Translate this page into:
Biological and microbiological activities of isolated Enterobacter sp. ACD2 exopolysaccharides from Tabuk region of Saudi Arabia
⁎Corresponding author. malmutari@ksu.edu.sa (Mikhlid H. Almutairi)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Several forms of bacterial exopolysaccharides (EPSs) have been reported as having industrial and medical benefits. Previously, our group had achieved a high yield of EPS production (8.6 gm/L) from Enterobacter sp. isolated from the marine environment of Haqel Beach, in the Tabuk region of Saudi Arabia, by optimizing its culture conditions. Then, the strain selected was identified using conventional methods and confirmed by molecular characterization that used 16S Ribosomal RNA (16S rRNA) gene sequencing. In the present study, we determined the composition of this EPS and evaluated its anticoagulation, fibrinolytic, antimicrobial, and prebiotic activities. We determined the monosaccharide moieties of this EPS using acid hydrolysis by qualitative and quantitative paper chromatography (PC). The monosaccharide composition of this EPS was almost 25% glucose, 25% galactose, 40% fucose, in addition to 10% uronic acid, with traces of fructose. Sulfation modification was applied to the EPS produced. The biological and microbiological (anticoagulation, fibrinolytic, antimicrobial, and prebiotic) activities of both the native and sulfated EPS were investigated and both exhibited higher antibacterial activity against Staphyllococcus aureus and Escherichia coli, although neither form displayed antifungal activity against Candida albicans. The minimum inhibitory concentration (MIC) of the native and sulfated EPS that sustained the highest bacterial inhibition zone was 15 mg/dl. No prebiotic activities were recorded for either EPS. In conclusion, the biopolymer produced has significant biological activities that require further adaptation for practical and industrial applications.
Keywords
Exopolysaccharide
16S rRNA
Enterobacter sp.
Marine bacteria
Prebiotic
Antimicrobial
Anticoagulation
Fibrinolytic
1 Introduction
An exopolysaccharide (EPS) is an exopolymer, meaning that it is a biopolymer secreted by an organism into the environment (i.e., external to the organism). These exopolymers include the biofilms produced by bacteria that anchor them and protect them from environmental conditions (Dupraz and Visscher, 2005). Most exopolymers consist of polysaccharides and proteins and may also include other molecules such as DNA and lipids (Braissant et al., 2009).
The marine environment harbors diverse bacterial species that can be exploited to produce valuable compounds, including EPSs, which hold promise for biotechnological applications (Aullybux et al., 2019). A bacterial population in marine ecosystems has immense potential to be a big approach to uncovering new natural products (Thornburg et al., 2010). In addition, EPSs appear to have a wide scope of applications, including emulsification, thickness, absorption, film development, gel arrangement, and anticancer treatments (Yahya et al., 2019). Natural products, including EPSs, are considered important sources of potential chemotherapy agents. In the hunt for novel bioactive agents, the investigations have extended to aquatic environments (Abdelhamid et al., 2020). EPSs possess significant biological activities, playing roles in controlling cell division, differentiation, immune regulation, antitumor, antioxidant, and antiviral activities. Due to their antioxidant activities, EPSs can prevent various diseases, inflammation, and atherosclerosis (Asker et al., 2015).
EPSs have been identified in medicinal uses such as the treatment of arthritis, wound dressings, surgery or tablets for pharmaceuticals (Moscovici, 2015). The health advantages that have been well reported of these compounds particularly from the species of Lactobacillus that use as probiotics (Khalil et al., 2018). In addition, EPSs have been recorded as antiviral, anti-oxidative, immunostimulatory, tumor-resistant and antibacterial properties (Balzaretti et al., 2017).
Neither rare monosaccharide sugars such as L-fucose and L-rhamnose, nor uronic acids are commonly found in nature, but both have many interesting properties, making them attractive in various fields of application as anti-inflammatories, antioxidants, and building blocks for synthesizing the nucleoside analogs that are used as antiviral agents, which justifies the efforts to produce them synthetically (Kumar et al., 2007).
