Keywords
Biodiversity
Conservation
Loss of habitats
Alien species
Red Sea
1 Introduction
As one of the main threats to biodiversity in all ecosystems e.g., terrestrial, marine, and freshwater, biological invasion attracts more attention and research efforts over the last decades (Mačić et al., 2018). Invasive species are generally defined as the organisms introduced to places out of their natural distribution (intentionally or unintentionally by humans) and followed by successful establishment and dispersion or spreading without the assistance of people in natural or semi-natural habitat that led to negative impacts via significant changes in the native species composition in their own ecosystem (Yair et al., 2020). For example in Saudi Arabia, Argemone sp. escaped from wheat farms and infest the natural habitats (Thomas et al., 2016). Invasive species have several harmful impacts on the local ecosystem including modifying habitats, affecting food web characteristics, modifying community composition, causing extinctions of local species and loss of native genotypes, and affecting the ecosystem processes and functioning (Vilà et al., 2010, Bellard et al., 2016, Mačić et al., 2018). According to the Convention on Biological Diversity (CBD), the ecological impacts of alien invasive species could be so severe that these species are considered among the major drivers of global biodiversity loss (CBD, 2022). Furthermore, introduction of invasive species may lead to severe negative socio-economic impacts that obviously hinder the ecosystem services and affect the human well-being (Vilà and Hulme, 2017).
Saudi Arabia is located between longitudes 34°40′E–55°45′E and latitudes 15°45′N–34°35′N (AlNafie, 2008) and consists mainly of a wide dry desert with an area of approximately 2,250,000 km2 occupying the greatest section of the Arabian Peninsula. The flora of Saudi Arabia shares similar characteristics with different geological areas i.e., the northern and northwestern Mediterranean, the southeastern and northeastern Asia, and the western Africa. The flora of Saudi Arabia was extensively investigated with the identification of 835 genera represented by 2,250 plant species (Collenette, 1985, 1998, 1999; Chaudhary, 1999-2001). Generally, the vegetation of Saudi Arabia belongs to the Saharo-Sindian phytogeographical region (Zohary, 1973) or to a mixture of different climatic conditions including Saharo-Arabian or Saharo-Sindian, Sindian, and Mediterranean regions (El-Sheikh et al., 2013, Al-Aklabi et al., 2016).
The western region of Saudi Arabia, with its diverse habitats, is considered among the richest regions of biodiversity in the Arabian Peninsula with the presence of large number of endemic, rare, threatened, and endangered plant species (Al-Abbasi et al., 2010, Al-Aklabi et al., 2016, Thomas et al., 2017, Al-Namazi et al., 2021). Biodiversity hotspots play pivot roles in the conservation strategic plans at both national and international levels (Hobohm et al., 2014). Although many studies examined the vegetation diversity and floristic structure of the western region of Saudi Arabia, no studies examined the presence and distribution of alien species in this area, nor their potential impacts on the plant diversity in this biodiversity hotspot.
The study of the impacts of alien invasive species on the biodiversity in the western Saudi Arabia is lacking. Nevertheless, it is considered as one of the most important aspect in planning of biodiversity conservation plans (Mačić et al., 2018). In Saudi Arabia, 55 alien or exotic species have been identified, most of them are limited to no more than 2 % in regions where they exist, but expectations show that they could potentially invade more than 10 or 15 % within the coming years (Alfarhan et al., 2021). Thomas et al. (2016) identified 48 exotic species in Saudi Arabia, of them only 6 species, namely Argemone mexicana L., Nicotiana glauca Graham, O. dillenii, Opuntia ficus-indica (L.) Mill., P. juliflora, and Trianthema portulacastrum L. showed negative impacts on the local habitats and species richness. Another study identified 42 alien plant species belong to 15 families and distribute among 11 different governorates in Saudi Arabia (Aljeddani et al., 2021). Calotropis procera (Aiton) W.T. Aiton as an invasive species in Taif region, Saudi Arabia showed a negative impact on the floristic composition and the associated plant communities (Al-Sodany et al., 2016). Similarly, the invasive shrub N. glauca showed a detrimental impact on the species richness and evenness in Taif region, western Saudi Arabia (Alharthi et al., 2021). Al-Robai et al. (2018) identified Cylindropuntia rosea (DC.) Backeb. for the first time near Jebel Hizna, Baljurashi region, southwestern Saudi Arabia as a serious invasive cactus species. Nevertheless, further studies are required to identify potential alien invasive species and their impacts on vegetation and flora of Saudi Arabia, especially in highly affected regions including western region of Saudi Arabia. Therefore, the current study was designed to examine the diversity of invasive alien species in the western region of Saudi Arabia and their existence in different habitats along the Red Sea coast.
2 Materials and Methods
2.1 Terminology
In the current study, the recorded alien plant species were defined and classified following Kowarik and Pyšek (2012), Richardson et al. (2000), and Vilà et al. (1999) into three categories as follows: 1) casual species indicating those plant species that may flourish or reproduce occasionally in an area, but do not form self-replacing populations, and rely on repeated introductions for their persistence, 2) naturalized species indicating plant species that reproduce consistently and sustain populations over many life cycles without direct intervention by humans; they often recruit offspring freely, usually close to adult plants, and do not necessarily invade natural, semi-natural or human-made ecosystems, and 3) invasive species indicating naturalized plant species that produce productive offspring, often in large numbers, at considerable distances from parent plants, and thus have the potential to spread over a considerable area.
