Translate this page into:
Biological activities and GC–MS based chemical profiling of polymolecular methanol extract of Alternaria alternata KUDB15
⁎Corresponding authors. sreenivasankud@gmail.com (Sreenivasa Nayaka), sraju@ksu.edu.sa (Raju Suresh Kumar)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
Objectives
The ecological niche of soil for microorganisms that produce beneficial naturally occurring and biologically active products suitable for pharmaceutical industries is widely recognized. The purpose of the current study is to isolate fungus from soil samples of Dandeli, Karnataka and its metabolic profiling and investigating biological activities.
Methods
Soil fungal isolates were screened for their antimicrobial potency against pathogens. The selected fungal isolate was morphologically characterized and ascertained by 18S rRNA gene sequencing, and the extract was investigated by gas chromatography-mass spectrometry (GC–MS) to identify the bio-molecules. Later the fungal extract was evaluated for antioxidant, antimicrobial, anti-mycobacterial and anti-proliferative activity against Human acute leukemia (K562) cell line.
Results
The potential fungal isolate, Alternaria sp. KUDB15 showed a number of bioactive compounds through GC–MS analysis. A significant antioxidant activity (IC50 = 37.60 µg/ml) was also reported for this organism. A significant inhibitory activity against tested pathogens was shown by Alternaria sp. KUDB15 along with a strong anti-mycobacterial activity with an IC50 value of 3.12 μg/ml. During anticancer assay, this organism showed a concentration dependent reduction in cell viability having IC50 of 112.09 µg/ml. Further purification of the crude extract with column chromatography and thin layer chromatography (TLC) recorded several bands and the particular band having antibacterial activity was determined by bioautography.
Conclusions
In the present research, the in vitro evaluation of biological activities of the fungal extract suggested that it might serve as a useful foundation for the discovery of compounds that have potential for medical applications. Additional research is required to identify and understand the structure of bio-molecules.
Keywords
Alternaria alternata
18S rRNA gene sequencing
Anti-mycobacterial
GC–MS
Bioautography
Anti-proliferative
1 Introduction
The abundance of diverse microorganisms in soil allows them to adapt to changing environmental conditions by synthesizing natural products as a means of surviving in harsh environments. Amongst microorganisms, fungi are the most abundant natural product producers. As a result, they could be regarded as a key component of the antimicrobial production industries because their metabolites may provide an alternative for health security (Afrah et al., 2021).
The ecosystem fungi are one of the largest biodiversity groups of microbial communities. They are available in almost all types of habitats, including terrestrial, aquatic, glaciers, deserts, etc. The terrestrial fungi are known as rich sources of biologically active components having low molecular weight called “secondary metabolites”. Besides secondary metabolites, they are the outstanding producers of organic acids, hydrolytic enzymes, polysaccharides, biofuels, etc. (Sergio and Arnold, 2017). Different classes of the secondary metabolites of fungi include terpenes, polyketides, non-ribosomal peptides, and siderophores (Grzegorz et al., 2021). Secondary metabolites have some of the major functions, such as they can be competitive weapons against pathogenic organisms and many of them are prescribed for doing biological activities, like enzyme inhibitors, antitumor agents, immunosuppressive agents, antimicrobials and antiparasitic agents, etc. (Thirumurugan et al., 2018).
The fungus Alternaria belongs to the family Pleosporaceae, it thrives as parasitic, saprophytic or endophytic in various ecosystems all over the world. A wide range of secondary metabolites, primarily polyketides, terpenes, quinones, and other compounds, are produced by Alternaria species. These metabolites have significant but underutilised applications in the chemical and pharmaceutical industries, as well as in the fields of medicine and biological control, among others (Zhao et al., 2023).
Nowadays pathogenic microorganisms have developed drug-resistant activity and this is one of the foremost problems in the world (Gauchan et al., 2020). Furthermore, the antibiotics existing today are limited by various factors, such as poor solubility, low potency, drug toxicity, and the emergence of the MDR strains. The search for the bioactive compounds from fungi is considered for inventing therapeutic drugs. Therefore there is a need for isolating and synthesizing antibiotic agents from fungi (Pavithra et al., 2014).
Similarly, the worldwide mortality rate of cancers is increasing quickly in recent years, as novel anticancer drugs are in huge demand (Ukwatta et al., 2020). According to studies, after the China and US, India has the third-highest reported rate of blood cancer cases, which, on a national level, affects above 70,000 women and men. Public health administrators in India are very concerned about this growing burden. Leukemia is a frequent malignant disease accounting for approximately 30% of all cancers occurring in children (Atashrazm et al., 2016).
Western ghats are home to a huge number of different groups of organisms, among them, microorganisms are unidentified and novel, thus they should be explored for potential applications (Nampoothiri et al., 2013). Hence this work describes isolation, identification, and screening of biologically active compounds from terrestrial fungi, for investigating antioxidative, antimicrobial, and anticancer properties.
2 Material and methods
2.1 Soil sample collection and isolation of fungi
Twenty samples were gathered at a depth of 5–10 cm in the inner forest land of Dandeli (latitude 15.247719 N and longitude 74.629678 W), Uttar-Kannada, Karnataka, India. In the laboratory, the collected soil samples were sieved and the debris like stones, twigs, etc., was eliminated. The samples were then air dried for 2 to 3 days at room temperature and stored at 4 ℃ until further use. To isolate the fungi, the sample suspensions (100 µl) were spread on potato dextrose agar (PDA) (#MH096, Himedia) medium, added 1% streptomycin and incubated at 27 ± 2 ℃ for one week.
2.2 Primary screening for antimicrobial activity
The fermented potato dextrose broth (PDB) (pH 5.1 ± 0.2) of isolated fungi were used for primary screening through well diffusion method against the pathogens, namely S.aureus, B. subtilis, E. coli, P. aeruginosa, and C. albicans. Hundred µl of culture filtrates were poured into respective wells of each fungal strain and the plates were placed in incubator for 24 h at 37 ℃. The fungus, Alternaria sp. KUDB15 showed the highest zone of inhibition and was used for further analysis.
2.3 Morphological characterizations
The colony features, like shape, margin, colour, etc. of Alternaria sp. KUDB15 were inspected. Then hyphal structures and conidial morphology were examined with a compound microscope (CX23, OLYMPUS, Tokyo) and a scanning electron microscope (SEM) (JSM-IT-500, JEOL, Tokyo). For SEM analysis, 6 mm diameter of fungal mycelia was treated for 2 h with 2.5% glutaraldehyde and washed with phosphate buffer saline (PBS). It was then gradually dehydrated for 30 min with rising ethanol concentrations (20, 40, 60, 80, and 100%). Final dehydration was done for 30 min in absolute ethanol. The sample was then dried in a critical point dryer, coated with gold sputtering for 2 min and examined under SEM (Uun and Rina, 2022).
2.4 Taxonomical characterization
The identification of Alternaria sp. KUDB15 was carried out by 18S rRNA gene sequencing in accordance with the method of Ibtisam et al. (2021). Based on the neighbour-joining method, the dendrogram was constructed. The total genomic DNA of the Alternaria sp. KUDB15 was extracted by using a spin column (#MB543, Himedia) and performed the amplification of the 18S rRNA gene using universal primers. The amplicons were purified and sequenced by Sanger DNA sequencing method using a genetic analyzer (ABI 3500xl). BLAST analysis of the sequence was done with the closest sequences retrieved from the National Center for Biotechnology Information (NCBI) database. A phylogenetic tree was built upon the neighbour-joining method utilizing MEGA7 software.
2.5 Preparation of methanol extract of Alternaria sp. KUDB15
The biomass of Alternaria sp. KUDB15 was washed thrice to eliminate traces of the growth media. This was followed by air drying to remove the water content. The obtained biomass (15 g) was then ground with 99.9% methanol (ratio 1:2, w/v) and filtered using Whatman no.1 filter paper. The concentration of the filtrate was done in a rotary evaporator at 40 ℃ for further characterization.