Marine EPSs possess significant potential as natural bioactive drugs for use in medical applications (Elsakhawy et al., 2017). The emergence of bacterial resistance to current antibiotics has created great interest in marine microorganisms as a new source of antimicrobial compounds (Pabba et al., 2011).
The prebiotic potential of some EPSs produced by lactic acid bacteria has been studied (Grosu-Tudor et al., 2013). Chitin or its sulfated derivative (chitosan) is one of the most abundant marine polysaccharide and can be used in biomedical applications such as blood anticoagulant after chemical sulfation (Deacon-Smith et al., 1985). Also, carrageenan’s polysaccharide showed high anticoagulation and fibrinolytic activities (Güven et al., 1991).
Previously, we isolated and selected a marine Enterobacter sp. and enhanced its production of EPS by optimizing different cultural conditions. Using these optimized conditions, we achieved a high yield of EPS (8.6 g/l) (Almutairi and Helal, 2020). Therefore, the aim of the present study was to evaluate the marine Enterobacter sp. native and sulfated EPS by studying their biological and microbiological activities, including anticoagulation, fibrinolytic, antimicrobial, and prebiotic activities.
2 Materials and methods
2.1 Sample collection
Five marine water samples were collected into sterile containers on 25 May 2019 (summer season) from Haqel Beach in Tabuk region of Saudi Arabia. These samples were collected at about 8–10 m from the beach with 0.5 and 1.0 m water depth (GPS location: 29.300886, 34.937985). All samples were labelled appropriately and taken to the laboratory for study.
2.2 Microorganisms studied
The study used three main groups of microorganisms: An isolated of marine bacterium, which was later defined as Enterobacter sp. Pathogenic microorganisms, including three pathogens, two of which were bacteria (E. coli and Staph. aureus), and the third of which was yeast (Candida albicans). These pathogens were isolated, identified and purchased from the microbiology lab of El-Demerdash Hospital in Cairo, Egypt. In addition, three probiotics (Bifidobacterium bifidum, Lactobacillus casei, and L. acidophilus) were used. The bifidogenic bacteria derived from the Chr. Hansen A/S Lab. Inc., in Fanøgade and Copenhagen, Denmark.
2.3 Bacteriological culture and isolation media
Potato dextrose agar (PDA) was used to grow inoculum and maintain the yeast strain Candida albicans. Nutrient broth (NB) and nutrient agar (NA) media were used to isolate, grow, and maintain the marine isolate and the pathogenic bacterial strains E. coli and S. aureus. De Man Rogosa Sharp (MRS) medium was used for probiotic growth and the prebiotic activity test.
2.4 Polysaccharide production medium (fermentation medium)
This medium was used to produce polysaccharides and consisted of sucrose (15%), peptone (0. 5%), K2HPO4 (0.4%), MgSO4·7H2O (0.07%), and MnSO4. 4 H2O (0.005%). It was incubated aerobically (shaken at 150 rpm) at 37 °C and a pH of 7.5 ± 0.2 (Almutairi and Helal, 2020).
2.5 Isolation methods
The marine samples were cultivated and isolated according to Viju et al. (2016).
2.6 Bacterial identification and isolate characterization
Morphology and microscopy: Colonies were examined for morphology, motility, spore formation, and Gram stain. Biochemical reactions: Oxidase, catalase, urea, indole, Voges-Proskaur, and citrate tests were performed in accordance with the taxonomic scheme of Barrow and Feltham (1993). 16S rRNA gene sequencing: The isolate was characterized according to Coenye and Vandamme (2003).
2.7 Determination of monosaccharide moieties of EPS
Complete acid hydrolysis of the bacterial EPSs was achieved according to the method of Haug and Larsen (1962). Qualitative examination of the hydrolysis product was performed by chromatography of the hydrolysates on Whatman No. 1 filter paper using the solvent system n-Butanol:acetone:water (4:5:1 v/v) (Jayme and Knolle, 1956). Spot detection was achieved by spraying with aniline phthalate reagent (Partridge, 1949). Quantitative PC to determine the monosaccharides in the investigated EPSs was conducted according to Jayme and Knolle (1956). For comparison, known amounts of authentic samples of galactose, glucose, fucose, sucrose, fructose, rhamnose, mannose, and glucuronic acid were spotted onto the chromatogram paper as references. The separated sugars were quantitatively determined using the method of Wilson (1959).