2.2 Study area
The current study was performed in the western region of Saudi Arabia and included the Tihama plain and Sarawat Mountains with different habitats (i.e., mountains, protected areas, wadis, farmlands, ruderal areas, and coastal areas) along the coastal region of the Red Sea from Rabigh in the north (N24°38′38″ E39°93′18″) to Jeddah in the south (N24°26′59.5″ E39°14′41.1″) and from Al Figrah mountains in the north (N24°25′32″ E39°23′36″) at elevation of 2100 m above sea level (a.s.l.) to Raidah Sanctuary in the south (N18°20′95″ E42°40′96″) at elevation of 1900 m a.s.l. (Fig. 1). The western region of Saudi Arabia is characterized by sedimentary rocks with the presence of several sand dunes and salt marshes sloping from the mountains towards the Red Sea. The study was performed over a whole year starting from winter 2018 to fall 2019. A total of 58 stands in the different habitats along the western region of Saudi Arabia were examined.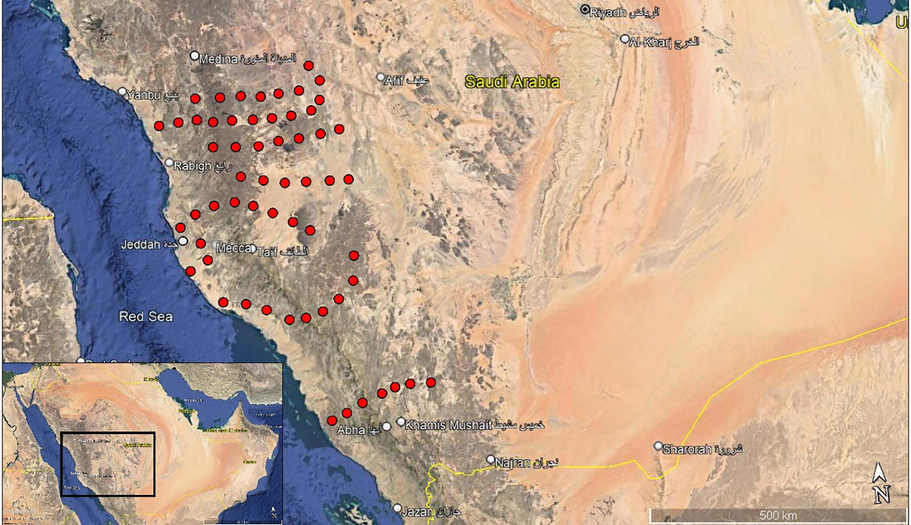
A map of Saudi Arabia (left bottom corner) showing the study area inside the black box with a zoomed-in map showing the distribution of the 58 stands (red dots) in the western region of Saudi Arabia.
2.3 Identification of alien plant species
A list of all the plants found in the study area was prepared. For the purpose of the current study, the alien species was defined according to Richardson et al. (2000) as “the alien species (exotic plants, non-native plants, non-indigenous plants) are plant taxa in a given area whose presence is due to intentional or accidental introduction as a result of human activity.” Identification of plants and their chorotypes and life forms, as well as the intentional and accidental introduction, were determined according to the flora references of Saudi Arabia (Collenette, 1985, 1998, 1999, Chaudhary, 1999-2001). The identification of alien species was revised in the King Saud Herbarium (KSUP). The habitats of each alien species were recorded during the different field trips.
2.4 Density and cover estimation
Quadrat method was applied to estimate the density of each recorded plant in the different habitats. In each selected sampling stand, 5 quadrats were applied and the number of individuals per each plant species in each quadrat was counted. The quadrat area varied according to the plants' sizes with a minimum of 5 × 5 m and a maximum of 50 × 50 m. The average quadrat area was 25 × 25 m. The density of each plant species was calculated following Ellenberg and Mueller-Dombois (1974). The density values were converted to number of individuals per 100 m2 and then this value was converted to number of individuals per hectare.
Pearson’s correlation analysis between the densities of native and alien plant species recorded in all the studied quadrats was performed using IBM SPSS Statistics 20 (IBM Corp., Armonk, NY).
The plant cover and relative cover were estimated according to Kent (2012).
2.5 Diversity indices
Several diversity indices were calculated (Pielou, 1975, Magurran, 1988, 2003). Briefly, α-diversity (species richness) was calculated as the average number of species per stand. Shannon-Wiener index was used to calculate species relative evenness as follows:
Species evenness was calculated using the following equation:
where is the number derived from the Shannon diversity index and is the maximum possible value of (if every species was equally likely).
Simpson index was used to measure the relative concentration of dominance as follows:
where (s) is the number of species and (pi) is the relative cover value of the i-th species.
Seeds of the identified plant species were collected. Moreover, the dispersal types for most of the collected diaspores from the recorded species were examined using the scheme proposed by Dansereau and Lems (1957).
2.6 Soil analysis
Soil samples were collected using a soil corer to the depth of 20 cm with tri-replicates for each stand. After air-drying of the soil samples, soil solution (1:5 w:v) was prepared to measure pH and electrical conductivity (EC). Soil texture was measured using Bouyoucos hydrometer (Allen, 1989). Organic matter (OM) content was measured using loss-on-ignition method (Allen, 1989). Atomic absorption spectroscopy was used to determine N and P contents in the soil. Contents of other nutrient elements (K, Ca, Mg, Na, F, and Mn) in the soil were analyzed using Inductively Coupled Plasma Mass Spectrometry (NexIONTM 300D ICP-MS, PerkinElmer, Inc., MA, USA) method (Allen, 1989).
2.7 Data analysis
The data matrix of species cover data was created with 58 stands, species cover values for the identified alien species and soil variables. Canonical correspondence analysis (CCA) was applied to examine the correlation between the soil variables and distribution of the identified alien species in the study area using the CANOCO software (Ter Braak and Smilauer, 2002). One-way ANOVA was performed to examine the variance in all diversity indices and soil parameters across different studied habitats via IBM SPSS Statistics 20 (IBM Corp., Armonk, NY). Means were compared using Duncan’s multiple range test (p < 0.05).