2.6 Characterizations of methanol extract of Alternaria sp. KUDB15
2.6.1 Fourier transform infrared (FTIR) analysis
The existing functional groups in methanol extract were inspected using FTIR instrument (NICOLET 6700, Thermo Fisher Scientific, Massachusetts, USA) in accordance with the method of Bidhayak et al. (2022).
The dried extract was ground with potassium bromide (5% of sample) and thin disc was made. The disc was later analysed in 400 to 4000 cm−1 wavelength range.
2.6.2 GC–MS analysis of methanol extract
The identification of compounds available in the extract of Alternaria sp. KUDB15 was achieved by GC–MS (QP2010S, Shimadzu, Japan) operated in electrospray ionization (ESI) mode and was fitted with an ELITE-5MS column (0.25 µ, 30 m × 0.25 mm). Column oven temperature was set at 80 ℃ and then raised to 450 ℃ at a rate of 20 ℃/min. Through a 2 mm injector, 2 µl of sample was injected directly into the capillary column. Identification of chromatographic peaks was guided by the relative retention times and mass spectra comparisons of the compounds obtained from the National Institute of Standards and Technology (NIST) library data (Chakraborty et al., 2022).
2.7 Biological applications of fungal methanol extract
2.7.1 Assessment of DPPH scavenging potential
The radical quenching potential was examined using a stable free radical, DPPH (2,2-diphenyl-1-picrylhydrazyl) as per Gauchan et al. (2020). Standard ascorbic acid was used in the assay. Various concentrations (25, 50, 75, 100, and 125 µg/ml) of methanol extract and ascorbic acid were taken in separate tubes and each received 3 ml of DPPH (100 µM) before incubation in dark for 30 min at room temperature. The absorbance at 517 nm was documented utilizing a spectrophotometer (UV-1800, Shimadzu, Kyoto, Japan); this was followed by calculation of the IC50.
2.7.2 Antimicrobial activity
The well diffusion method was employed to determine the antimicrobial potential of the methanol extract according to Chakraborty et al. (2022). The pathogenic organisms, such as Staphylococcus aureus (MTCC 6908), Bacillus subtilis (MTCC 6633), Bacillus cereus (MTCC 11778), Proteus morganii (MTCC 2983), Pseudomonas aeruginosa (MTCC 9027), Proteus vulgaris (MTCC 4587), Escherichia coli (MTCC 40), Shigella flexneri (MTCC 1457), and Candida albicans (MTCC 227) were set to 0.5 Mc-Ferland standards (1.5 × 108 CFU/ml) and were taken for antimicrobial activity. Muller Hinton media plates were swabbed with 100 µl of test microorganisms and six mm wells were made. Methanol extract in 25, 50, 75, and 100 µl were filled in the respective wells for each pathogen. Positive controls were amphotericin-B and streptomycin (10 mg/ml) and sterilized distilled water was negative control. Incubation of petri plates was done at 37 ℃ for about 24 h and inhibition zones were determined in mm.
2.7.3 Anti-tubercular activity
Anti-tubercular capacity against the pathogen Mycobacterium tuberculosis strain H37 RV (ATCC 27294) was assessed based on microplate alamar blue assay (MABA). A total of 5 standard drugs (isoniazid, ethambutol, pyrazinamide, rifampicin, and streptomycin) and methanol extract were taken for analysis. Both the drugs and methanol extract were diluted serially, starting from a concentration of 100 μg/ml down to 0.2 μg/ml. For 5 days, the microplate was incubated at 37 °C, this was followed by the addition of 10% tween 80 and 25 μl of alamar blue reagent (1:1) for 24 h. Finally, MIC of methanol extract was determined (Gauri et al., 2021).
2.7.4 Anti-proliferative assay
The anti-proliferative assay was conducted by 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)–2H-tetrazolium (MTS) method against K562-Human blood cancer (leukemia) cell line according to the method of Chakraborty et al. (2021). Briefly, a cell suspension of 100 µl was seeded in a microtiter plate containing the required concentration of cells (̴20,000 cells/well). Various doses (12.5, 25, 50, 100, and 200 µg/ml) of the extract were given to the cancer cells, which were then cultured for 24 h under a 5% CO2 environment at 37 ℃. Doxorubicin (3 µM/ml) was positive control and the cells without any treatment were negative control. At the end of incubation, 20 µl of MTS reagent (5 mg/ml) was added and incubated for 3 h. The produced formazan crystals were dissolved completely in DMSO (100 μl) after incubation. At 450 nm, absorbance was measured and 630 nm served as the reference wavelength. Cell viability percentage and IC50 were estimated.
2.7.5 Assessment of cell apoptosis
The extract was applied to the K562 cells (0.5 × 106 cells/ml) at IC50 concentration and kept for 24 h for incubation. The cells that were not treated with any substance were referred to as the control. After treatment, the cells were rinsed with PBS and combined with 5 µl of Propidium iodide (PI) and Fluorescein isothiocyanate- AnnexinV (FITC-AnnexinV) along with 1X binding buffer (400 µl) and incubated in dark for 15 min at room temperature. Emitted fluorescence was analyzed further by flow-cytometer (BD FACS Calibur) (Razali et al., 2021).
2.8 Purification and characterization
2.8.1 Column chromatography, thin layer chromatography and bioautography
The methanol extract obtained from Alternaria sp. KUDB15 was purified using column chromatography. A glass column (1.8 × 70 cm) was filled with silica gel (230–400 mesh, #GRM7484, Himedia) and equilibrated with ethyl acetate. The fungal extract (0.5 g) in methanol was added to the silica gel column and subsequently eluted with a gradient solvent system of ethyl acetate and methanol (v/v). The collected fractions were concentrated in a rotary evaporator at 40 ℃ and checked for antibacterial activity against P. vulgaris (Talwinder et al., 2016).
The eluted column fraction, which exhibited good antibacterial efficacy, was further purified by silica gel TLC on aluminium plate (1.5 × 10 cm) layered with silica gel (60 F254, Merck, Darmstadt, Germany). The active fraction of 30 µl was applied on TLC plates and were developed using the mobile phase (toluene, ethyl acetate, methanol, and acetic acid) in the ratio of 5:3:1:0.5. Finally, the bands were examined in a TLC chamber under a long wavelength (366 nm) and a short wavelength (254 nm) (Praptiwi et al., 2018).
The agar overlay bioautography was performed against the pathogens S. aureus and P. vulgaris to determine the bioactive compound present on the TLC plates. Nutrient agar medium was overlaid onto the developed TLC plates and 100 µl of the pathogens (0.5 Mc-Farland standards) were swabbed thoroughly on respective petri plates and incubated for 24 h at 37 ℃. The clear zones were marked and were scrapped from reference TLC plates based on similar Rf values (Praptiwi et al., 2018).
2.9 Statistical analysis
Experiments were carried out three times (n = 3), and each value was the mean value of three similar studies. The outcome was shown as the mean and standard deviation (±SD).
3 Results
3.1 Soil sample collection and isolation of fungi
Twenty samples were gathered and totally eighteen fungi were isolated. Isolated fungal colonies were having characteristic features, such as cottony, flat, concave, convex, and powdery appearance, and of different sizes and colours ranging from yellow, black, brownish, greyish, white, and green.
3.2 Primary screening of antimicrobial activity
The fungus Alternaria sp. KUDB15 expressed the best antimicrobial activity in primary screening (Table 1). Among the fungal isolates, 9 isolates (50%) displayed activity against S. aureus, 10 isolates (55.55%) displayed activity against B. subtilis, 6 isolates (33.33%) were active against E. coli, 7 isolates (38.88%) expressed activity against P. aeruginosa, and 8 isolates (44.44%) expressed activity against C. albicans. Note: -= No activity, += Weak activity, ++=Moderate activity, and +++=Good activity.