2.8 Sulfation modification of the EPS
Isolated bacterial EPSs were subjected to chemical modification using sulfation to produce new polysaccharide products that had improved properties. The sulfation process was achieved using Hussein’s (1994) method.
2.9 Biological activities of the native and sulfated bacterial isolate EPSs
The anticoagulation activities of the isolated extracts were investigated using the method for the assay of sodium heparin from U.S. Pharmacopeial Convention (1960). Fibrinolytic activity was investigated by exposing a plasma clot to the effect of an aqueous solution of the investigated samples at suitable concentrations. Preparation of the plasma clot was done under the same conditions used to determine anticoagulation activity (U. S. Pharmacopeial Convention, 1960). Antimicrobial activity was evaluated using the agar-diffusion technique described by Mitcher et al. (1972). The NA medium was inoculated using an overnight culture of the test organisms Escherichia coli (example of Gram-negative bacteria) and Staphylococcus aureus (example of Gram-positive bacteria). The PDA medium was inoculated using an overnight culture of Candida albicans. The MICs of the isolated native bacterial EPS and the sulfated modified EPS were determined against E. coli, Staphylococcus aureus, and C. albicans. The NA medium was inoculated using an overnight culture of the test organisms E. coli and Staphylococcus aureus. Aliquots of 0.1 ml of the tested native and sulfated isolated EPS solutions were applied into 0.5-cm pores in the cultured plates at concentrations of 22.5, 15, 7.5, 3.75, and 1.875 mg/ml and incubated for 48 h at 37 °C for the bacteria and 28 °C for the yeast. Then, the inhibition-zone diameters were determined. Prebiotic activity was investigated by growing the probiotics B. bifidum, L. acidophilus, L. casei, and L. bulgaricus on MRS medium and E. coli on NB medium, both at 37 °C for 24 h. Aliquots of 0.1 ml of each resulting bacterial culture were used as inoculum for 10 ml of the studied medium containing polysaccharides as a carbohydrate source. Such media were prepared at concentrations of 150 mg of carbohydrate source per 10 ml of MRS medium. After incubation at 37 °C for 24 h, the resulting bacterial growth expressed as optical density (OD) and measured at 625 nm against a blank composed of uninoculated EPS containing medium (Hussein et al., 2010). The prebiotic activity was calculated using the Prebiotic Index (I);
Prebiotic Index (I) = OD of bifidogenic bacterial culture growth/OD of E. coli culture growth.
2.10 Statistical analysis
The data were statistically analyzed using SPSS, version 10.00 for Windows (Nie et al., 1975). The data were presented as mean ± standard deviation.
3 Results
According to the morphological and biochemical tests (Fig. 1), the selected marine isolate was a Gram-negative mucoid bacillus, motile and non-spore forming. The results of the 16S rRNA gene-sequence technique used showed this isolate’s gene sequence as CAGGATTAGATACCCTGGTAGTCCACGCCGTAAACGATGTCGATTTGGAG, which was compatible with the Enterobacter sp. ACD2 strain.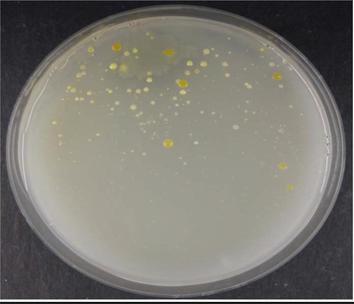
Colony morphology of marine isolates obtained from the marine sample cultured on NA plates.
The PC of the EPS acid hydrolysates identified the monosaccharide components as almost 25% galactose, 25% glucose, 40% fucose, and 10% uronic acid, with traces of fructose (Table 1 and Fig. 2).
