Translate this page into:
Biological and toxicological evaluation of aerial parts extracts of locally grown Cleome austroarabica
⁎Corresponding author. amzad@unizwa.edu.om (Amzad Hossain)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The current study was conducted to prepare different polarities extracts and evaluate their antimicrobial and cytotoxic activities from the aerial parts of Cleome austroarabica (C. austroarabica) which was collected from the initial campus of the University of Nizwa. The coarse powder was extracted with direct methanol by using a maceration method. The extract residue was fractionated by using various organic solvents with increasing polarity. The agar disc and brine shrimp lethality (BSL) bioassays were used to assess the antimicrobial and cytotoxic activities of the aerial extracts of C. austroarabica. Four concentrations (2000, 1000, 500, 250 µg/ml) of each extract were used to determine the antimicrobial activity against two Gram (+) bacteria: Staphylococcus aurous (S. aurous), Enterococcus faecalis (E. aurous) and two Gram (−) bacteria: Haemophilus Influenza (H. Influenza), and Escherichia coli (E. coli). Brine shrimp organisms were used to assess the cytotoxic activities of various polarities aerial extracts and the fractions of chloroform extract. The antimicrobial activity results showed that all polarities extracts at different concentrations did not give any activity against the tested bacteria. The cytotoxic activity of all polarities aerial extracts displayed activity within the value of LC50 385.25–640.25 μg/ml in the order of chloroform > hexane > hydro alcoholic > ethyl acetate > methanol extract. However, the isolated all chloroform fractions showed high LC50 activity compared to control. The further extensive study will be needed to confirm the antimicrobial and cytotoxic activities of the crude extracts and to isolate the active ingredients from the highest activity aerial extracts.
Keywords
Cleome austroarabica
Muqabil al shams
Antimicrobial
Cytotoxicity
Oman
1 Introduction
Cancer is an incurable disease. Recently, it is global burden and it has a major impact on communities across the world. It is a multiple disease involving abnormal cell growth and rapidly spread to the other parts of the human body (Greenwell and Rahman, 2015). The most commonly listed cancers globally available are, breast cancer, lung cancer, prostate cancer, pancreatic cancer, colorectal cancer, leukemia cancer etc. The major causes of cancer are tobacco, alcohol, obesity, poor diet, lack of physical exercise, radiation, environmental pollution, and different types of virus and bacterial infections (Roy et al., 2017). Nowadays, these diseases are creating a main health problem in the third world countries. The demand for new drugs to prevent or cure these diseases is abnormally increasing. Plants as traditional medicines are one of the basic and needful sources to provide remedies continuously to save people since the ancient times. People prefer plant derived remedies to treat those incurable diseases without side effect. Some of the plants and plant ingredients showed the potential role against cancer treatment. Therefore, the scientists are working on the natural sources to find out the cancer remedies to treat different types of cancer (Willis, 1996).
Cleome L. is a large genus included 150 species which grown well in the tropical and subtropical countries (Stevens, 2012). One of the most interesting plant family that is under the researchers lance is Cleomaceae family. They are interested, especially on Cleome genus because the selected genus extracts showed significant anticancer and antimicrobial activities (Bose et al., 2011).