S. aureus
B. subtilis
E. coli
P. aeruginosa
C. albicans
KUDB1
+
–
–
–
–
KUDB2
–
++
+
–
–
KUDB3
++
+
–
–
–
KUDB4
–
–
–
–
–
KUDB5
–
–
+
–
–
KUDB6
++
+
–
–
+
KUDB7
–
–
+
+
+
KUDB8
+
–
–
+
–
KUDB9
–
+
–
–
–
KUDB10
–
++
+
+
+
KUDB11
–
+
–
–
+
KUDB12
++
–
–
+
+
KUDB13
–
+
–
–
–
KUDB14
+
++
+
+
+
KUDB15
+++
+
++
+++
++
KUDB16
–
–
–
+
–
KUDB17
+
–
–
–
–
KUDB18
+
+
–
–
–
3.3 Morphological and molecular characterizations
The Alternaria sp. KUDB15 was greyish to black on the aerial side (Fig. 1A) and black on the reverse (Fig. 1B). The colonies were circular, filamentous and mycelia were branched, multicellular and septate. Conidia were brownish with various shapes like ellipsoidal or obclavate and having tapered apex with transverse and longitudinal septa (Fig. 1C). The SEM micrograph displayed obclavate-shaped individual conidia tapering to the apex and having verruculose surface (Fig. 1D).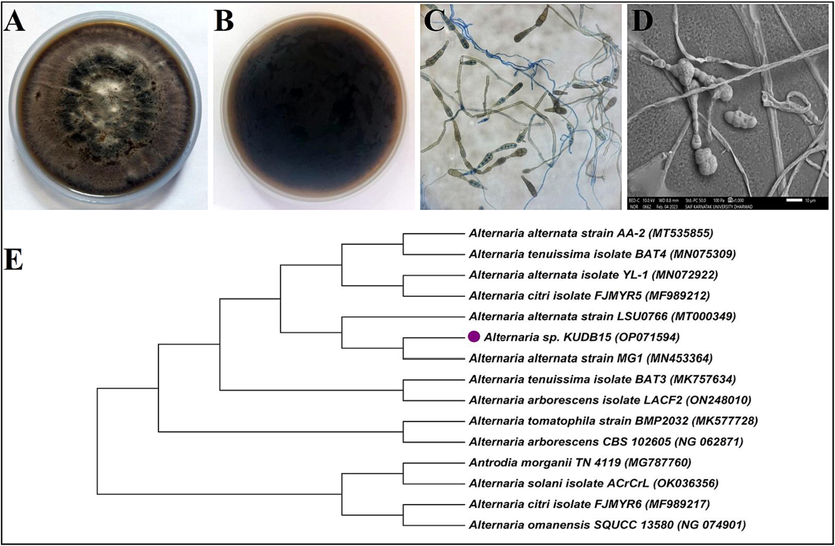
Characterizations of Alternaria sp. KUDB15: (A) aerial side of the fungal colony, (B) reverse side of the fungal colony, (C) light microscopic image showing long chains of conidia, and (D) SEM micrograph of conidia and mycelia, and (E) Phylogenetic tree of Alternaria sp. KUDB15 showing evolutionary relationship with Alternaria alternata strain MG1.
The partial 18S rRNA gene sequencing of the Alternaria sp. KUDB15 was 890 base-pairs (NCBI accession no. OP071594). The BLAST analysis showed 99.78% sequence similarity with Alternaria alternata strain MG1. The phylogenetic tree of the Alternaria sp. KUDB15 constituted a single clad with Alternaria alternata strain MG1 (Fig. 1E).
3.4 Characterizations of methanol extract
The intracellular metabolites of Alternaria sp. KUDB15 was achieved by grinding the mycelial mass with methanol followed by drying it in a rotary evaporator. The intracellular extract (yield = 55.2%) was slightly oily in consistency and reddish-brown in colour.
3.4.1 FTIR spectroscopy analysis
The FTIR spectroscopy of methanol extract was shown in Fig. 2A. The broad and strong peak at 3372 cm−1 indicated alcohol with O–H stretching, the peak at 2935 cm−1 was corresponded to C–H stretching of alkane. A peak at 1654 cm−1 showed C=N stretching of imine/ oxime, the peak at 1414 cm−1 was because of O–H bending of carboxylic acid and peak 1302 cm−1 arose owing to C-N stretching of aromatic amine. An absorption peak at 1079 cm−1 was attributed to C-O stretching of primary alcohol, the peak at 861 cm−1 indicated P-O-C stretching of aromatic phosphates, the peak at 779 cm−1 indicated C-Cl stretch of aliphatic chloro compounds and the peak at 636 cm−1 was assigned to C–H bending of alkyne.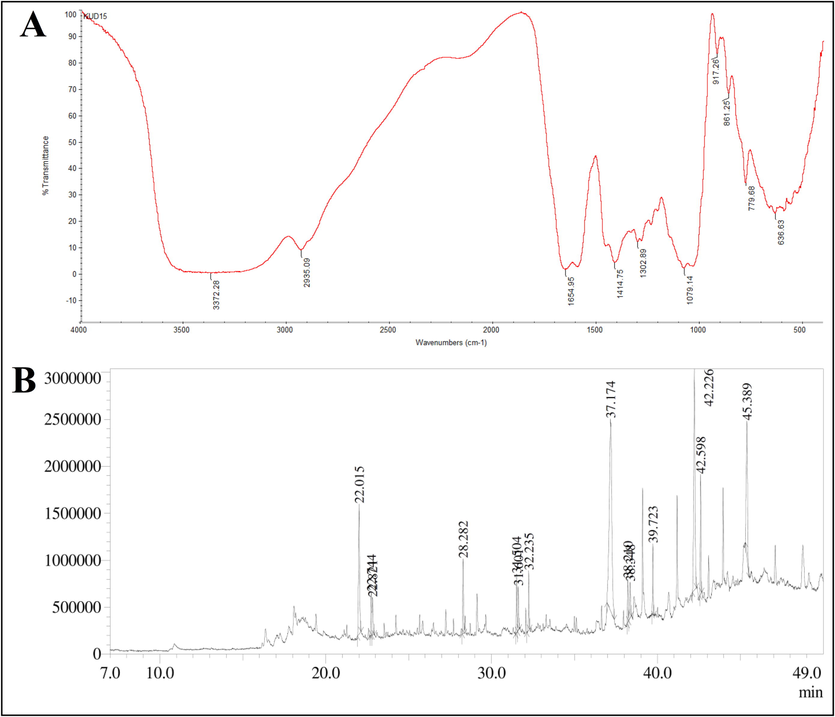
(A) FTIR spectrum of methanol extract and (B) GC–MS chromatogram of methanol extract of Alternaria sp. KUBD15.
3.4.2 Detection of compounds by GC–MS analysis
The intracellular metabolite from Alternaria sp. KUDB15 was subjected to GC–MS analysis. The chromatogram manifested the occurrence of 14 compounds (Fig. 2B), which were listed with their molecular formula, molecular weight, area%, height%, and biological activities in the Table 2. Among the 14 compounds, the major compounds were Tetratetracontane with the highest area% of 32.39, Pentatriacontane with an area% of 17.43, Eicosane with an area% of 11.40, Isosorbide,bis(tert-butyldimethylsilyl)ether with an area% of 9.06. The compounds were identified as having major bioactivities, such as antioxidant, antimicrobial, anticancer, etc.