Monosaccharide
Percentage
Glucose
25%
Galactose
25%
Fucose
40%
Glucuronic acid
10%
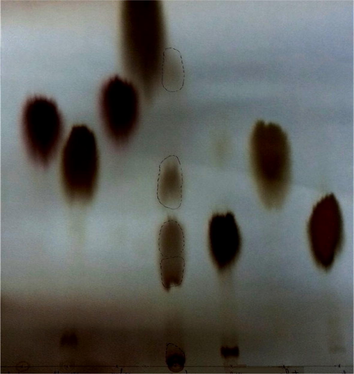
Paper chromatograph of the hydrolyzed produced EPS.
The fibrinolytic activities of both the native and sulfated isolated EPS were determined as lysis percentages of plasma clots. For comparison, a commercial preparation of pentosan sulfuric polyester, hemoclar, was used as a standard fibrinolytic agent in a simultaneous experiment. In contrast, the anticoagulation activities of the EPSs were evaluated by measuring the clotting times for mixtures of tested EPS, saline as a blank and standard heparin. Table 2 shows the fibrinolytic and anticoagulation activities of the investigated native and sulfated EPSs, both of which showed significant anticoagulation activity as the coagulation time was prolonged more than 24 h. This exceeds the coagulation time of standard heparin (90 min). It also showed 100% lysis of both plasma clots, which exceeds the fibrinolytic activity of standard hemoclar (75% lysis of the plasma clot). *Prolonged means a time of more than 24 h.
EPS
Anticoagulation activity
Fibrinolytic activity
Hour
Minute
Second
Native EPS
Prolonged*
–
–
100%
Sulfated EPS
Prolonged*
–
–
100%
Standard heparin (1.4 IV)
–
90
–
–
Saline blank
–
4
–
–
Standard hemoclar (2000 µ/ml)
–
–
–
75%
The antimicrobial activity was evaluated by measuring the inhibition zone against the tested organisms. Table 3 shows that the native and sulfated EPS created an inhibition zone against the studied bacteria (E. coli and Staph. aureus) but no inhibition zone against Candida albicans. The antibacterial activity of the native EPS showed higher values (25.1 and 30.0 mm) against the tested bacteria (E. coli and Staph. aureus, respectively) than the sulfated form (24.3 and 29.2 mm for E. coli and Staph. aureus, respectively). The sulfated EPS created an inhibition zone diameter that was the same as or slightly less than the native one against E. coli and S. aureus but showed no antifungal effect. *Control: Penicillin 200 mg/ml for bacteria and fluconazole 33.3 mg/ml for yeast.
Tested material
Inhibition zone diameter (mm) at 48 hr.
E. coli
Staphylococcus aureus
Candida albicans
Native EPS
25.1 ± 0.2
30 ± 0.2
zero
Sulfated EPS
24.3 ± 0.2
29.2 ± 0.1
zero
Control*
26 ± 0.0
31 ± 0.0
35 ± 0
Table 4 shows the MIC for the native and sulfated isolated bacterial exopolysaccharides against E. coli, Staphylococcus aureus, and Candida albicans. The MIC of the native and sulfated EPSs that sustained the highest inhibition zone was 15 mg/dl. The highest antibacterial activity was observed after 48 h of incubation at different EPS concentrations. *Control: Penicillin 200 mg/ml for bacteria and fluconazole 33.3 mg/ml for yeast.
Tested material
Concentrations mg/dl
Inhibition zone diameter (mm) at 48 hrs.
E. coli
Staphylococcus aureus
Candida albicans
Native EPS
22.5
25.1 ± 0.1
30.0 ± 0.1
zero
15
25.1 ± 0.1
30.0 ± 0.2
zero
7.5
10.1 ± 0.1
19.0 ± 0.2
zero
3.75
9.0 ± 0.3
10.0 ± 0.1
zero
1.875
zero
4.0 ± 0.1
zero
Sulfated EPS
22.5
24.3 ± 0.2
29.1 ± 0.2
zero
15
24.3 ± 0.1
29.2 ± 0.1
zero
7.5
16.2 ± 0.2
17.1 ± 0.1
zero
3.75
9.0 ± 0.2
10.1 ± 0.2
zero
1.875
zero
zero
zero
Control*
26.0 ± 0.1
31.1 ± 0.0
34.0 ± 0.2
Prebiotic activity was expressed as an index calculated from the study’s probiotic growth relative to its E. coli growth using the isolate EPS preparations. Table 5 shows that neither the native nor the sulfated EPSs exhibited any probiotic activity. Their prebiotic indices were less than 1.0, which reflected the growth densities for the bifidogenic bacteria tested (L. casei, L. acidophilus, and Bifidobacterium bifidum) as compared to E. coli.