3 Results
3.1 Floristic diversity and ecological analysis of the alien plant species
A list of all the 146 plant species (either alien or native) recorded in the study area along with their families and life forms is provided (Table S1). The results of the current study revealed the presence of 52 alien plant species (4 casual, 45 naturalized, and 3 invasive) in the western region of Saudi Arabia belonging to 25 different plant families with Asteraceae being the most represented family with 9 species followed by Gramineae (8 species). Several families were represented by only 1 species while Aizoaceae, Chenopodiaceae, Malvaceae, and Solanaceae were represented by 3 species each. Amaranthaceae, Lamiaceae, Leguminosae, and Zygophyllaceae were represented by 2 species each (Table 1). Interestingly, the identified casual and invasive species (except P. juliflora) belonged to families that were represented by one species only.
Species
Family
Life form
Chorotype/
Native range*
Sex form
Propagation/ Dispersal type
Flowering & fruiting period
Casual
Conocarpus erectus
Combretaceae
Shrub
TR AF-TR AM
Bisexual
Seeds/ Auxochore
March – May
Cupressus sempervirens
Cupressaceae
Tree
AM
Bisexual
Seeds/ Barochore
March – May
Heliotropium curassavicum
Boraginaceae
Subshrub
SA-SM
Bisexual
Nutlets/ Microsclerochore
January – May
Lantana camara
Verbenaceae
Shrub
AM
Bisexual
Seeds/ Sarochore
April –September
Naturalized
Abutilon pannosum
Malvaceae
Shrub
SM
Bisexual
Seeds
June – September
Aeluropus lagopoides
Gramineae
Perennial grass
EU-Sib-Med-IT
Bisexual
Seeds, Cuttings
December – July
Aerva javanica
Amaranthaceae
Perennial herb
TR
Bisexual
Seeds, Cuttings
June – December
Aizoon canariensis
Aizoaceae
Perennial herb
SM
Bisexual
Seeds/ Pterochore
August – December
Aristida adscensionis
Gramineae
perennial grass
Med-IT-SA
Bisexual
Seeds/ Pogonochore
April – September
Amaranthus hybridus
Amaranthaceae
Herb
Pleiotropic
Bisexual
Seeds/ Microsclerochore
April – November
Bassia eriophora
Chenopodiaceae
Perennial herb
SA-SM
Bisexual
Seeds/ Microsclerochor
September – November
Boerhaevia diffusa
Nyctaginaceae
Annual grass
TR AF
Bisexual
Seeds/ Sarochore
August – January
Calendula arvensis
Asteraceae
Annual herb
Med-IT
Bisexual
Spikelet/ Pogonochore
November – March
Calotropis procera
Asclepiadaceae
Shrub
SA-IT
Bisexual
Seeds/ Cyclochore
June – October
Cenchrus ciliaris
Gramineae
Perennial grass
TR AF
Bisexual
Seeds/ Microsclerochore
March – November
Centaurea pseudosinaica
Asteraceae
Annual herb
SA
Bisexual
Seeds/ Pterochore
April – November
Centaurea sinaica
Asteraceae
Subshrub
SA
Bisexual
Seeds/ Pogonochore
April – November
Chenopodium murale
Chenopodiaceae
Annual herb
SA-IT
Bisexual
Burrs/ Pogonochore
August – March
Chrozophora oblongiflolia
Euphorbiaceae
Subshrub
SM
Bisexual
Seeds/ Desmochore
December – March
Citrullus colocynthis
Cucurbitaceae
Perennial herb
SA
Bisexual
Seeds/ Desmochore
March – July
Convolvulus arvensis
Convolvulaceae
Subshrub
TR
Bisexual
Seeds/ Sporochore
January – April
Conyza stricta
Asteraceae
Subshrub
TR AF
Bisexual
Seeds/ Sarochore
May – September
Cynodon dactylon
Gramineae
Perennial grass
SA-IT
Bisexual
Seeds/ Sarochore
March – December
Cyperus rotundus
Cyperaceae
Subshrub
Med-IT-TR AF
Bisexual
Seeds and suckers/ Microsclerochore
June – August
Datura innoxia
Solanaceae
Shrub
Med.
Bisexual
Seeds/ Desmochore
June – November
Dichanthium annulatum
Gramineae
Perennial grass
TR
Bisexual
Seeds/ Pogonochore
April – August
Dipterygium glaucum
Capparaceae
Subshrub
SA
Bisexual
Seeds and vegetative parts/ Auxochore
June – September
Emex spinosus
Polygonaceae
Annual herb
Med.-SA
Bisexual
Seeds and tubers/ Auxochore
October- January
Fagonia bruguieri
Zygophyllaceae
Subshrub
SM
Bisexual
Seeds/ Pogonochore
July – December
Fagonia indica
Zygophyllaceae
Annual herb
SA
Bisexual
Seeds/ Microsclerochore
August – January
Hibiscus micranthus
Malvaceae
Shrub
Pleiotropic
Bisexual
Seeds/ Desmochore
June – August
Launaea mucronata
Asteraceae
Annual herb
SA-SM
Bisexual
Seeds/ Microsclerochore
March – August
Malva parviflora
Malvaceae
Annual herb
SA-SM
Bisexual
Seeds/ Microsclerochore
December – May
Marrubium vulgare
Lamiaceae
Subshrub
Med-IT
Bisexual
Seeds/ Pterochore
June – September
Nicotiana glauca
Solanaceae
Subshrub
AM
Bisexual
Seeds/ Sarochore
April – November
Panicum turgidum
Gramineae
Perennial grass
SA
Bisexual
Seeds/ Pogonochore
July – December
Phragmites australis
Gramineae
Perennial grass
Med.-IT-SA
Bisexual
Seeds/ Pterochore
September – October
Picris cyanocarpa
Asteraceae
Annual herb
SA
Bisexual
Seeds/ Desmochore
January – April
Portulaca oleracea
Portulacaceae
Annual herb
Cosm.