Compound names
Retention Times
Area%
Height%
Chemical formulas
Molecular weights
Biological activities
References
Isosorbide, bis(tert-butyldimethylsilyl) ether
22.015
9.06
10.28
C23H48O5Si3
488.8
Not reported
–
Alpha.-d-galactopyranose, 1,2,3-tris-o-(trimethylsilyl)-, cyclic methylboronate
22.744
2.36
3.39
C11H22B2O6Si
300.0
Not reported
–
1,4-di-o-acetyl-2,3,5-tri-o-methylpentitol
22.821
2.46
2.95
C12H22O7
278.30
Not reported
–
Methyl palmitate
28.282
3.71
5.92
C17H34O2
270.5
Antiinflammatory, antifibrotic
El-Demerdash, 2011
Methyl linolelaidate
31.504
2.66
4.63
C19H34O2
294.5
Catechol-O-Methyl-transferase-inhibitor and methyl-guanidine-inhibitor
Igwe et al., 2016
9-octadecenoic acid (z)-, methyl ester
31.601
2.24
3.50
C19H36O2
296.4879
Antioxidant, anticancer, enzyme inhibitor
Eman et al., 2021
2,2′,2′'-nitrilotriethanol, triethyl ether
32.235
2.56
4.73
C12H27NO3
233.3477
Not reported
–
Tetratetracontane
37.174
32.39
15.17
C44H90
619.2
Hypoglycaemic, anti-oxidant, antibacterial
Nurbani et al., 2016, Rhetso et al., 2020
Tetracosane
38.210
1.72
3.41
C24H50
338.7
Cytotoxic activity
Uddin et al., 2012
Methyl 11-docosenoate
38.348
2.08
2.83
C23H44O2
352.6
Not reported
–
Heneicosane
39.723
3.16
5.59
C21H44
296.6
Antimicrobial,
bio-pesticide
Vanitha et al., 2020, Rhetso et al., 2020
Pentatriacontane
42.226
19.33
17.43
C35H72
492.9
Antibacterial, antiviral, herbistat
Soosairaj and Dons, 2016, Nurbani et al., 2016
Octacosane
42.598
4.87
9.44
C28H58
394.8
Mosquitocidal, Cytotoxic
Rajkumar and Jebanesan, 2004, Figueiredo et al., 2014
Eicosane
45.389
11.40
10.73
C20H42
282.5
Cytotoxicity, antifungal, antioxidant, antiinflamatory
Rhetso et al., 2020, Patrick, 2020.
3.5 Biological applications of methanol extract
3.5.1 DPPH scavenging potentiality
DPPH scavenging potentiality was estimated using the methanol extract of Alternaria sp. KUDB15 (Fig. 3). The result indicated a steady increase in the scavenging activity in a concentration-dependent approach. The scavenging activity was done with different concentrations of methanol extract, such as 25, 50, 75, 100, and 125 µg/ml and the inhibition ratios were obtained as 45.09%, 54.60%, 64.85%, 76.92%, and 85.21%, respectively. The IC50 of methanol extract obtained from the graph was 37.60 µg/ml.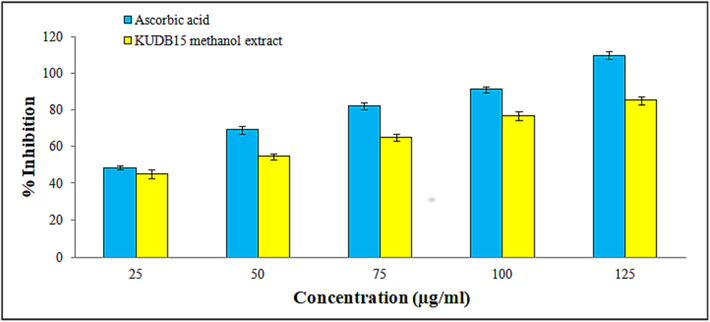
Histogram representing a gradual increase in the DPPH free radical scavenging potential of methanol extract of Alternaria sp. KUDB15.
3.5.2 Antimicrobial activity of methanol extract
A total of 9 pathogens were taken to check the extract’s antimicrobial activity (Fig. 4A to I). The inhibition zones formed with 25, 50, 75, and 100 µl of the methanol extract were measured in mm against pathogens. Methanol extract exhibited the highest inhibitory activity against P. vulgaris (20.58 ± 1.55), followed by S. aureus (20.43 ± 1.28), and S. flexneri (19.68 ± 1.9), the lowest activity was noticed against E. coli (13.42 ± 1.24). The antimicrobial activity was increased with an increase in volumes of methanol extract (Fig. 4J).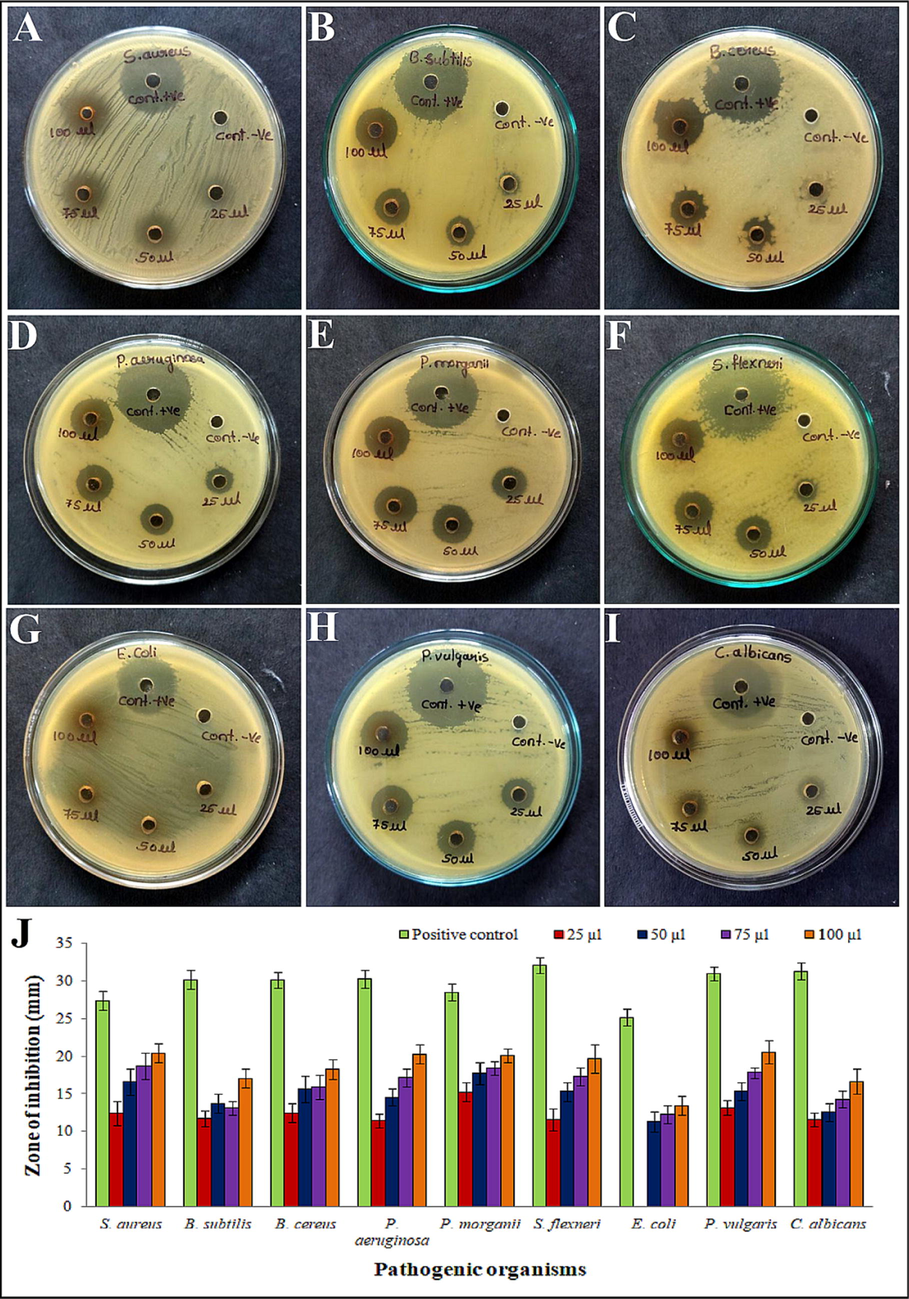
Antimicrobial activity of methanol extract of Alternaria sp. KUDB15 against pathogens: (A) S. aureus, (B) B. subtilis, (C) B. cereus, (D) P. aeruginosa, (E) P. morganii, (F) S. flexneri, (G) E. coli, (H) P. vulgaris, (I) C. albicans, and (J) Histogram representing the zones of inhibition formed by methanol extract against pathogens.