Organisms
Prebiotic index (I)
Native EPS
Sulfated EPS
L. casei
0.71
0.70
L. acidophilus
0.53
0.50
Bifidobacterium bifidum
0.28
0.27
4 Discussion
The current study focused on assessing and altering the structure of an EPS isolate through sulfation and then examining its anticoagulation, fibronogenic, antimicrobial, and prebiotic activities. Such EPS can be subjected to acid hydrolysis and the resulting monomers separated using paper chromatographic methods to yield pure monosaccharides. Then, the sugar contents can be used as precursors for synthesizing molecules for use in high-value applications (Roca et al., 2015). Full acid hydrolysis was used to determine the sugar contents of the EPSs formed by the selected bacteria. The results indicated 25% galactose, 25% glucose, 40% fucose, and 10% uronic acid, with traces of fructose. This result agrees with those of Iyer et al. (2005), who found that the composition of Enterobacter cloacae are glucose, galactose, fucose, and glucuronic acid in the molar ratio of 2:1:1:1. Apart from these sugar moieties, the EPS product has two rare monosaccharides: fucose and uronic acid (Verhoef et al., 2005).
In the current study, both native and sulfated EPSs showed strong anticoagulation (prolonged anticoagulation time) and fibrinolytic (100% clot lysis) effects. These results agree with those of Ghosh et al. (2009), who stated that sulfated polysaccharides, including mannan sulphate, chondroitin sulphate, heparin, and sulphoevernan, have well-known anticoagulant activities. However, Cao et al. (2019) demonstrated that certain carbohydrates also interfere in the prothrombin pathway, and hence, are not ready to affect the outward coagulation pathway.
The antimicrobial activity of both the native and sulfated derivative EPSs on E. coli and S. aureus showed nearly the same antimicrobial effect as the controls. However, neither the native nor the sulfated EPS had any such activity against Candida albicans. This antibacterial activity agrees with that found by Onbasli and Aslim (2008), who assayed Pseudomonas aeruginosa B1 and B2 strains for the ability to produce inhibitory substances, including EPSs, against the growth of B. subtilis, E. coli, S. aureus, and C. albicans and reported activity against only E. coli and S. subtilis. Other researchers have reported the antibacterial activities of EPSs isolated from the Aeromonas hydrophila strain An4 from marine catfish (Anju et al,. 2010) and from four biofilm bacteria (Galionella sp, Alteromonas sp, S. aureus, and Klebsiella sp) from marine waters (Shankar et al., 2010).
The MIC is the minimum inhibitory concentration of a substance required to inhibit or kill a microorganism (Murray, 1995). The MIC of both the native and sulfated EPSs that sustained the highest inhibition zone was 15 mg/dl. This result indicates that this isolated EPS may hold promise for use as an antibacterial agent against both Gram-positive and Gram-negative bacteria. “Probiotic” is a broad term covering many strains of microbes, the majority of which belong to Gram-positive bacilli as Lactobacilli or Bifidobacteria. Some recent studies have reported that a sizable and growing number of hospitalized patients have received probiotics as part of their care. These findings given the lack of sufficient evidence for the efficacy and safety of probiotic use in hospitalized patients, suggest that research is critically necessary to guide the use of these agents in the hospital setting (Sarah et al., 2016). However, studying the prebiotic activity of our EPS product showed that neither it’s native nor sulfated forms had any probiotic activity, as the probiotic index was less than 1.0 for all studied EPSs. On other hand, a recent study found that an L. delbrueckii bulgaricus EPS had the highest prebiotic indices (I), which varied from 7.9 to 10.1. In contrast, L. helveticus and L. casei EPSs have been found to have the lowest prebiotic indices (I), which varied from 1.4 to 2.4 (Hussein et al., 2015). The marine Enterobacter sp. ACD2 EPS provided a renewable range of materials used in industrial and pharmaceutical applications. Therefore, this EPS can also be used in a wide variety of industrial applications in biomedical products, including antibacterial antibiotics, as a thrombolytic anticoagulant, and as a clot solvent and this agree with Abdelhamid et al. (2020).
5 Conclusions
Our produced biopolymer in both its native and sulfated forms has significant, promising biological activities as antibacterial, anticoagulation, and fibrinolytic products. In addition, the EPS composition contains rare monosaccharide moieties as fucose and uronic acid which has significant interest in medical applications and it surely requires further investigation and optimization for industrial applications as antitumor, antiviral, immunomodulatory and antioxidant activities.
Authors contributions
Mikhlid Almutairi was responsible for the study design. Mikhlid Almutairi and Mohamed Helal performed all practical experiments. Mohamed Helal performed data analyses and wrote the first draft of the manuscript. Mikhlid Almutairi wrote the final draft of the manuscript. All authors read and approved the final submitted manuscript.
Acknowledgment
The authors extend their appreciation to the Research Support Project (number RSP-2020/191), King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Medical application of exopolymers produced by marine bacteria. Bull. Natl. Res. Centre. 2020;44(1):1-14.
- [Google Scholar]
- Almutairi M.H., Helal, M.M.I., 2020. Exopolysaccharide Production from Isolated Enterobacter sp. strain ACD2 from the Northwest of Saudi Arabia. Journal of King Saud University Science. (Article in press).
- Hemolysin, protease, and EPS producing pathogenic Aeromonas hydrophila strain An4 shows antibacterial activity against marine bacterial fish pathogens. J. Marine Biol.. 2010;2010:1-9.
- [Google Scholar]
- Production and characterization of exopolysaccharide from novel Bacillus sp. M3 and evaluation on development sub-chronic aluminum toxicity induced Alzheimer’s disease in male rats. Am. J. Biochem. Biotechnol.. 2015;11(2) 92.103
- [Google Scholar]
- Phylogenetics and antibacterial properties of exopolysaccharides from marine bacteria isolated from Mauritius seawater. Ann. Microbiol.. 2019;69(9):957-972.
- [Google Scholar]
- A novel rhamnose-rich hetero-exopolysaccharide isolated from Lactobacillus paracasei DG activates THP-1 human monocytic cells. Appl. Environ. Microbiol.. 2017;83(3)
- [Google Scholar]
- Characters of Gram-positive bacteria. In: Cowan and Steel’s Manual for the Identification of Medical Bacteria. New York, NY: Cambridge Univ. Press; 1993. p. :52.
- [Google Scholar]
- Characteristics and turnover of exopolymeric substances in a hypersaline microbial mat. FEMS Microbiol. Ecol.. 2009;67(2):293-307.
- [Google Scholar]
- Anticoagulant and antithrombotic properties in vitro and in vivo of a novel sulfated polysaccharide from marine green alga Monostroma nitidum. Marine Drugs. 2019;17(4):247.
- [Google Scholar]
- Diversity and significance of Burkholderia species occupying diverse ecological niches. Environ. Microbiol.. 2003;5(9):719-729.
- [Google Scholar]
- Anticoagulant activity in extracts of British marine algae. Bot. Mar.. 1985;28(8):333-338.
- [Google Scholar]
- Microbial lithification in marine stromatolites and hypersaline mats. Trends Microbiol.. 2005;13(9):429-438.
- [Google Scholar]
- Marine microbial polysaccharides environmental role and applications: an overview. Environ. Biodiver. Soil Sec.. 2017;1(2017):61-70.
- [Google Scholar]
- Focus on antivirally active sulfated polysaccharides: from structure–activity analysis to clinical evaluation. Glycobiology. 2009;19(1):2-15.
- [Google Scholar]
- Prebiotic potential of some exopolysaccharides produced by lactic acid bacteria. Roman. Biotechnol. Lett.. 2013;18(5):8666-8676.
- [Google Scholar]
- Anticoagulant, fibrinolytic and antiaggregant activity of carrageenans and alginic acid. Bot. Mar.. 1991;34(5):429-432.
- [Google Scholar]
- Quantitative determination of uronic acid composition of alginates. Acta. Chem. Scand.. 1962;16:1908-1918.
- [Google Scholar]
- Hussein, M.M., 1994. Methods for Preparation of Pentosan Sulfuric Polyester: A Fibrinolytic Agents. Egyptian, Patents, (19381).
- Production and prebiotic activity of exopolysaccharides derived from some probiotics. Egypt Pharm. J. 2015;14(1):1.
- [Google Scholar]
- The prebiotic activities of oligosaccharides derived from partial hydrolysis of commercial algal polysaccharides. Egypt Pharm.. 2010;9:1-23.
- [Google Scholar]
- Characterization of an exopolysaccharide produced by a marine Enterobacter cloacae. Indian J. Exp. Biol.. 2005;43(5):467-471.
- [Google Scholar]
- Paper chromatography of sugar mixtures on glass fiber papers. Angew. Chem. Int. Ed.. 1956;68(7):243-246.
- [Google Scholar]
- Probiotic characteristics of exopolysaccharides-producing Lactobacillus isolated from some traditional Malaysian fermented foods. CyTA J. Food. 2018;16(1):287-298.
- [Google Scholar]
- Present and future medical applications of microbial exopolysaccharides. Front. Microbiol.. 2015;6:1012.
- [Google Scholar]
- Manual of Clinical Microbiology (Sixth ed.). American Society of Microbiology; 1995. p. :1327-1335.
- SPSS: Statistical Package for the Social Sciences. Vol Vol. 227. New York: McGraw-Hill; 1975.
- Determination of antimicrobial activity and production of some metabolites by Pseudomonas aeruginosa B1 and B2 in sugar beet molasses. Afr. J. Biotechnol.. 2008;7(24):4614-4619.
- [Google Scholar]
- Isolation and screening of marine bacteria for antimicrobial activity along Vishakapatanam Coast. J. Microbiol. Biotech. Res. 2011;1(2):86-89.
- [Google Scholar]
- Aniline hydrogen phthalate as a spraying reagent for chromatography of sugars. Nature. 1949;164(4167):443.
- [Google Scholar]
- Exopolysaccharides enriched in rare sugars: bacterial sources, production, and applications. Front. Microbiol.. 2015;6:288.
- [Google Scholar]
- Prevalence of probiotic use among inpatients: a descriptive study of 145 U.S. hospitals. Am. J. Infect. Control. 2016;44(5):548-553.
- [Google Scholar]
- Antimicrobial activity of marine bacteria associated with Polychaetes. Biores. Bull.. 2010;1:24-28.
- [Google Scholar]
- Deep-sea hydrothermal vents: potential hot spots for natural products discovery? J. Nat. Prod.. 2010;73(3):489-499.
- [Google Scholar]
- The Pharmacopeia of the United States of America. Sixteenth Revision; 1960.
- Sugar composition and FT-IR analysis of exopolysaccharides produced by microbial isolates from paper mill slime deposits. Biotechnol. Bioeng.. 2005;91(1):91-105.
- [Google Scholar]
- Antibiofilm and antifouling activities of extracellular polymeric substances isolated from the bacteria associated with marine gastropod Turbo sp. Oceanol. Hydrobiol. Stud.. 2016;45(1)
- [Google Scholar]
- Quantitative determination of sugars on paper chromatograms. Anal. Chem.. 1959;31(7):1199-1201.
- [Google Scholar]
- Newly isolated marine bacterial exopolysaccharides enhance antitumor activity in HepG2 cells via affecting key apoptotic factors and activating toll like receptors. Mol. Biol. Rep.. 2019;46(6):6231-6241.
- [Google Scholar]