Bisexual
Seeds/ Pogonochore
June – October
Reichardia tingitana
Asteraceae
Annual herb
Med-IT-SA
Bisexual
Seeds/ Pogonochore
January –June
Rosmarinus officinalis
Lamiaceae
Shrub
Med
Bisexual
Seeds/ Microsclerochore
August – December
Salsola imbricate
Chenopodiaceae
Subshrub
SA
Bisexual
Seeds/ Pogonochore
June – September
Senna italic
Leguminosae
Subshrub
SM
Bisexual
Seeds/ Microsclerochore
March – October
Sesuvium portulacastrum
Aizoaceae
Herb
AM
Bisexual
Seeds, Cutting/ Auxochore
March – August
Solanum nigrum
Solanaceae
Annual herb
Cosm.
Bisexual
Seeds/ Pogonochore
May – September
Sonchus oleraceus
Asteraceae
Herb
Eu-Sib-Medi-IT
Bisexual
Seeds/ Peterochore
December – April
Stipa capensis
Gramineae
Annual grass
SA-SM
Bisexual
Seeds/ Megasclerochore
April – December
Trianthema portulacastrum
Aizoaceae
Subshrub
AM
Bisexual
Seeds, Cutting/ Auxochore
March – August
Xanthium spinosum
Asteraceae
Subshrub
TR
Bisexual
Spiny heads/ Desmochore
May – September
Invasive
Argemone mexicana
Papaveraceae
Herb
AM
Bisexual
Seeds/ Desmochore
February –May
Opuntia dillenii
Cactaceae
Shrub
AM
Bisexual
Vegetative parts, cutting
March – April
Prosopis juliflora
Leguminosae
Tree
AM
Bisexual
Seeds/ Ballochore
March – May
The dominant life forms observed in the identified alien species were annual herbs (11 species) followed by subshrubs and shrubs (8 and 7 species, respectively). The three identified invasive species belonged to annual herbs, shrubs, and trees; while the casual identified species were 2 subshrubs, a shrub, and a tree.
The majority of the recorded alien species in the western region of Saudi Arabia belonged to the American and the Saharo-Arabian regions (8 species each). The Somali Masai region and the biregional Saharo-Arabian – Somali Masai are represented by 5 species each, while the tropical region was represented by 4 species. The Tropical African region and the biregional Saharo-Arabian – Irano Turanian were represented by 3 species each. It is worth noting that all the identified invasive alien species and two (half) of the identified casual species belonged to the American region.
Pearson’s correlation analysis revealed a negative correlation (r2 = -0.586, p = 0.001) between the density of native and alien species recorded in the study area. Moreover, the ecological analysis of the identified alien species revealed that 9 species showed high frequencies along the study area namely A. mexicana, Conocarpus erectus L., Datura innoxia Mill., Heliotropium curassavicum L., Lantana camara L., Nicotiana glauca Graham, O. dillenii, P. juliflora, and Xanthium spinosum L. The other 5 species (Amaranthus hybridus L., Cupressus sempervirens L., Rosmarinus officinalis Spenn., Sesuvium portulacastrum (L.) L., and T. portulacastrum) showed lower frequencies. Therefore, further analyses regarding the distribution of the high-frequent species along different habitats were performed.
3.2 Density of alien species in different habitats
In mountain habitats, O. dillenii showed the highest density (1722.2 individual hectare-1) followed by A. mexicana (955.6 individual hectare-1) and D. innoxia (11.1 individual hectare-1; Fig. 2). Similarly, O. dillenii dominated the protected area habitats with 1516.7 individual hectare-1 followed by N. glauca (483.3 individual hectare-1). N. glauca plants were not observed in any other habitat. Moreover, A. mexicana and C. erectus were found in protected area habitats but with lower densities (66.7 and 16.7 individual hectare-1). In wadi (valley) habitats, P. juliflora showed the highest frequency (590.8 individual hectare-1) followed by O. dillenii (227.7 individual hectare-1). C. erectus and H. curassavicum were found in wadi habitats with 12.31 individual hectare-1 (Fig. 2), while A. mexicana and L. camara showed 6.2 individual hectare-1. D. innoxia had the lowest frequency in wadi habitats (6.12 individual hectare-1). The highest number of unique alien species was observed in the farmland habitats as 8 out of the 9 high-frequent species were observed in farmlands. Similar to mountain and protected area habitats, O. dillenii showed the highest frequency (800 individual hectare-1) in the farmlands (Fig. 2). P. juliflora and C. erectus showed 181.82 and 121.2 individual hectare-1, respectively. The lowest frequency in farmlands was observed for H. curassavicum (12.12 individual hectare-1). Interestingly, X. spinosum plant was observed in farmlands with a frequency of 36.4 individual hectare-1. This plant was not recorded in any other habitat (Fig. 2). In ruderal habitats, only P. juliflora was recorded with a frequency of 1600 individual hectare-1. Furthermore, P. juliflora and C. erectus dominated the coastal area habitats with 1200 and 66.7 individual hectare-1, respectively (Fig. 2).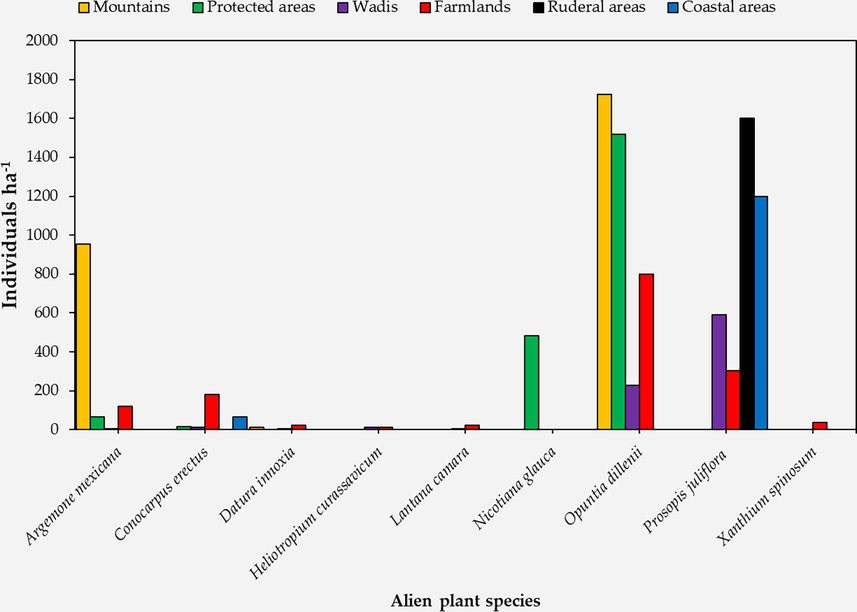
Density of each identified alien plant species in different habitats measured as average number of individuals per hectare of land area.