3.5.3 Anti-mycobacterial activity of methanol extract
The methanol extract was used to test its anti-mycobacterial activity by MABA method in 96 well plate (Fig. 5). The minimum inhibitory concentration (MIC) was calculated for standard antibiotics were as follows: isoniazid (1.6 μg/ml), ethambutol (1.6 μg/ml), pyrazinamide (3.12 μg/ml), rifampicin (0.8 μg/ml), and streptomycin (0.8 μg/ml). The methanol extract revealed MIC 3.12 μg/ml and expressed considerable anti-mycobacterial activity.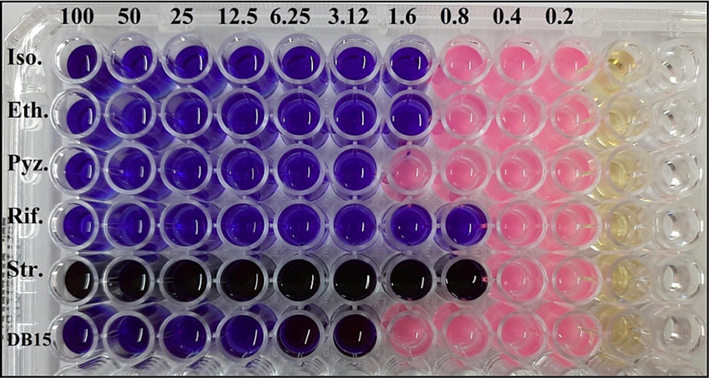
Anti-mycobacterial activity of methanol extract of Alternaria sp. KUDB15 by MABA method showing MIC of 3.12 μg/ml.
3.5.4 Anti-proliferative activity of methanol extract
MTS cell proliferation assay was carried out against the leukemia K562 cell line. Cell viability in the untreated control was 100 % and the standard drug, doxorubicin exhibited 51.00% cell viability (Fig. 6A and B). The K562 cell line was exposed to the extract for 24 h, which evidenced a steady decrease in the viability of cancer cells with the increase in the extract concentration. The cancer cells treated with extract of 12.5, 25, 50, 75, 100, and 200 µg/ml showed 91.54%, 84.56%, 63.90%, 53.26%, and 37.66% of cell viability, respectively (Fig. 6C to G). Among all the concentrations of methanol extract, the 200 µg/ml showed more efficiency in destroying more number of cancer cells, while the 12.5 µg/ml concentration showed the lowest efficiency. Further, the results indicated a significant anti-proliferative activity of the leukemia (K562) cell line and IC50 of 112.09 µg/ml (Fig. 6H).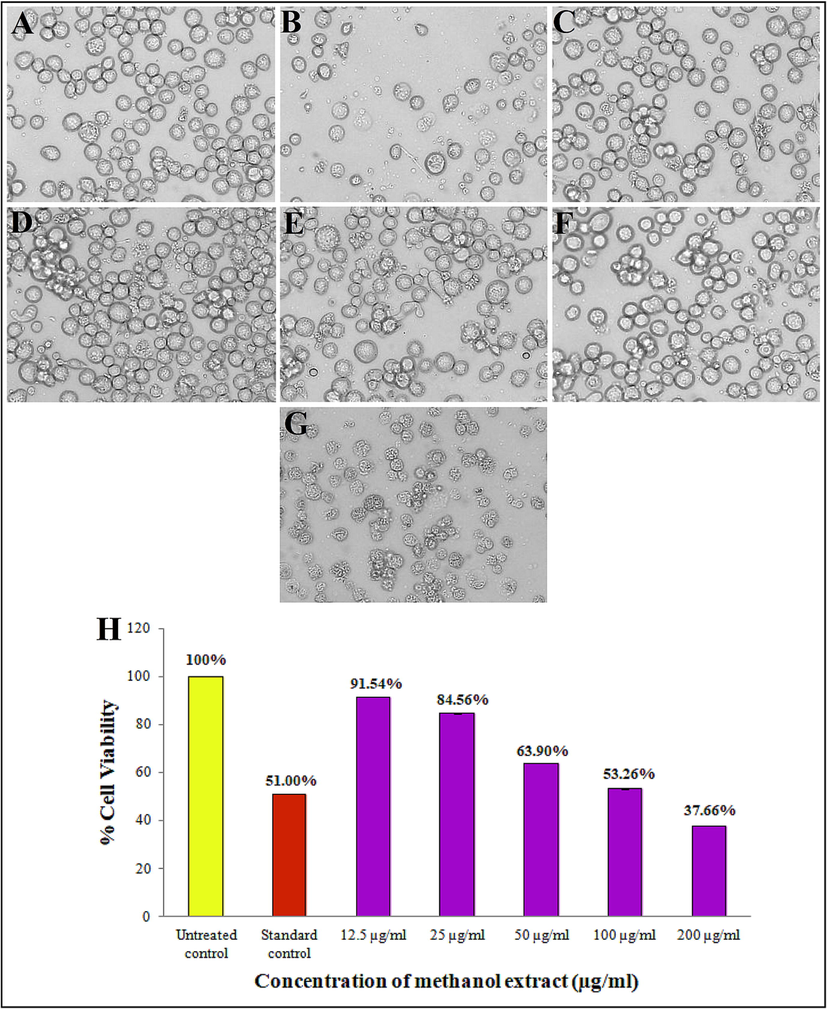
Anti-proliferative activity of different concentration of Alternaria sp. KUDB15 methanol extract: (A) Untreated control, (B) standard control (Doxorubicin), (C) 12.5 µg/ml, (D) 25 µg/ml, (E) 50 µg/ml, (F) 100 µg/ml, (G) 200 µg/ml, and (H) Histogram depicting comparison in percentage cell viability of K562 cells treated with methanol extract.
3.5.5 Apoptosis assay
The K562 cells were exposed to IC50 of methanol extract for 24 h. The untreated cells showed 0.0% necrosis, 4.28% late apoptosis, 7.93% early apoptosis, and 87.79% viable cells (Fig. 7A), whereas the treated cells showed 21.76% late apoptosis, 32.66% early apoptosis, and 45.58% viable cells (Fig. 7B). No necrotic cell population was recorded. Hence, the shift in the percentage of viable cells signified that the methanol extract of fungi triggered apoptosis in K562 leukemia cell line. The untreated cells’ cell cycle analysis revealed 87.79% viable cells in marker 1 (M1) and 12.21% apoptotic cells in M2 (Fig. 7C), while the treated cells showed 45.58% viable cells in M1 and 54.42% apoptotic cells in M2 (Fig. 7D). The bar graph (Fig. 7E) represents the percentage of different apoptotic cell populations.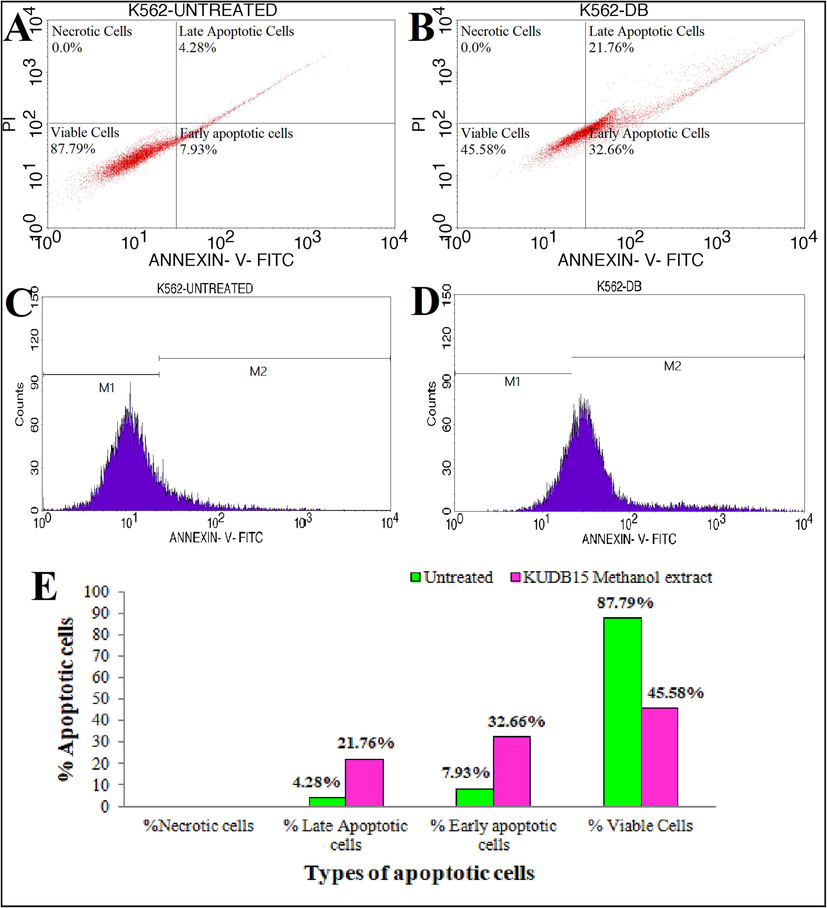
Flow cytometric analysis showing quadrangular plot of Annexin-V/PI expression of methanol extract of Alternaria sp. KUDB15 against K562 cancer cell line: (A) Untreated control, (B) Cancer cells treated with IC50 of methanol extract, (C) Cell cycle analysis of untreated control cells, (D) Cell cycle analysis of treated cells, and (E) Graph showing % of different apoptotic cell population.
3.6 Purification, TLC and bioautography
The methanol extract of Alternaria sp. KUDB15 was purified and a total of twenty fractions were collected. The fraction 9 (ethyl acetate: methanol, 2:8, v/v) was selected owing to its good antibacterial activity and further purified by TLC.
The TLC analysis of fraction 9 exhibited 3 bands under the fluorescent light (Fig. 8A), 7 bands under the short wavelength (254 nm) (Fig. 8B), and 13 bands under the long wavelength (366 nm) (Fig. 8C). Later, agar overlay bioautography analysis indicated the band with the Rf value of 0.80 expressed antibacterial activity against the pathogens S. aureus (Fig. 8D) and P. vulgaris (Fig. 8E). The band was therefore scrapped from the reference TLC plate and analysed further for identification of the antibacterial compound.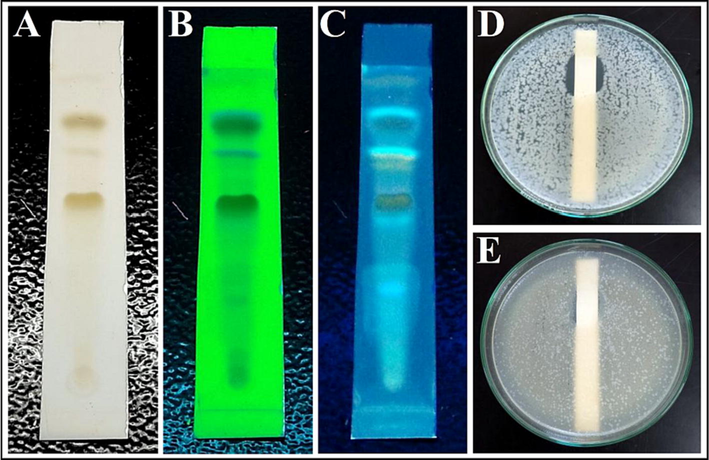
TLC and bioautography of column purified fraction 9: (A) TLC plate under fluorescent light, (B) TLC plate under short wavelength 254 nm, (C) TLC plate under long wavelength 366 nm, (D) Bioautograpgy against S. aureus, and (E) Bioautograpgy against P. vulgaris showing inhibition zones.
4 Discussion
Fungi are considered one of the most successful inhabitants of soil because of their ability to thrive in harsh and unfavorable conditions. Dandeli forest region is covered by tropical semi-evergreen and moist deciduous type of trees. Eighteen fungal strains were isolated from soil samples by serial dilution on PDA medium. This is compared to a previous study, where serial dilution of desert soil samples from Saudi Arabia gave rise to isolation of fungal strains (Ameen et al., 2022). The primary screening result conferred that Alternaria sp. KUDB15 was the most potent among all the isolates. Morphologically Alternaria sp. KUDB15 produced circular colonies, greyish to black coloured mycelia. The SEM analysis revealed septate mycelia and long chains of club-shaped conidia. The result obtained here can be linked to the findings of Madonna et al. (2018), where SEM investigation of the soil fungus Aspergillus aculeatus showed swollen vesicles and ellipsoidal to globose or subglobose conidia.
The 18S rRNA gene sequencing has emerged as the most popular technique to identify the fungal community up to the species level due to its sequence conservation and high specificity. The 18S rRNA gene sequencing of Alternaria sp. KUDB15 showed 99.78% similarity to Alternaria alternata strain MG1. Eukaryotic ribosome contains 18S rRNA as the small subunit. The gene frequently employed in phylogenetic investigations, widely recognized as important marker for conducting environmental biodiversity screening with random target PCR. It is primarily employed for detailed taxonomic investigations of fungi and it contains both conserved and variable regions that can reflect the discrepancy among eukaryotic species (Shu et al., 2015). The phylogenetic analysis of Alternaria sp. KUDB15 represented its evolutionary relationship with Alternaria alternata strain MG1 forming a single clad. This outcome is compared with the findings of Ibtisam et al. (2021), where the fungal isolates: AS1, AS2, and AS6 were identified as Aspergillus oryzae, Penicillium chrysogenum, and Aspergillus niger, respectively.
To obtain the intracellular metabolites, the biomass of Alternaria sp. KUDB15 was ground with methanol followed by filtration. The cell envelope or cell membrane functions as a physical barrier responsible to contain the internal metabolites within the cell. Therefore, it is imperative to extract metabolites from within the cell compartment in order to quantify and identify intracellular metabolites. This is typically accomplished using extraction solvents (organic, inorganic, non-aqueous or a mixture of the two). The solvents create permeability or porosity in the cell's membrane, enabling the entry of these solvents into the intracellular medium and enhanced recovery of intracellular metabolites (Farhana et al., 2017).
FTIR is a highly useful technique for identifying functional groups and chemical bonds in compounds, relying on the analysis of absorbed light wavelengths. The annotated spectrum provides informations and by interpreting the peak values, the functional groups can be determined. The FTIR analysis of Alternaria sp. KUDB15 methanol extract identified functional groups were alkane, alkyne, alcohols, carboxylic acids, aromatic phosphates, aromatic amines, aliphatics, etc. According to Muruganantham et al. (2009), the existence of functional groups like carboxylic acids, amines, and phosphates are responsible for various medicinal properties.
The methanol extract was subjected to GC–MS chemical profiling, resulting in the identification of 14 compounds in 49 min. The literature search of these chemicals indicated that they were documented for numerous biological activities like antimicrobial, antioxidant, anticancer, and other pharmacological activities as well as enzyme inhibitory and herbicidal properties. Therefore, it could be assumed that the antimicrobial, antioxidant, and antiproliferative properties of Alternaria sp. KUDB15 could be ascribed to the presence of these intracellular bioactive metabolites. In a similar study by Skanda & Vijayakumar, (2022), the crude extract of a soil fungus Periconia sp. was analysed by GC–MS, which revealed the availability of 25 compounds. The major compounds identified were 2-phenylethanol, 1-tetracosanol, p-menthan-3-one, etc.
Numerous diseases, persistent inflammation, and infections are associated with overproduction of reactive oxygen species (ROS) or free radicals. This abundance of ROS is strongly linked to the mechanisms underlying tumour initiation, promotion, and progression. The DPPH assay is a very straightforward assay and delivers the first indication on the capacity of compounds to scavenge free radicals (Chakraborty et al., 2021). In our study, a gradual increase in DPPH scavenging potentiality of methanol extract was shown having the IC50 of 37.60 µg/ml. This indicated a promising antioxidant activity. In the DPPH assay, the methanol extract was added, which transformed the violet-colored DPPH solution to diphenylpicryl hydrazine, a pale yellow-coloured product, and this change in colour was influenced by the concentration of the extract. The acceptance of hydrogen atoms or electrons supplied by antioxidant compounds present in the extract was the cause of this change in colour. The outcome was in agreement with the result, where the antioxidant capacity of endophytic fungi, A. fumigatus, A. nidulans, and A. flavus isolated from Ocimum basilicum, revealed IC50 of 166.3, 68.4, and 151.2 μg/ml, respectively (Sharaf et al., 2022).
The antimicrobial activity of methanol extract showed pronounced inhibitory activity against pathogens. The pathogen, P. vulgaris was the most susceptible and the pathogen E. coli was the most resistant one. The result showed a steady increase in the diameter of zone of inhibitions along with a concomitant increase in concentration of methanol extract. According to various reports the antimicrobial activity of crude extracts, containing multiple compounds, can be more potent than individual antimicrobial molecules. Hence, this pronounced antimicrobial activity was due to a synergistic interaction among the constituents of the extracts. The antimicrobial activity of compounds primarily involves actions such as disrupting cell wall and protein biosynthesis, damaging cell membranes, interfering with DNA replication and repair, and inhibiting metabolic pathways. Moreover, bacteria can develop resistance to antibacterial agents through mechanisms like porin and efflux pump structural modifications, destruction of antibacterial agents, antibiotic modification, and alteration of drug targets (Bidhayak et al., 2022). A similar finding was documented by Meghashyama et al. (2023), where the fungi, A. niger demonstrated various levels of inhibitory activity against B. subtilis, S. aureus, S. flexneri, C. albicans, C. glabrata, and E. coli.
Mycobacterium tuberculosis causes the contagious disease called TB, which is one of the leading factors in death across the globe. The methanol extract of Alternaria sp. KUDB15 showed moderate anti-mycobacterial activity at IC50 of 3.12 μg/ml. The work was in accordance with the findings of Kudzanai et al. (2022), where the methanol extract of a fungus, C. rogersoniana showed antitubercular activity against M. tuberculosis and M. smegmatis having MIC of 0.2 and 0.125 mg/ml, respectively.
The methanol extract showed a significant anti-proliferative activity against K562 cell line with different concentrations of extract (12.5 to 200 µg/ml). This expressed a reduction in cell viability from 91.54% to 37.66% with IC50 of 112.09 µg/ml. The specific mechanism responsible for the anti-proliferative activity is unclear. Along with the anti-proliferative property, a significant antioxidant activity of Alternaria sp. KUDB15 extract was also recorded. It is assumed that methanol extract, a potent antioxidant, can affect the redox state of cancer cells, which in turn inhibits cell proliferation. Redox alterations have a noteworthy impact on the signal transduction pathways, which is crucial for the regulation of cell growth. It was proposed that protein prenylation and antioxidant properties, in addition to cytotoxicity could potentially have a role in the manifestation of antitumor activity (Jelena et al., 2020). The result was compared with Elavarasi et al. (2018), where the Fusarium sp. exhibited strong cytotoxicity with IC50 of 0.03 and 0.06 μg/ml, against HeLa and K562 cell lines, respectively.
The quantification of apoptosis using FITC-AnnexinV/PI dyes relies on the properties of membrane disintegration of cancer cells. Results of apoptosis induction demonstrated that percentage of early and late apoptotic cells were increased after treatment with IC50 of Alterneria sp. KUDB15 methanol extract. The untreated control experiment showed 12.21% apoptotic cells and 87.79% viable cells, whereas the treated cells showed 52.42% apoptotic cells and 45.58% viable cells without any necrotic cell population. The significant variation after treatment in the apoptotic and viable cells’ percentage was attributed to the combined impact of individual molecules present in the methanol extract. In the staining process, viable cells remained unstained, whereas AnnexinV dye selectively stained early apoptotic cells upon exposure of phosphatidylserine on the cell membrane. On the other hand, both AnnexinV and PI can stain the late apoptotic cells and the necrotic cells get stained only with PI (Hugo et al., 2008). The result was compared with the report of Meghashyama et al. (2023).
Gravity column chromatography technique employed for purification of methanol extract resulted in twenty fractions. Among the fractions, the fraction 9 exhibited good antibacterial activity against P. vulgaris. The obtained band, which showed antibacterial activity, was checked by agar overlay bioautography. Here, the TLC band with an Rf value of 0.80 exhibited antibacterial activity against S. aureus and P. vulgaris. The result was similar to the report of Sedjati et al. (2022), where the extract of Trichoderma longibrachiatum was purified by TLC and bioautography. TLC analysis revealed 5 bands and the band with the smallest Rf value (0.14) showed antibacterial activity against K. pneumoniae through bioautography.
5 Conclusion
The soil fungi constitute the most prominent group of microorganisms because of their active role in nutrient cycling and decomposition in soil ecosystems and produce vast range of bioactive compounds. In this study, Alternaria sp. KUDB15 was obtained from soil and characterized morphologically. The methanol extract of the fungus revealed the presence of different compounds having biological activities. The organism showed pronounced antioxidant, antimicrobial, and anti-proliferative activities. Further, the extract was purified by column chromatography and TLC. Bioautography analysis revealed the TLC fraction with antibacterial activity against pathogens. Therefore, further studies on this organism for identification and structure elucidation of antimicrobial and anticancer compounds will be carried out. In future, comprehensive studies on biosynthetic pathways of Alternaria sp. KUDB15 metabolites can optimize the synthesis of bioactive compounds and can also inhibit the production of unwanted, toxic metabolites.
CRediT authorship contribution statement
Bidhayak Chakraborty: Methodology, Writing – original draft, Writing – review & editing. Kariyellappa Nagaraja Shashiraj: Methodology. Dhanyakumara Shivapoojar Basavarajappa: Validation. Meghashyama Prabhakara Bhat: Validation. Sreenivasa Nayaka: Investigation, Supervision. Raju Suresh Kumar: Investigation, Funding acquisition. Abdulrahman I. Almansour: Investigation, Funding acquisition. Karthikeyan Perumal: Investigation, Funding acquisition.
Acknowledgement
The authors express their gratitude to the USIC and SAIF, Karnatak University, Dharwad, for granting necessary instrument facilities.
Funding
The project was funded by Researcher Supporting Project number (RSP2023R231) King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Investigation of biological activity of soil fungal extracts and LC/MS-QTOF based metabolite profiling. Scientific Reports. 2021;11:4760.
- [CrossRef] [Google Scholar]
- Desert soil fungi isolated from Saudi Arabia : cultivable fungal community and biochemical production. Saud. J. Biol. Sci.. 2022;29(4):2409-2420.
- [CrossRef] [Google Scholar]
- Fucoidan suppresses the growth of human acute promyelocytic leukemia cells in vitro and in vivo. Journal of Cellular Physiology. 2016;231(3):688-697.
- [CrossRef] [Google Scholar]
- Bioprospection and secondary metabolites profiling of marine Streptomyces levis strain KS46. Saud. J. Biol. Sci.. 2022;29(2):667-679.
- [CrossRef] [Google Scholar]
- Evaluation of antioxidant, antimicrobial and antiproliferative activity of silver nanoparticles derived from Galphimia glauca leaf extract. J. King Saud Univ. Sci.. 2021;33(8):101660
- [CrossRef] [Google Scholar]
- Streptomyces filamentosus strain KS17 isolated from microbiologically unexplored marine ecosystems exhibited a broad spectrum of antimicrobial activity against human pathogens. Process Biochem.. 2022;117:42-52.
- [CrossRef] [Google Scholar]
- Cytotoxic and antibacterial activities of endophytic fungi isolated from mangroves. World Journal of Pharmaceutical Research. 2018;7(1):954-961.
- [CrossRef] [Google Scholar]
- Anti-inflammatory and anti fibrotic effects of methyl palmitate. Toxicol. Appl. Pharmacol.. 2011;254:238-244.
- [CrossRef] [Google Scholar]
- Evaluation of antioxidant and anticancer activity of crude extract and different fractions of Chlorella vulgaris axenic culture grown under various concentrations of copper ions. BMC Complement. Med. Ther.. 2021;21:51.
- [CrossRef] [Google Scholar]
- Analysis of intracellular metabolites from microorganisms: Quenching and extraction protocols. Metabolites. 2017;7(4):53.
- [CrossRef] [Google Scholar]
- Pyrustegia venusta heptane extract containing saturated aliphatic hydrocarbons induces apoptosis on B16F10-Nex2 melanoma cells and display antitumor activity in vivo. Pharmacognosy Magazine. 2014;10(Suppl 2):S363-S376.
- [CrossRef] [Google Scholar]
- Gauchan, D.P., Kandel, P., Tuladhar, A., Acharya, A., Kadel, U., Baral, A., Shahi, A. B., García-Gil, M.R., 2020. Evaluation of antimicrobial, antioxidant and cytotoxic properties of bioactive compounds produced from endophytic fungi of Himalayan yew (Taxus wallichiana) in Nepal. F1000Res. 9, 1-28. https://doi.org/10.12688/f1000research.23250.2.
- In-vitro profiling of antitubercular compounds by rapid, efficient, and nondestructive assays using autoluminescent Mycobacterium tuberculosis. Antimicrobial Agents and Chemotherapy. 2021;65(8):e00282-e321.
- [CrossRef] [Google Scholar]
- Terpenoid biosynthesis dominates among secondary metabolite clusters in mucoromycotina genomes. J. Fungi.. 2021;7(4):285.
- [CrossRef] [Google Scholar]
- Extracellular annexin A5: functions of phosphatidylserine-binding and two-dimensional crystallization. Biochim. Biophys. Acta, Mol. Cell Res.. 2008;1783(6):953-963.
- [CrossRef] [Google Scholar]
- Identification and antibacterial characterization of endophytic fungi from Artemisia sieberi. International JournalMicrobiol.. 2021;2021:6651020.
- [CrossRef] [Google Scholar]
- Screening for secondary metabolites in Huru crepitans bark ethanol extract using GC-MS analysis : a preliminary study approach. J. Sci. Technol. Adv.. 2016;1(2):64-71.
- [Google Scholar]
- Evaluation of anticancer activity of Satureja montana supercritical and spray-dried extracts on Ehrlich’s Ascites Carcinoma bearing mice. Plants.. 2020;9(11):1532.
- [CrossRef] [Google Scholar]
- Antimycobacterial activity and molecular docking of methanolic extracts and compounds of marine fungi from Saldanha and False Bays. South Africa. Heliyon.. 2022;8(12):e12406.
- [Google Scholar]
- Identification of lipase producing fungus isolated from dairy waste contaminated soil and optimization of culture conditions for lipase production by the isolated fungus. J. Microbiol. Biotechnol. Food Sci.. 2018;8(1):698-704.
- [Google Scholar]
- Aspergillus niger CJ6 extract with antimicrobial potential promotes in-vitro cytotoxicity and induced apoptosis against MIA PaCa-2 cell line. Environmental Research. 2023;229:116008
- [CrossRef] [Google Scholar]
- FTIR and SEM-EDS comparative analysis of medicinal plants, Eclipta alba hassk and Eclipta prostrata Linn. Rom. J. Biophys.. 2009;19(4):285-294.
- [Google Scholar]
- Western ghats of india: Rich source of microbial biodiversity. Journal of Scientific and Industrial Research. 2013;72(9–10):617-623.
- [Google Scholar]
- Phytochemical profiles of propolis Trigona spp., from three regions in Indonasia using GC-MS. J. Biol. Agric. Health Care. 2016;6(14):2224-3208.
- [Google Scholar]
- Evaluation of anti-inflammatory, analgesic, antipyretic effect of eicosane, pentadecane, octacosane, and heneicosane. Asian J. Pharm. Clin. Res.. 2020;13(4):29-35.
- [CrossRef] [Google Scholar]
- Antimicrobial and enzyme activity of endophytic fungi isolated from Tulsi. Journal of Pharmaceutical and Biomedical Research. 2014;16(12):1-6.
- [Google Scholar]
- Antibacterial and antioxidant activities of endophytic fungi extracts of medicinal plants from Central Sulawesi. J. Appl. Pharm. Sci.. 2018;8(8):69-74.
- [CrossRef] [Google Scholar]
- Mosquitocidal activities of octacosane from Moschosma polystachyum Linn. (Lamiaceae) J. Ethnopharmacol.. 2004;90(1):87-89.
- [CrossRef] [Google Scholar]
- An in-vitro anticancer activity evaluation of Neolamarckia cadamba (Roxb.) Bosser leaves’ extract and its metabolite profile. Front. Pharmacol.. 2021;12:741683
- [CrossRef] [Google Scholar]
- Chemical constituents, antioxidant, and antimicrobial activity of Allium chinense G. Don. Futur. J. Pharm. Sci.. 2020;6:102.
- [CrossRef] [Google Scholar]
- Antibacterial activity of the fungal metabolite Trichoderma longibrachiatum against multidrug-resistant Klebsiella pneumoniae and methicillin-resistant Staphylococcus aureus. Jordan Journal of Biological Sciences. 2022;15(1):107-113.
- [CrossRef] [Google Scholar]
- Antimicrobial, antioxidant, cytotoxic activities and phytochemical analysis of fungal endophytes isolated from Ocimum Basilicum. Applied Biochemistry and Biotechnology. 2022;194(3):1271-1289.
- [CrossRef] [Google Scholar]
- Taxonomic resolutions based on 18S rRNA genes: A case study of subclass Copepoda. PLoS One.. 2015;10(6):e0131498.
- [CrossRef] [Google Scholar]
- Antioxidant and antibacterial potential of crude extract of soil fungus Periconia sp. (SSS-8) Arabian Journal for Science and Engineering. 2022;47:6707-6714.
- [CrossRef] [Google Scholar]
- Bio-active compounds analysis and characterization in ethanolic plant extracts of Justicia tranquebariensis L. (Acanthaceae) using GC-MS. International JournalChemTech Res.. 2016;9(7):260-265.
- [Google Scholar]
- Purification and characterization of a new antifungal compound 10-(2,2-dimethyl-cyclohexyl)-6,9-dihydroxy-4,9-dimethyl-dec-2-enoic acid methyl ester from Streptomyces hydrogenans strain DH16. Frontiers in Microbiology. 2016;7:1004.
- [CrossRef] [Google Scholar]
- Thirumurugan, D., Cholarajan, A., Raja, S.S.S., Vijayakumar, R., 2018. An Introductory Chapter: Secondary Metabolites, in: Vijayakumar, R., Raja, S.S.S. (Eds.). Secondary Metabolites- Sources and Applications. https://doi.org/10.5772/intechopen.79766.
- Evaluation of cytotoxic activity of patriscabratine, tetracosane and various flavonoids isolated from the Bangladeshi medicinal plant Acrostichum aureum. Pharmaceutical Biology. 2012;50(10):1276-1280.
- [CrossRef] [Google Scholar]
- Antimicrobial, anti-cancer, anti-filarial and anti-inflammatory activities of Cowabenzophenone A extracted from the endophytic fungus Aspergillus terreus isolated from a mangrove plant Bruguiera gymnorrhyza. Mycol.. 2020;11(4):297-305.
- [CrossRef] [Google Scholar]
- Morphological, molecular characterization and physico-chemical analysis of Trichoderma yunnanense as indigosol golden yellow dye-decolorizing fungus. The Philippine Journal of Science. 2022;151(6B):2459-2470.
- [Google Scholar]
- Heneicosane, A novel microbicidal bioactive alkane identified from Plumbago zeylanica L. Industrial Crops and Products. 2020;154:112748
- [CrossRef] [Google Scholar]
- Secondary metabolites of Alternaria: A comprehensive review of chemical diversity and pharmacological properties. Front. Microbiol.. 2023;13:1085666.
- [CrossRef] [Google Scholar]