The Cleome plant is a genus of flowering plants also belongs to Cleomaceae family. Previously, it had been placed in the same family. However, DNA studies found that the Cleomaceae genera is closely related to Brassicaceae family than Capparaceae family (Samout et al., 2015). The selected plant is a herbaceous sticky plant with unpleasant odor (Abdullah et al., 2016). The C. austroarabica is locally known as Muqabil al shams in Arabic (Ravindra, 2010). The selected plant is endemic to Southern Arabia, including Oman. In Oman, there are 12 Cleome species available. The height of the selected plant is about 1 m (Fig. 1). The leaves are alternate and the shape is oval to round with entire margins. It is fully covered with sticky glands (Ravindra, 2010). The flowers are a pale yellow colour and the petals folded back with yellow stamens (Ravindra, 2010). The flowering period throughout the year. The fruits and seeds are oblong and erect capsule size (Ravindra, 2010). According to the literature showed that many plant species belong to this genus are very rich content of essential oils. Several compounds which are responsible for biological activities such as terpenes, sterols, flavonoids, glucosinolates and isothiocyanates were found in various Cleome species (Jordheim et al., 2009; Chopra et al., 1972). In Oman, it is used traditionally as an eye drops to treat cataracts (Ghazanfar, 1999). Most of the Cleome species are generally used as folk medicine for stomach aches, scabies, inflammation, rheumatism and cytotoxic (Tsichritzis et al., 1993; Ahmad et al., 1990; Nagaya et al., 1997). However, as an extract of the entire plant is used in Southern Arabia to treat cataracts (Ghazanfar, 1999). In the literature, there are no reports available on the C. austroarabica for the determination of phytochemical, biological and toxicological activities. Therefore, the target of this current study is to prepare various polarities aerial extracts and to estimate their antimicrobial and cytotoxic activities by the available disc diffusion and brine shrimp lethality (BSL) bioassays.
Plant of Cleome austroarabica.
2 Materials and method
2.1 Chemicals and materials
The standards and chemicals such as chloroform, dimethyl sulphoxide (DMSO, purity 99%) and methanol were used in this study obtained from Fisher Chemical Company, UK. Hexane was collected from Daejung, Korea. Ethyl acetate was collected from Carbon Group, Ireland. Filter papers were purchased from Whatman, UK. The control ciprofloxacin antibiotic, silica gel and TLC plate were obtained from E. Merck, Germany. Brine shrimp eggs was purchased from the USA. Sea water was collected from Wave beach, Muscat, Sultanate of Oman.
2.2 Microorganism
The clinically isolated selected microorganism such as Escherichia coli (E. coli), Haemophilus influenza (H. influenza), Enterococcus faecalis (E. faecalis), and Staphylococcus aureus (S. aureus) were obtained from one of local hospitals in Nizwa, Sultanate of Oman.
2.3 Sample collection
The aerial parts of C. austroarabica were collected from the initial campus of the University of Nizwa, Birkat Al Mouz, Nizwa, Oman, in November 2016. The morphological authentication was done using the microscopic method by Dr. Syed Abdullah Gilani, Department of Biological Sciences and Chemistry, College of Arts and Sciences, University of Nizwa. The collected aerial parts were cleaned and dried at ambient temperature.
2.4 Extraction
The dried plant was ground into coarse powder by using blender machines and the yield was around 414.1 gm. The powdered samples (400 gm) were macerated in about 1 L of methanol solvent for one week (Hossain et al., 2013). The extracts were filtered by using a Buchner funnel and concentrated at 40 °C using a rotary evaporator to give yield 37.35 gm. Then the residue (35 gm) was suspended in ethanol and water mixture (1:1) and finally fractioned with hexane, chloroform, and ethyl acetate consecutively to give the corresponding extracts. The mother solvent was evaporated completely and the weight of each residue was recorded.
2.5 Antibacterial activity
The antibacterial activity of each polarity aerial extract of C. austroarabica was evaluated by using the disc diffusion method (Abdullah et al., 2016; Matani et al., 2015). In this experiment, four concentrations of each polarity extract (2000, 1000, 500, 250 µg/ml) were prepared by the dilution method using dimethyl sulphoxide (DMSO). Filter paper discs of 5 mm diameter size were dipped in each concentration. The discs were put on agar plates that were inoculated with the clinically isolated Gram (+) positive and Gram (−) bacteria. The solvent DMSO as a negative control and ciprofloxacin (250 µg/ml) as a positive control were used. After inoculated, the plates were kept in the incubator for 24 h at 37 °C. An antibacterial activity of each concentration of each polarity extract was evaluated manually by measuring the diameter of the inhibited zone.
2.6 Cytotoxic activity
The toxicity of each prepared aerial extract of C. austroarabica was done by using shrimp assay, which is usually called as sea monkeys. Shrimp invertebrate organisms about 1 mm in size with brownish colour was used for the calculation of cytotoxic activity (Lieberman, 1999).
2.6.1 Hatching of shrimp larvae
About (50 mg) of cysts (shrimp eggs) was placed into the sea water which were taken in a plastic container which was divided into two compartments with few holes. The compartment was separated into two parts by a polyethylene glycol wall. One compartment of the plastic container was covered with aluminium foil to create artificial darkness and the other compartment under light. Both the compartments were maintained at an ambient temperature. After hatching shrimp mature nauplii were attracted to the other lighted compartment that was illuminated. These nauplii was taken for the cytotoxic bioassay.
2.6.2 Brine shrimp lethality assay
The cytotoxic activity of various aerial extracts of C. austroarabica was evaluated by well-established BSL assay (Matani et al., 2015; Rehab and Hossain, 2016). Five different concentrations such as 10, 100, 250, and 500 μg/ml of each extract were prepared by using DMSO. After properly labelling the glass vials, 0.1 ml of each concentration was placed in each vial and the volume was adjusted to five millilitres by using the sea water. Then, ten nauplii were added to each vial with the help of a dropper. Similar concentrations of potassium permanganate were prepared in the same way without sample and used as a positive control. DMSO (0.1 ml), nauplii (10 nos) and 4.9 ml of sea water in a vial was used as a negative control. All experimental vials were kept under the light for 24 h. If the nauplii did not exhibit any internal or external movement during the time of observation then it was considered dead. Each concentration vial and positive and negative control were checked by using a magnifying glass. The number of surviving nauplii was counted after 24 hrs. The mortality (%) was calculated at each concentration and usually expressed as a median lethal concentration (LC50). Probit analysis method was used to calculate the LC50 value (Finney, 1971). The LC50 value represents the concentration of the chemical that produces death in half of the subjects after a certain exposure period.
The mortality (%) of each experimental dose and the positive and negative controls were evaluated by using the formula:
2.7 Fractionation of lowest LC50 by column chromatography
The chloroform aerial extract gave lowest LC50 value. The chloroform extract (5 gm) was subjected to column chromatography over silica gel (25 × 75 cm, 100 gm) by using n-hexane, n-hexane: chloroform, chloroform, chloroform: ethyl acetate, ethyl acetate, ethyl acetate: methanol mixtures as a mobile phase (Sohail et al., 2017). All collected fractions were on TLC and those with similar pattern were combined and screened for their cytotoxic activity.
3 Results and discussion
3.1 Yield of extracts
The aerial course powdered of C. austroarabica were extracted directly with methanol by maceration method. The residue was reextracted by different solvents with increasing polarity. The approximate yields and their percentage of yield of each individual extract are given in Table 1. The highest percentage of the yield of methanol extract was about 10.6% obtained from 414.1 gm of aerial parts of coarse powder of C. austroarabica (Table 1). After fractionation, the amount and yield of hexane and chloroform was higher than the ethyl acetate and hydro alcoholic extracts (Table 1). Therefore, the aerial parts of C. austroarabica contained relatively higher amounts of nonpolar ingredients compared to the polar fractions.
Extract
Amount (gm)
Yield (%)
Methanol
44.3 ± 0.09
10.6
Hexane
8.5 ± 0.19
2.05
Chloroform
11.5 ± 0.27
2.87
Ethyl acetate
0.97 ± 0.51
0.23
Hydro alcoholic
11.6 ± 0.18
2.80
3.2 Antimicrobial activity
The activity of methanol extract and its derived different polarities aerial fractions of C. austroarabica were determined against clinically isolated Gram (+and −) bacterial strains (Najwa and Hossain, 2018). In our current experiment, two Gram (+) bacteria: Staphylococcus aurous (S. aurous), Enterococcus faecalis (E. aurous) and two Gram (−) bacteria: Haemophilus Influenza (H. Influenza), and Escherichia coli (E. coli) were used against various polarities aerial extracts at different concentrations. Unfortunately, the experimental results showed that there was no growth inhibition obtained against the tested Gram (+and −) bacteria at any concentrations (Table 2). However, the ethanol extract of leaves and flowers of other species of C. viscosa belongs to this family showed activity against E. coli, P. vulgaris and P. aeruginosa (Abreu et al., 2013). Another study conducted by Muhaidat et al on the essential oils of C. droserifolia and C. trinervia species belong to the same family and showed that it gave a significant inhibition against different pathogen bacterial strains (Muhaidat et al., 2015). But, the selected plant species which were grown in Oman did not give any activity, it could be due to the sensitivity of applied bacterial strains or the dose of the extract samples (Najwa and Hossain, 2018). There is no reports and data available on antimicrobial studies of the selected species in Oman. Therefore, we are unable to compare our results with the reported values. nd = not detected.
Extract
Conc. (µg\m)
E. coli
H. influnza
E. faecalis
S. aureus
Methanol
250
nd
nd
nd
nd
500
nd
nd
nd
nd
1000
nd
nd
nd
nd
2000
nd
nd
nd
Ciprofloxacin
250
30
22
23
23
Hexane
250
nd
nd
nd
nd
500
nd
nd
nd
nd
1000
nd
nd
nd
nd
2000
nd
nd
nd
nd
Ciprofloxacin
250
31
22
20
21
Chloroform
250
nd
nd
nd
nd
500
nd
nd
nd
nd
1000
nd
nd
nd
nd
2000
nd
nd
nd
nd
Ciprofloxacin
250
27
22
23
20
Ethyl acetate
250
nd
nd
nd
nd
500
nd
nd
nd
nd
1000
nd
nd
nd
nd
2000
nd
nd
nd
nd
Ciprofloxacin
250
29
26
23
21
Hydro alcoholic
250
nd
nd
nd
nd
500
nd
nd
nd
nd
1000
nd
nd
nd
nd
2000
nd
nd
nd
nd
Ciprofloxacin
250
29
30
23
21
3.3 Cytotoxic activity
The cytotoxicity of the prepared aerial extracts of C. austroarabica was evaluated by brine shrimp lethality (BSL) assays reported by several authors (Matani et al., 2015; Rehab and Hossain, 2016). All the prepared aerial extracts at different concentrations have shown activity against the brine shrimp larvae. Among the five prepared different polarities aerial extracts, the hexane, chloroform, ethyl acetate and hydro alcoholic extracts have displayed significant activity against the brine shrimp larvae. The mortality percentages of the shrimp larvae exposed to different aerial extracts of C. austroarabica are shown in Table 3. The order of activity was chloroform > methanol > ethyl acetate > hexane > hydro alcoholic extract. Moreover, there is an increase in the mean percentage of mortality with increase in concentration of aerial extract. Both the hexane and hydro alcoholic extracts gave the highest LC50 value 640.26 µg/ml (Table 3). According to the definition of LC50, the high LC50 value means it has less toxicity. That means, both the hexane and hydro alcoholic extracts contained less toxic compounds (high LC50 value means less toxic). The minimum LC50 value was obtained from the chloroform extracts compared to positive control KMNO4 (Fig. 2). In our present experiment, the chloroform extract showed the lowest LC50 value. Therefore, the chloroform extract is the most potent toxic aerial extract among the five prepared extracts from the C. austroarabica.
Extract Conc. (µg/ml)
Mean percent mortality of brine shrimp larvae (%)
Standard KMnO4
Methanol
Hexane
Chloroform
Ethyl acetate
Hydro alcoholic
DMSO
500
60 ± 0.19
60 ± 0.17
40 ± 0.09
60 ± 0.25
60 ± 0.17
40 ± 0.18
0
250
50 ± 0.10
30 ± 0.12
30 ± 0.18
40 ± 0.18
30 ± 0.55
30 ± 0.27
0
100
30 ± 0.23
20 ± 0.14
20 ± 0.15
20 ± 0.44
20 ± 0.21
20 ± 0.42
0
10
30 ± 0.08
10 ± 0.45
10 ± 0.29
0
10 ± 0.44
10 ± 0.76
0
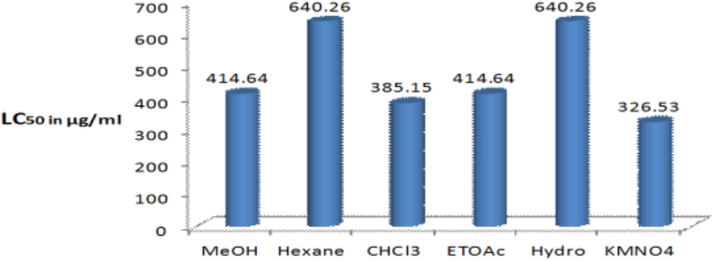
Comparison of LC50 values of methanol (MeOH), hexane, chloroform (CHCl3), ethyl acetate (EtOAc), hydro alcoholic (Hydro) and potassium permanganate (KMNO4).
According to the LC50 value, chloroform extract was selected for the separation of toxic ingredients. The column chromatography method was used to separate the ingredients from the chloroform extract. After fractionation of the chloroform extract by column chromatography, seven fractions (Fraction 1 (0.45gm); Fraction 2 (0.15gm), Fraction 3 (0.14gm), Fraction 4 (0.47gm), Fraction 5 (0.42gm), Fraction 6 (0.48gm) and Fraction 7 (0.2 gm) were obtained by using the mobile phase e.g., n-hexane, n-hexane: chloroform, chloroform, chloroform: ethyl acetate, ethyl acetate, and ethyl acetate: methanol mixtures. All the isolated fractions from chloroform aerial extract were tested for toxicity in the same way mentioned by using brine shrimp napulii. All chloroform fractions gave cytotoxic activity at very high concentrations as compared to the original chloroform extract (Fig. 3). Some cytotoxic activity was done by the authors of the other species such as C. droserifolia, C. viscosa and C. burmanni belongs to the selected family. They reported that the C. droserifolia plant species showed significant activity against breast cancer cell (MGF7) and colon adenocarcinoma (HCT116) (Ezzat and Adel Motaal, 2012). Another study conducted on cytotoxic activity on the C. viscosa and C. burmanni species by Pillali and Nair. They mentioned that methanol extracts of both the plant species exhibited significant toxicity against the shrimp nauplii when compared to standard potassium permanganate (positive control) and thus is possibly a good indicator of toxicity (Pillai and Nair, 2011). There are no reports and data available on the selected species. Therefore, we are unable to compare our results with the reported data due to the lack of literature on the toxicological activity of C. austroarabica.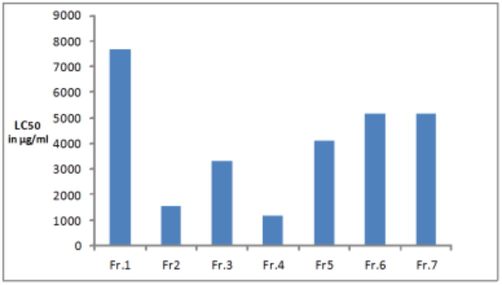
LC50 values of different fractions of chloroform extract.
4 Conclusion
The pharmacological (antimicrobial activity) and toxicological (cytotoxic activity) were measured by well-established bioassays. Among the five extracts from C. austroarabica, none of them showed antibacterial activity due to the sensitivity and the dose. However, all five aerial extracts at various doses showed cytotoxic activities against brine shrimp larvae (BSL). Among them chloroform aerial extract showed the highest toxicity. In addition, seven chloroform fractions obtained from the chloroform extract by column also showed significant activities. Therefore, further comprehensive phytochemical and pharmacological analyses are needed for the selected locally grown plant of C. austroarabica for the consideration of this plant as medicinal plant. It is also needed to isolate and identify the toxic ingredients which will be the pharmacological and toxicological potential for the treatment of diseases.
Acknowledgement
One of the authors is grateful to the University of Nizwa for providing all facilities to complete her graduation project. The authors are also grateful to the Nizwa Hospital to providing us the necessary bacterial strains.
References
- Chemical constituents and biological activities of Cleome Genus: a brief review. Inter. J. Pharm. Phytochem. Res.. 2016;8(5):777-787.
- [Google Scholar]
- Antibacterial activity of phenyl isothiocyanate on Escherichia coli and Staphylococcus aureus. Med. Chem.. 2013;9(5):756-761.
- [Google Scholar]
- Efficacy of formalinized liver-organ-vaccine against Angara disease in broilers. Veterinarski Arhiv.. 1990;60(3):131-138.
- [Google Scholar]
- Chopra's Indigenous Drugs of India. Calcutta, India: Dhur & Sons Private Limited; 1972.
- Isolation of New Cytotoxic Metabolites from Cleome droserifolia Grown in Eygpt. Zeitschrift fuer Naturf.. 2012;67C:266-274.
- [Google Scholar]
- Probit Analysis (third ed.). London UK: Cambridge University Press; 1971.
- Handbook of Arabian Medicinal Plants. Boca Raton-London: CRC Press; 1999.
- Medicinal plants: their use in anticancer treatment. Int. J. Pharm. Sci. Res.. 2015;6:4103-4112.
- [Google Scholar]
- Effect of temperature and extraction process on antioxidant activity of various leaves crude extracts of Thymus vulgaris. J. Coast Life Med.. 2013;1(2):118-122.
- [Google Scholar]
- Acylated anthocyanins in inflorescence of spider flower (Cleome hassleriana) Phytochem.. 2009;70(6):740-745.
- [Google Scholar]
- A brine shrimp bioassay for measuring toxicity and remediation of chemicals. J. Chem. Educ.. 1999;76(12):1689-1691.
- [Google Scholar]
- In vitro evaluation of the total phenolic and flavonoid contents and the antimicrobial and cytotoxicity activities of crude fruit extracts with different polarities from Ficus sycomorus. Pac. Sci. Rev. A: Nat. Sci. Eng.. 2015;17:103-108.
- [Google Scholar]
- Phytochemical investigation and in vitro antibacterial activity of essential oils from Cleome droserifolia (Forssk.) Delile and C. trinervia Fresen. (Cleomaceae) South Afr. J. Bot.. 2015;99:21-28.
- [Google Scholar]
- Chemical composition and antimicrobial potency of locally grown lemon essential oil against selected bacterial strains. J. King Saud Univ. Sci.. 2018;30:14-20.
- [Google Scholar]
- A comparative study on the anthelmintic potential of Cleome viscosa L and C. burmanni. Indian J. Pharm. Sci.. 2011;73(1):98-100.
- [Google Scholar]
- Cleome viscosa (wild mustard): a review on ethnobotany, phytochemistry, and pharmacology. Pharm. Biol.. 2010;48(1):105-112.
- [Google Scholar]
- Evaluation of antioxidant, antimicrobial and cytotoxic activities of seed crude extracts of Ammi majus grown in Oman. Egypt J. Basic Appl. Sci.. 2016;3(4):329-334.
- [Google Scholar]
- A review on medicinal plants against cancer. J. Plant Sci. Agric. Res.. 2017;2(1):1-5.
- [Google Scholar]
- Antihypercholesterolemic effect of Cleome Arabica L. on high cholesterol diet induced damage in rats. Excli J.. 2015;14:791-794.
- [Google Scholar]
- Isolation and characterization of antimicrobial compound from the stem-bark of the traditionally used medicinal plant Adenium obesum. J. Tradit. Complement. Med.. 2017;7:296-300.
- [Google Scholar]
- Stevens, P.F., 2012. Angiosperm Phylogeny <http://www.mobot.org/MOBOT/research/APweb/> accessed on (6.11.18).
- Antinociceptive, cytotoxic and antibacterial activities of Cleome viscosa leaves. Braz. J. Pharmacogn.. 2011;21(1):165-169.
- [Google Scholar]
- A Dictionary of the Flowering Plant and Ferns. USA: Cambridge University Press; 1996. p. :64-120.