3.3 Distribution of high-frequent alien species in different habitats
A. mexicana plants were mainly observed in mountain habitats (955.6 individual hectare-1). This species was also recorded in wadi, protected area, and farmland habitats (Fig. 3). The highest frequency of C. erectus was recorded in farmland habitats (181.82 individual hectare-1) followed by coastal area, protected area, and wadi habitats. Nevertheless, C. erectus was not recorded in mountain or ruderal habitats. D. innoxia was recorded in 3 different habitats (mountains, wadis, and farmlands). D. innoxia showed lower frequencies as compared to other alien species in these habitats. H. curassavicum and L. camara were found in wadis and farmlands. N. glauca was found in protected areas only with a frequency of 483.3 individual hectare-1. O. dillenii was recorded in 4 different habitats (mountains, protected areas, wadis, and farmlands). Interestingly, O. dillenii showed high frequencies in all habitats where it was found. P. juliflora was also found in 4 different habitats (wadis, farmlands, ruderal, and coastal areas). The highest frequency of P. juliflora was found in ruderal habitats where it was the only recorded alien plant species. X. spinosum was recorded only in farmlands with relatively low frequency (36.4 individual hectare-1).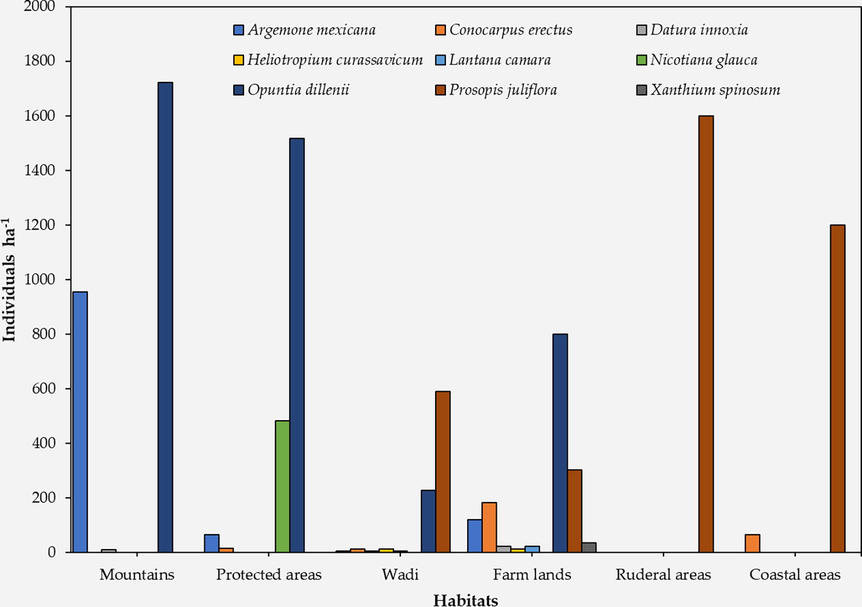
The most frequently identified alien plant species in different habitats measured in individuals per hectare.
3.4 Variation in diversity indices and soil variables across different habitats
The results of the current study revealed that all the diversity indices (i.e., number of species, species richness, species evenness, Shannon index, and Simpson index) except species cover had significant variation across the studied habitats (Table 2). The highest number of species, species richness, and species evenness (11.56, 2.76, and 0.81, respectively) were recorded in farmland habitats. Furthermore, farmlands showed the highest Shannon index (0.85) while coastal areas showed the highest Simpson index (0.57). Unsurprisingly, the soil of farmlands was characterized by high OM contents. Contents of N and P didn’t show variation among the studied habitats. Nevertheless, K, Mg, Mn, and Na showed significant variation across different habitats with coastal areas having the highest Mg and K values and the second highest Na values after ruderal areas.
Habitats*
Mountains
Protected areas
Wadi
Farmlands
Ruderal areas
Coastal areas
F value
P value
No. of species
9.73 ± 0.78
6.00 ± 0.78
8.00 ± 0.60
11.56 ± 1.03
7.00 ± 1.08
6.50 ± 0.50
4.618
0.001***
Species cover (m 100 m−1)
67.82 ± 16.49
70.88 ± 11.85
64.63 ± 8.81
58.33 ± 13.77
41.75 ± 14.59
114.50 ± 9.50
0.839
0.528
Species richness
2.38 ± 0.26
1.29 ± 0.26
1.92 ± 0.18
2.76 ± 0.27
1.70 ± 0.24
1.16 ± 0.13
3.836
0.005***
Species evenness
0.68 ± 0.07
0.54 ± 0.06
0.72 ± 0.03
0.81 ± 0.04
0.74 ± 0.10
0.48 ± 0.08
2.667
0.032*
Shannon index (Ĥ)
0.67 ± 0.08
0.41 ± 0.06
0.63 ± 0.04
0.85 ± 0.05
0.60 ± 0.06
0.39 ± 0.05
4.741
0.001***
Simpson index (C)
0.33 ± 0.07
0.55 ± 0.06
0.32 ± 0.03
0.20 ± 0.04
0.33 ± 0.09
0.57 ± 0.09
4.095
0.003**
pH
7.70 ± 0.09
7.74 ± 0.10
7.79 ± 0.05
7.62 ± 0.14
7.46 ± 0.19
7.35 ± 0.14
1.718
0.147
EC (ms cm−1)
0.22 ± 0.05
0.34 ± 0.19
0.30 ± 0.08
1.46 ± 0.57
1.47 ± 0.99
1.06 ± 0.22
3.603
0.007**
Sand (%)
55.17 ± 6.25
74.95 ± 2.92
72.35 ± 2.81
64.68 ± 6.22
76.34 ± 0.31
60.51 ± 3.89
2.737
0.029*
Clay (%)
15.14 ± 1.85
9.32 ± 0.79
11.49 ± 0.87
12.16 ± 1.49
10.91 ± 1.03
11.98 ± 1.96
1.830
0.123
Silt (%)
29.69 ± 4.76
15.72 ± 2.30
16.16 ± 2.26
23.16 ± 4.90
12.76 ± 0.85
27.51 ± 1.93
2.754
0.028*
OM (%)
7.83 ± 1.12
9.17 ± 1.06
5.39 ± 0.59
10.29 ± 1.65
5.36 ± 1.12
5.62 ± 0.67
3.752
0.006**
Ca (ppm)
14.65 ± 3.21
13.03 ± 1.72
11.73 ± 1.61
18.04 ± 2.90
26.53 ± 10.99
21.97 ± 0.00
2.172
0.071
K (ppm)
2.35 ± 0.52
2.74 ± 0.53
4.60 ± 0.70
6.96 ± 1.30
6.28 ± 1.74
9.12 ± 0.83
4.088
0.003**
Na (ppm)
11.57 ± 1.95
10.95 ± 0.70
10.58 ± 0.98
13.55 ± 2.11
26.02 ± 9.79
19.93 ± 0.00
4.110
0.003**
Fe (ppm)
0.23 ± 0.05
0.12 ± 0.04
0.50 ± 0.19
0.39 ± 0.23
0.31 ± 0.15
0.08 ± 0.01
0.548
0.739
Mg (ppm)
2.43 ± 0.54
3.52 ± 0.33
2.75 ± 0.41
5.33 ± 0.83
6.17 ± 1.57
7.03 ± 0.04
5.451
0.000***
Mn (ppm)
0.02 ± 0.00
0.05 ± 0.00
0.03 ± 0.00
0.03 ± 0.01
0.09 ± 0.07
0.08 ± 0.01
2.819
0.025*
N (ppt)
6.61 ± 0.96
3.99 ± 0.43
4.62 ± 0.62
5.59 ± 1.21
7.69 ± 2.82
3.60 ± 0.50
1.437
0.226
P (ppt)
0.18 ± 0.05
0.14 ± 0.01
0.15 ± 0.02
0.18 ± 0.04
0.25 ± 0.10
0.12 ± 0.01
0.740
0.597
3.5 Relationships between alien plant species, diversity indices, and soil variables
The relationships between alien plants distribution along the ordination axes and the soil variables were examined via CCA (Fig. 4). The results revealed that the distribution of A. hybridus, H. curassavicum, and O. dillenii was correlated with soil pH and OM content. Similarly, the distribution of A. mexicana, N. glauca, and X. spinosum was correlated with silt content in the soil on the AX1 positive axis. On the other hand, the distribution of C. erectus and D. innoxia showed correlation with Mn content, EC, and sand content in the soil. The distribution of P. juliflora and T. portulacastrum showed correlation with the soil contents of K, Ca, and Mn on the AX1 negative axis. Nevertheless, the distribution of H. curassavicum showed correlation with soil contents of N and P (Fig. 4).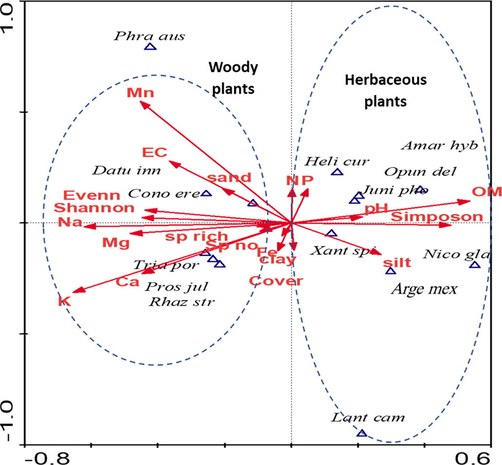
A biplot showing the correlation between distribution of the alien species and the significant soil variables based on the canonical correspondence analysis (CCA) ordination. Plant scientific names are shown as the first 4 letters of genus and the first 3 letters of the species.
4 Discussion
According to Collenette (1999), the Saudi Arabian flora includes 2250 species belonging to 835 genera and about 142 families, of them 147 species are “endemic”, 721 species are “endangered”, and 22 species are “completely extinct”. In the current study, 52 alien plant species that related to 50 different genera and 25 plant families were recorded in the western region of Saudi Arabia, representing 2.3 %, 6.0 %, and 17.6 % of total species, genera, and families in the flora of Saudi Arabia, respectively. In United Arab Emirates, 8 alien pant species were recorded (Soorae et al., 2015). In Oman, 111 alien plant species were identified, of them 77 species are naturalized (Patzelt et al., 2022). In the Middle East region, 136 alien species were recorded in Egypt (Shaltout et al., 2016), 60 species in Tunisia, and 143 species in Algeria (Vilà et al., 1999). These results shows that the western region of Saudi Arabia has an average occurrences of alien species introduction. Alfarhan et al. (2021) stated that the southwestern region of Saudi Arabia is the most affected area in terms of invasive infestations. Similarly, Al-Baha region was among the most affected areas by invasive alien species in Saudi Arabia (Aljeddani et al., 2021). The analysis of life forms of the identified alien species revealed that they were mainly annual herbs, subshrubs, and shrubs. In general, alien shrub and tree species have higher potential to survive and spread comparing to other life forms (Shaltout et al., 2016). Most of the alien plant species identified in the western region of Saudi Arabia belong to the American and the Saharo-Arabian regions. This could be due to the active trade and transportation activities happen from these regions via the Red Sea. Moreover, imported crop seeds e.g., wheat and some plants introduced for afforestation, ornamental and shading e.g., Prosopis sp., Conocarpus sp. etc. could be polluted with strange seeds.
The results of the current study showed a negative correlation between the density of alien and native species and that only 9 alien species had high frequencies in the study area. In 2016, A. mexicana, N. glauca, O. dillenii, and P. juliflora were classified as dangerous invasive alien species in Saudi Arabia (Thomas et al., 2016). In the current study, A. mexicana was found mainly in mountain habitats. Previous results showed that A. ochroleuca and A. mexicana are alien species that found in Saudi Arabia at high altitude areas and not found below the heights of 1500 m a.s.l. (Thomas et al., 2016, Alfarhan et al., 2021). The results of the current study reported the presence of N. glauca in the protected areas only. This could be an indicator of the potential control over the spread of this plant as an invasive species. In principle, P. juliflora was introduced to Saudi Arabia as a part of one of the Ministry of Agriculture’s afforestation programs. In the current study, P. juliflora was found in all the studied habitats except mountains and protected areas. This could be attributed to the efforts made to remove the plant from the urban areas; however, it continues to spread in natural habitats in addition to its persistent usage as a shade tree in the suburban areas. Such infestations were also reported from different parts of Africa (Berhanu and Tesfaye, 2006). Furthermore, P. juliflora in Saudi Arabia is usually found in lower altitudes (less than 200 m). The poor performance of this plant at high altitudes could be attributed mainly to its inability to withstand the sudden changes in climate e.g. low temperatures, fogs, extreme humidity, and frosts (Tomas et al., 2010). The results of the current study showed that P. juliflora was not present in mountain habitats. On the other hand, P. juliflora was the only alien invasive plant species identified in the ruderal areas in the current study in a clear indication of its ability to survive under harsh conditions and the highly competitive characteristics of this plant.
The CCA results approved the correlation between the distribution of P. juliflora and the soil contents of K, Ca, and Mn. The results of the current study revealed an important and unique phenomenon in most wadis of western region (e.g., wadi Rabegh) which were invaded by P. juliflora. This tree is more abundant in wadis and its invasions cover a large area of the cultivated farms in the wadis by changing its growth habit from erect trees into a prostrate shrub extending its vegetative shoot parts and covering the soil surface as a tangle mat, and thus prevent any farming operations. Moreover, it produces thousands of active germinating seeds. The removal of this plant via cutting is not solving the problem due to its active ability to regrow after a short time. This will cause several problems in cultivated farms and deplete the ground water; therefore, converting the cultivated farms into abandoned areas. In addition, the same phenomena were noted in Farsan Islands, located at southwest of Saudi Arabia in Red Sea, as the vegetation composition was changed after replacing many wild plants by this invasive tree.
O. dillenii was the most abundant alien species in the studied area. It was found in all habitats except ruderal and coastal areas. Opuntia spp. could be found in all communities at various altitudes, particularly at heights between 800 and 2200 m (Thomas et al., 2016). The results of CCA coordination analysis in the current study revealed that the distribution of O. dillenii correlated with soil pH and OM content. The results of the current study recorded an important phenomenon about Opuntia spp. that cause several dangerous problems by their invasions into the rose farms in Taif region (with high economic values in Saudi Arabia). After removal of these plants, several thousands of small vegetative parts which have active ability for regeneration again in few days remain in the soil, and therefore may convert the rose farms into abandoned areas with lower productivity. In addition, Opuntia spp. invade the protected areas e.g., Rida escarpment (that has many rare, endangered, and endemic species) and within few years, this plant is expected to replace the majority of wild plant species at different habitats leading to potential loss of diversity in these unique protected areas. On the other hand, the distribution of N. glauca correlated with the silt content in the soil. As one of the threaten invasive plant species, and due to the existence in protected areas only, N. glauca should get more attention to increase the control among its spread that could negatively affect the biodiversity in these areas that planned, in principle, to protect local biodiversity and natural habitats.
5 Conclusions
Alien and invasive plant species threaten the local biodiversity in several countries. In Saudi Arabia, the western region is exposed to frequent introduction of alien species because of the continuous trade and transportation activities along the Red Sea coast. The results of the current study identified 52 alien plant species with the identification of 3 invasive, 45 naturalized, and 4 casual species in the western region of Saudi Arabia and showed the most dangerous species among them and examined their distribution in different habitats. A. mexicana, O. dillenii, and P. juliflora were identified in the current study as invasive species. Their presence showed a negative correlation with the presence of other native species. The results of the current study will significantly help in reshaping the strategic plants with the aim of conserving biodiversity and combat the spread of alien species. Further studies are surely needed to examine the potential impacts of the identified alien plant species (especially the invasive ones) on the associated plant communities in terms of floristic structure and vegetation of the invaded habitats.
Funding
This scientific paper contains studies and research results supported by King Abdulaziz City for Science and Technology Grant No. (1-18-01-001-0076).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Main vegetation types and plant species diversity along an altitudinal gradient of Al Baha region. Saudi Arabia. Saudi J. Biol. Sci.. 2016;23(6):687-697.
- [CrossRef] [Google Scholar]
- Impact Assessment and Management of Invasive Species in Plant Diversity Centers and Agriculture Fields of Saudi Arabia. In: Pullaiah T., Ielmini M.R., eds. Invasive Alien Species: Observations and Issues From Around the World. NJ, USA, John: Wiley & Sons Ltd.; 2021. p. :207-225.
- [Google Scholar]
- Influence of the invasive shrub Nicotiana glauca Graham on the plant seed bank in various locations in Taif region, western of Saudi Arabia. Saudi J. Biol. Sci.. 2021;28(1):360-370.
- [CrossRef] [Google Scholar]
- Inventory of some introduced and invasive plant species in some governorates of the Kingdom of Saudi Arabia. Appl. Ecol. Environ. Res.. 2021;19(6):4373-4388.
- [Google Scholar]
- Chemical analysis of ecological materials. Oxford: Blackwell Scientific; 1989.
- Natural plant species inventory of hotspot areas in Arabian Peninsula: Southwest Al-Baha region. Saudi Arabia. Saudi J. Biol. Sci.. 2021;28(6):3309-3324.
- [CrossRef] [Google Scholar]
- Cylindropuntia rosea (DC.) Backeb, (Cactaceae): a new generic alien record in the flora of Saudi Arabia. J. Asia-Pacific Biodiversity. 2018;11(2):320-323.
- [CrossRef] [Google Scholar]
- Ecology of invasive species in Saudi Arabia, Calotropis procera (Ait.) WT Ait.: floristic composition and associated plant communities. Int. J. Ecotoxicol. Ecobiol.. 2016;1(3):127-140.
- [Google Scholar]
- Alien species as a driver of recent extinctions. Biol. Lett.. 2016;12(2):20150623.
- [CrossRef] [Google Scholar]
- The Prosopis dilemma, impacts on dryland biodiversity and some controlling methods. J. Drylands. 2006;1(2):158-164.
- [Google Scholar]
- CBD, 2022. Convention on Biological Diversity, Programme of Work on Invasive Alien Species. Retrieved 06.03.2022, from https://www.cbd.int/invasive/.
- Chaudhary, H., 1999-2001. Flora of Kingdom of Saudi Arabia vol. I, II and III. Riyadh, Saudi Arabia, Ministry of Agriculture and Water, National Herbarium, National and Water Research Center.
- An illustrated guide to the flowers of Saudi Arabia. Riyadh: Scorpion publishing Ltd; 1985.
- A Checklist of Botanical Species in Saudi Arabia. West Sussex (UK), International Asclepiad Society; 1998.
- Wildflowers of Saudi Arabia. Riyadh: National Commission for Wildlife Conservation and Development (NCWCD); 1999.
- The grading of dispersal types in plant communities and their ecological significance. Institut Botanique de L’Universitè deMontrèal.. 1957;71:1-52.
- [Google Scholar]
- Aims and methods of vegetation ecology. New York: Wiley; 1974.
- Vegetation of Thumamah Nature Park: a managed arid land site in Saudi Arabia. Rendiconti Lincei.. 2013;24(4):349-367.
- [CrossRef] [Google Scholar]
- Hobohm, C., M. Janišová, J. Jansen, et al., 2014. Biogeography of endemic vascular plants–overview. Endemism in vascular plants. Switzerland, Springer Nature: 85-163.
- Vegetation description and data analysis: a practical approach. Oxford: UK, John Wiley & Sons Ltd.; 2012.
- The first steps towards unifying concepts in invasion ecology were made one hundred years ago: revisiting the work of the Swiss botanist Albert Thellung. Divers. Distrib.. 2012;18(12):1243-1252.
- [CrossRef] [Google Scholar]
- Biological invasions in conservation planning: a global systematic review. Front. Mar. Sci.. 2018;5
- [CrossRef] [Google Scholar]
- Ecological diversity and its measurement. Princeton University Press; 1988.
- Measuring biological diversity. John Wiley & Sons; 2003.
- Alien flora of Oman: invasion status, taxonomic composition, habitats, origin, and pathways of introduction. Biol. Invasions. 2022
- [CrossRef] [Google Scholar]
- Pielou, E.C., 1975. Ecological diversity.
- Naturalization and invasion of alien plants: concepts and definitions. Divers. Distrib.. 2000;6(2):93-107.
- [CrossRef] [Google Scholar]
- Composition and pattern of alien species in the Egyptian flora. Flora - Morphology, Distribution, Functional Ecology of Plants.. 2016;222:104-110.
- [CrossRef] [Google Scholar]
- Alien species recorded in the United Arab Emirates: an initial list of terrestrial and freshwater species. J. Threatened Taxa. 2015;7(12):7910-7921.
- [CrossRef] [Google Scholar]
- Ter Braak, C. J. and P. Smilauer, 2002. CANOCO reference manual and CanoDraw for Windows user's guide: software for canonical community ordination (version 4.5), http://www.canoco.com.
- Impact of alien invasive species on habitats and species richness in Saudi Arabia. J. Arid Environ.. 2016;127:53-65.
- [CrossRef] [Google Scholar]
- Endemics and endangered species in the biodiversity hotspot of the Shada Mountains, Saudi Arabia. J. Arid Land. 2017;9(1):109-121.
- [Google Scholar]
- Floristic composition of the Farasan Archipelago in Southern Red Sea and its affinities to phytogeographical regions. Arab Gulf J. Sci. Res.. 2010;28(2):79-90.
- [Google Scholar]
- How well do we understand the impacts of alien species on ecosystem services? A pan-European, cross-taxa assessment. Front. Ecol. Environ.. 2010;8(3):135-144.
- [CrossRef] [Google Scholar]
- Non-native Species, Ecosystem Services, and Human Well-Being. In: Vilà M., Hulme P.E., eds. Impact of Biological Invasions on Ecosystem Services. Cham: Springer International Publishing; 2017. p. :1-14.
- [Google Scholar]
- Preliminary analysis of the naturalized flora of northern Africa. Orsis: organismes i sistemes.. 1999;14:9-20.
- [Google Scholar]
- Differential germination and growth response to temperature of three Ambrosia Weed Species—Implications for future spread. Front. Agronomy. 2020;2
- [CrossRef] [Google Scholar]
- Geobotanical foundations of the Middle East. Lisse, Netherlands: Swets & Zeitlinger; 1973.
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2022.102496.
Appendix A
Supplementary material
The following are the Supplementary data to this article:







