Translate this page into:
Biogenic synthesis, characterization and antimicrobial activity of Ixora brachypoda (DC) leaf extract mediated silver nanoparticles
⁎Corresponding author. sreenivasankud@gmail.com (Sreenivasa Nayaka)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
The present investigation was aimed at the evaluation of synthesis, characterization and antimicrobial activity of Ixora brachypoda DC. leaf extract mediated silver nanoparticles (AgNPs). The AgNPs were characterized by UV–Visible (UV–Vis) spectrophotometer, Fourier transform infrared spectroscopy (FTIR), Atomic force microscopy (AFM), Scanning electron microscopy (SEM) with Energy dispersive X-ray spectrometry (EDS), Transmission electron microscopy (TEM), Zeta potential and X-ray diffractometry (XRD). Finally, the antimicrobial activity of synthesized AgNPs was investigated against pathogenic microorganisms. The absorption peak, obtained at 423 nm in UV–Vis analysis, confirmed the synthesis of AgNPs and the presence of biological functional groups involved in the capping and stabilization were determined by FTIR analysis. The other characterizations revealed the details about the AgNPs as spherical, poly-dispersed and size ranging from 18 to 50 nm with an average diameter of 27.76 nm. The zeta potential was calculated to be −30.4 mV and the typical Bragg’s planes in the metallic silver range indicated the confirmation of AgNPs formation. The in-vitro analysis confirmed the antimicrobial potential of I. brachypoda leaf aqueous extract synthesized AgNPs, which effectively inhibited the growth of pathogens and it can be concluded that the I. brachypoda AgNPs can be used as broad-spectrum antimicrobials against multi-drug resistant microbial pathogens.
Keywords
Ixora brachypoda
Plant extract
Biosynthesis
Nanoparticles
Antimicrobial activity
1 Introduction
The new and improved properties of nanoparticles with respect to their size, shape, and distribution lead to the development of several nano-technological methods, for which it has become a major theme of research in the scientific field. There are diverse metal nanoparticles, derived from platinum, gold, silver, copper, titanium, zinc, etc., having extensive uses in the field of biomedicine, pharmaceutics, engineering, electronics, chemical industries, cosmetics, optics and biological research (Zhang et al., 2016; Abdelghany et al., 2018).
The synthesis of nanoparticles from noble metals have received profound attention since past few decades and recently, gold and silver among others are most commonly being used for the synthesis. The AgNPs have attracted special attention because of their unique properties like conductivity, catalytic activity, stability and antimicrobial activity (Chung et al., 2016; Moodley et al., 2018). In addition to this, AgNPs are well-known antibacterial, antiviral and antifungal agents used to reduce the infections caused during surgery. In recent years, the AgNPs are even being used as an anticancer drug for diagnosis and treatment of various types of cancers (Ahmed et al., 2016; Ravichandran et al., 2019).
The synthesis of nanoparticles can be done by means of chemical, physical and biological methods. The physical and chemical methods require chemicals, which are toxic and harmful to human health and environment. The synthesis of metal nanoparticles, using biological sources like plants, bacteria, actinomycetes, fungi, etc., leads to clean, nontoxic and eco-friendly nanoparticles (Iravani et al., 2014; Burdusel et al., 2018). The principles of green chemistry are applied in the synthesis procedure of biogenic nanoparticles, which makes it simple, economic and easily scalable for larger production. Generally, the nanoparticles synthesis from plant-based preparations is of greater advantage over other biogenic synthesis procedures due to its rapid production in less time (Keat et al., 2015; Ali et al., 2020).
Fungi are considered as one of the larger producer compared to its microbial embodiments due to extensive secretory components, which are involved in the reduction and capping of AgNPs. Although, the use of plant extracts for the synthesis of AgNPs is much easier considering the presence of a number of secondary metabolites for the reduction and capping of nanoparticles (Beyene et al., 2017; Burlacu et al., 2019). The synthesis of AgNPs using various plant parts eliminates the extra work of culture maintenance, which is common in case of microbial synthesis and makes it rapid and easily approachable in comparison to the counterparts (Gudikandula and Maringanti, 2016).
The metal NPs especially, AgNPs have long been documented as antibacterial and antifungal agents due to their toxicity towards microbial cell membrane. In today’s world, the ascending resistance ability of microbial pathogens against multiple drugs is indicating the need for an alternate or broad spectrum antimicrobial agent to battle against many diseases of plants and animals including humans (Gumel et al., 2019). The AgNPs as an antimicrobial agent rupture the microbial cell membrane by releasing silver ions that generate reactive oxygen species (ROS) followed by apoptosis. However, the mechanism of antimicrobial action of AgNPs is not completely decoded (Rajeshkumar and Bharath, 2017; Kalwar and Shan, 2018; Barros et al., 2018).
The plant Ixora brachypoda DC. (I. brachypoda) belongs to the family Rubiaceae and also known as Ixora radiata Hiern. (synonym). The plant is glabrous, single-stemmed shrub, growing up to 6 m height and usually grows in swamps and other damp areas. The fruit (berry) is edible, generally used as food. In the traditional medicine system, the plant parts like, roots and bark are used. The bark is used as medicine for malnutrition, debility, wound healing and as pain killer, whereas roots are used to cure weakness, healing and as vermifuges to expel parasitic worms and other internal parasites from the body. Therefore, keeping these values in view, the leaves of I. brachypoda were utilized for the synthesis and evaluation of AgNPs via a green synthesis approach.
2 Materials and methods
2.1 Collection of microorganisms and plant materials
Gram-positive and Gram-negative bacterial strains like, Staphylococcus aureus (MTCC6908), Bacillus subtilis (MTCC2393), Pseudomonas aeruginosa (MTCC424) and Escherichia coli (MTCC40) and the fungal strains such as, Alternaria alternata (MTCC2060), Candida albicans (MTCC227), Magnaporthe grisea (ATCC64557) and Fusarium oxysporum (MTCC2087) were obtained from Institute of Microbial Technology, Chandigarh, India. The fresh leaves of I. brachypoda (Fig. 1) were collected from Karnatak University Campus, Dharwad, Karnataka, India and authenticated by comparing with the herbarium specimen at the depository of plants and animals museum, KU Dharwad along with the literature of Flora of Dharwad.
Ixora brachypoda twig with flower.
2.2 Leaf extract preparation
The freshly collected leaves of I. brachypoda were washed with tap water for 2 to 3 times to remove dust impurities. Later, the leaves were cut into smaller pieces and air dried at room temperature. About 20 g of leaves were weighed and plunged in 100 ml of deionized water in a 500 ml conical flask. The suspension was boiled at 60 °C for about 1 h and allowed to cool at room temperature, which was filtered with Whatmann No.1 filter paper. The filtrate was centrifuged for 15 min at 5000 rpm and the leaf extract was stored in refrigerator at 4 °C for further studies.
2.3 Synthesis of AgNPs
For the preparation of 1 mM AgNO3 solution, 0.1698 g of AgNO3 was dissolved in 1L of distilled water in a conical flask. 100 ml of leaf aqueous extract of I. brachypoda was taken in a clean 1L conical flask and added with 900 ml of freshly prepared AgNO3 solution. The pH of the mixture was set at 8.5 followed by incubation in a dark for 48 h to nullify the photo activation of AgNO3 at room temperature. The change in the colour of reaction mixture after the incubation period was an initial indication for the synthesis of AgNPs. The reaction mixture was further centrifuged and AgNPs were collected, air-dried and refrigerated for further use.
2.4 Characterizations of silver nanoparticles
The reduction of ionic silver into the elemental form was monitored by measuring the UV–Vis spectrum using a double beam UV–Visible spectrophotometer (UV-9600A, METASH Instruments Co. Ltd., Shanghai, China). The presence of probable biological functional groups involved in the formation and stabilization of AgNPs were recorded in transmittance mode in the range of 4000 –400 cm−1 with the aid of FTIR spectrophotometer (Nicolet 6700, Thermo Fisher Scientific, Waltham, Massachusetts, USA). The surface morphology and distribution of AgNPs was analysed using AFM instrument (Nano surf Flex-Bio AFM system). In addition to this the morphology, size, average diameter and presence of different elements were determined using SEM in conjunction with EDS (JEOL, JSM-IT500 LA, Japan) and HR-TEM (FEI, TECNAI G2, F30) instruments. The hydrodynamic particle size, zeta potential and electrostatic stability of the AgNPs were investigated (HORIBA nanoparticle analyser SZ-100, Kyoto, Japan) along with X-ray Diffractometer (Rigaku SmartLab SE, Austin, Texas, US) to determine the nature of synthesized AgNPs.
2.5 Antimicrobial activity of silver nanoparticles
The I. brachypoda leaf extract synthesized AgNPs were washed and resuspended in distilled water and tested for antimicrobial activity against bacterial and fungal pathogens on nutrient agar and potato dextrose agar medium respectively, using agar well diffusion method. A 5 ml of AgNPs stock suspension with 50 µg/ml concentration was prepared and different volumes like 25, 50 and 100 µl of stock suspension were poured in their respective wells. Appropriate volumes of positive controls such as, streptomycin (antibacterial) and fluconazole (antifungal) were used along with distilled water as negative control. After the incubation time, the inhibition zones were measured in mm using standard antibiotic zone scale.
2.6 Evaluation of minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC)
The MIC test was conducted to determine the concentration of AgNPs required to prevent the bacterial growth using 96 well micro-titre plate using resazurin. For MBC test, the suspension taken from each well of micro-titre plate was spread onto Muller-Hinton agar plates. The plates were incubated at 37℃ for 48 h and the MBC value was indicated by the concentration where no bacterial growth was observed.
3 Results and discussion
3.1 Visual and UV–Vis analysis
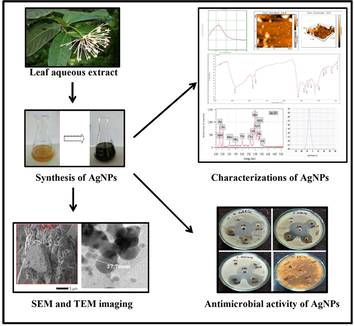
The synthesis of AgNPs was initially evidenced by colour change of the leaf extract from yellow to brown when mixed with colourless AgNO3 solution at pH 8.5 (Fig. 2A). The colour change of the reaction mixture was because of the reduction of silver ions (Ag+) into elemental silver (Ag0) by the secondary metabolites present in the leaf extract that act as reducing, stabilizing and capping agents during the synthesis of AgNPs (Lakshmanan et al., 2017; Kumar et al., 2018). The characteristic UV absorption spectrum of the AgNPs colloidal suspension was observed at 423 nm, when scanned between the wavelengths ranging from 200 to 700 nm (Fig. 2B). The characteristic peak observed in UV–Vis spectrum is due to the surface plasmon resonance (SPR) of the AgNPs and the presence of single narrow peak indicated the synthesis of small sized AgNPs (Valsalam et al., 2019; Alharbi and Alarfaj, 2020).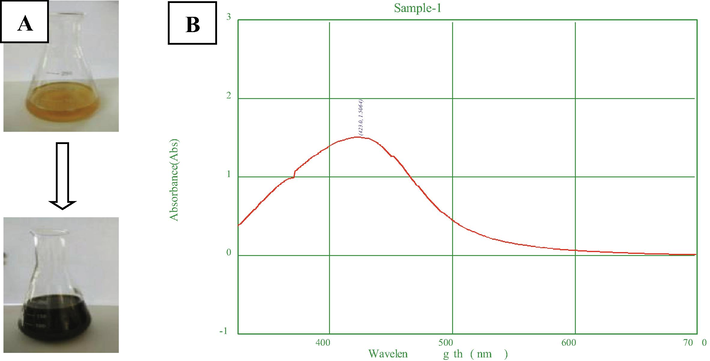
Visual and UV–Vis analysis of AgNPs A) Visual observation of colour change of reaction mixture, B) UV–Vis spectrum analysis.
3.2 FTIR analysis
The FTIR spectrum recorded from 4000 – 400 cm−1 conveyed different peaks indicating the presence of various biological functional groups, which were involved in the reduction and stabilization during biogenic AgNPs synthesis (Fig. 3). The strong intensity peak at 3403 cm−1 corresponded to free stretching carboxylic acids (COOH), the medium intensity peaks at 2923 cm−1 and 2853 cm−1 depicted the presence of C–H bond stretching vibrations from alkane (CH4) and aldehydes (CHO) respectively. The strong intensity peak at 1603 cm−1 indicated N–H bending primary amines (NH2), medium intensity peaks at 1403 and 1384 cm−1 illustrated the presence of C–H bending alkanes (CH3) and N⚌O bonded nitro compound (NO2) respectively. The weak intensity peaks at 1318 and 1262 cm−1 corresponded to S⚌O bending sulfates (SO4-2) and esters (COOR1) respectively. The peak at 1117 cm−1 shows the presence of flourides and some weak intensity peaks at 859, 788, 620 and 467 cm−1 were also identified as C-X bond stretching aromatic compounds like bromides and iodides. Similar findings of FTIR spectrum results indicated that, the AgNPs were surrounded by plant secondary metabolites such as, alkaloids, terpenoids, carbonyl groups, esters, halides and alcohol which act as active binding sites for AgNPs to give stabilization (Femi-Adepoju et al., 2019; Algebaly et al., 2020).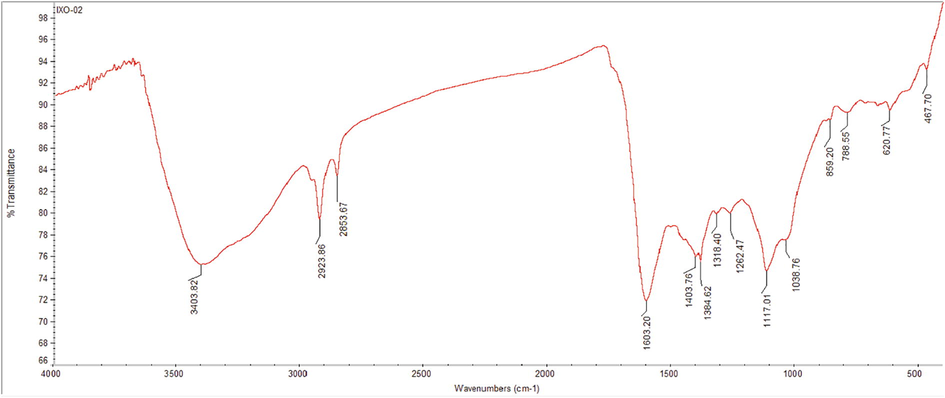
FTIR analysis of AgNPs.
3.3 AFM analysis
AFM data depicted that, the biological AgNPs were spherical, poly-dispersed with the size ranging from 18.36 nm to 50.04 nm. In three dimensional view, the height of AgNPs was found to be about 18.6 nm with around 38 nm distance from each other (Fig. 4A–C). These findings were in accordance with the previous studies on surface morphology of AgNPs in AFM analysis (Yugandhar and Savithramma, 2016; Nayaka et al., 2020a; 2020b).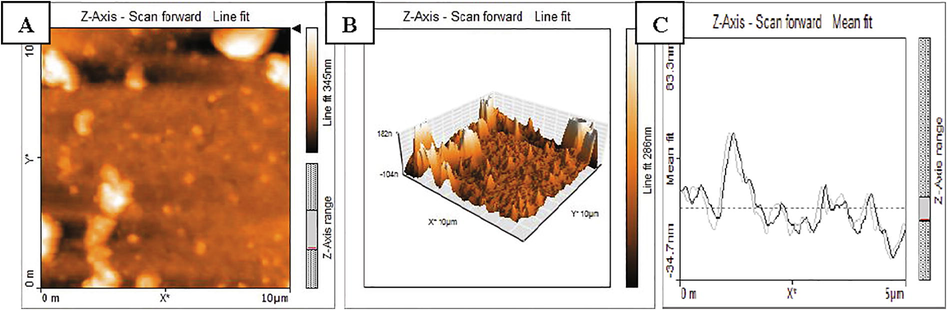
AFM analysis of AgNPs A) 2D structure of AgNPs, B) 3D structure of AgNPs, C) Particle size distribution.
3.4 SEM and EDS analysis
SEM image manifested the morphology of AgNPs as spherical, non-uniformly distributed up to some degree of aggregation (Fig. 5A). The size determined by SEM analysis was in accordance with size determined by AFM analysis. The presence of chemical elements was evaluated using EDS analysis from 0 to 5 keV and exhibited the characteristic peaks for pure metal silver and the presence of 38.47% silver illustrated the synthesis of AgNPs was good. Apart from silver, the pattern also indicated the presence of carbon, oxygen, sodium, potassium and chlorine (Fig. 5B). These findings were supported by the previous studies on the morphology and chemical elements of AgNPs using SEM/EDS and the EDS pattern with sharp peak at 3 keV, which was typical for crystalline metallic silver due to SPR (Kgatshe et al., 2019; Yadav et al., 2019).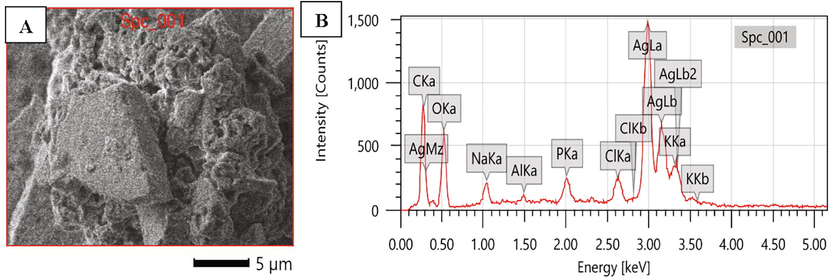
SEM with EDS image of AgNPs A) SEM image showing surface morphology, B) EDS spectrum indicating the presence of silver.
3.5 High resolution-transmission electron microscope analysis
HR-TEM imaging was done for understanding the details in morphology, size and distribution of AgNPs and the data illustrated morphological details. The synthesized AgNPs were spherical, poly-dispersed with few aggregations (Fig. 6A) and the size ranged from 18 to 50 nm with average diameter of 27.76 nm (Fig. 6B). Similarly, the TEM images of biogenic AgNPs showed that most of them were topographically spherical, discreet and poly-dispersed with size varying between 15 and 33 nm (Ezealisiji et al., 2017; Gomathi et al., 2017; Masum et al., 2019).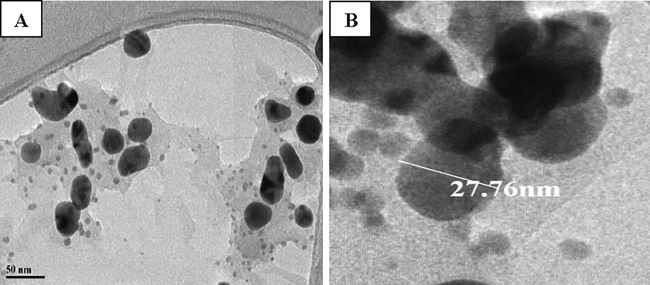
TEM analysis of AgNPs A) Morphology and distribution, B) Image showing average diameter.
3.6 Zeta potential analysis
Zeta potential indicates the stability of synthesized AgNPs and higher negative value attributed to the higher stability and high dispersivity of well-defined colloids. The zeta potential of I. brachypoda AgNPs was found to be −30.4 mV (Fig. 7) and that conveyed the phenomenon of repulsion among the synthesized AgNPs and their stable, distribution state in water. This result was well supported by the previous findings of zeta potential in AgNPs synthesized from Hyptis suaveolens (Botcha and Prattipati, 2020), Azadirachta indica (Haroon et al., 2019), and Scoparia dulcis (Parvataneni, 2018).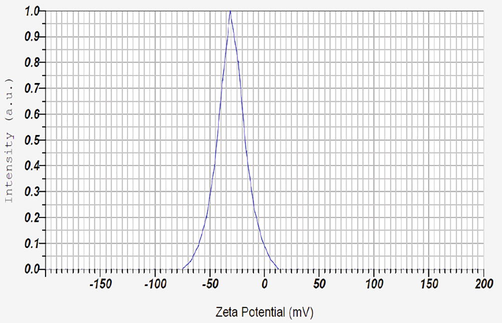
Zeta potential analysis of AgNPs.
3.7 XRD analysis
Further, the formation of I. brachypoda AgNPs was confirmed by the XRD patterns, which indicated the crystalline nature of AgNPs by characteristic diffraction peaks in 2θ range at 30° to 80° angles. The diffraction peaks observed at 2θ values of 38.07°, 46.32°, 64.47° and 77.34° corresponded to (1 1 1), (2 0 0), (2 2 0), and (3 1 1) Bragg’s planes of the face-centered cubic (FCC) structure of metallic silver, respectively (Fig. 8). The results of previous studies by Sangaonkar and Pawar, (2018), and Uddin et al., (2020) also conveyed the crystalline structure of AgNPs synthesized using Garcinia indica and Cocos nucifera extracts. These results matched with the Joint Committee on Powder Diffraction Standards (JCPDS) database for the confirmation of AgNPs formation.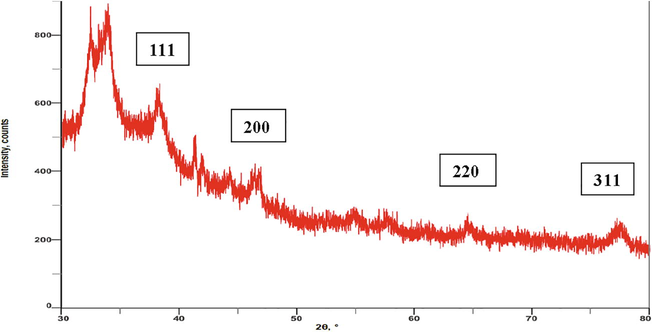
XRD pattern of AgNPs.
3.8 Antimicrobial activity
The AgNPs exhibited significantly increased antibacterial activity against the test pathogens, and the highest activity was found against B. subtilis with inhibition zones of 18, 20 and 21 mm at volumes of 25, 50 and 100 µl AgNPs suspension, respectively. The least activity was recorded against P. aeruginosa with 14, 14 and 16 mm inhibition zones at 25, 50 and 100 µl of AgNPs suspension, while AgNPs showed moderate activity against the remaining two bacterial pathogens. In antifungal activity, the highest activity was observed against C. albicans with 16, 22 and 24 mm of inhibition zones at 25, 50 and 100 µl of AgNPs suspension, and other three fungal pathogens showed moderate inhibition zones at different volumes of AgNPs suspension (Fig. 9).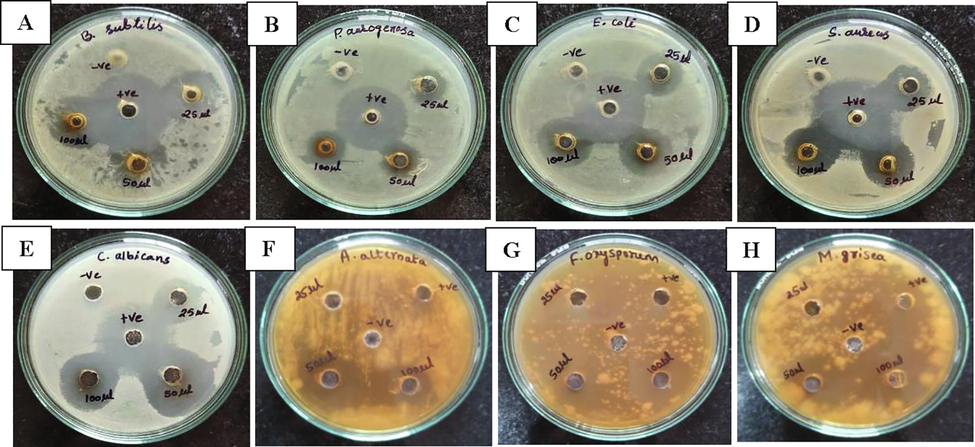
Antimicrobial activity of AgNPs; A) B. subtilis, B) P. aeruginosa, C) E. coli, D) S. aureus, E) C. albicans, F) A. alternata, G) F. oxysporum and H) M. grisea.
When the AgNPs get attached to the cell wall, they release silver ions and that leads to the change in the composition of cell wall of the microorganism by affecting the permeability. Apart from cell wall rupture, Ag+ ions disrupt DNA replication, expression of cellular and ribosomal subunit proteins as well as inactivation of enzymes required for ATP synthesis (Pei et al., 2019; Hemlata et al., 2020). The smaller size of the AgNPs also plays an influential role in the mechanism of antimicrobial activity, which allows them to efficiently pass across the membrane barriers and disrupt the physio-chemical functions of the microorganism (Majoumouo et al., 2019).
3.9 Evaluation of MIC and MBC
The MIC of I. brachypoda synthesized AgNPs was determined for all the four bacterial pathogens. The lowest MIC value was recorded for B. subtilis at 6 µg/ml and in P. aeruginosa the MIC value was 9 µg/ml. The MBC test showed the susceptibility of pathogenic bacteria towards the AgNPs and the values were observed as 6, 7, 8 and 9 µg/ml for B. subtilis, S. aureus, E. coli and P. aeruginosa, respectively (Table 1). Similar results were obtained in the previous studies by Loo et al., (2018) and Nayaka et al., (2020a; 2020b), where Pu-erh tea leaves and Zanthoxylum rhetsa seed coat extract mediated AgNPs exhibited the MIC and MBC values of about 4 to 8 µg/ml against the pathogenic bacteria by effectively inhibiting the growth at lower concentrations.
Sl. No.
Name of the pathogen
MIC (µg/mL)
MBC (µg/mL)
1.
B. subtilis
6
6
2.
P. aeruginosa
9
9
3.
E. coli
8
8
4.
S. aureus
7
7
4 Conclusion
The I. brachypoda AgNPs were synthesized using leaf aqueous extract through green chemistry method and the overall process was rapid, eco-friendly, non-toxic and cost effective. The bio-fabricated AgNPs showed the characteristic absorption maximum at 423 nm, the FTIR analysis suggested the presence of biological functional groups involved in the reduction, capping and stabilization of AgNPs. The Bragg’s planes in XRD confirmed the synthesis of AgNPs, the spherical shaped AgNPs ranged from 18 to 50 nm in size with 27.76 nm of average diameter and the higher negative zeta potential of −30.4 mV depicted the high stability of AgNPs. The biogenic AgNPs exhibited high antimicrobial activity against the human pathogens with considerable MIC and MBC values. The biogenic AgNPs offer applications in diverse fields due to less toxicity and high biological activities. The present in-vitro analysis further suggests the use of AgNPs as controlling agents against wide range of plant and human pathogens after successful clinical trials.
Acknowledgments
The authors are thankful to the University Scientific Instrumentation Centre (USIC), Karnatak University Dharwad, Karnataka, for providing essential instrumentation facilities. The project was supported by Researchers Supporting Project number (RSP-2020/231), King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Recent advances in green synthesis of silver nanoparticles and their applications: about future directions. A review. BioNanoSci.. 2018;8(1):5-16.
- [CrossRef] [Google Scholar]
- A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: a green expertise. J. Adv. Res.. 2016;7(1):17-28.
- [CrossRef] [Google Scholar]
- Biogenic synthesis of silver nanoparticles: antibacterial and cytotoxic potential. Saudi J. Biolog. Sci.. 2020;27(5):1340-1351.
- [CrossRef] [Google Scholar]
- Green synthesis of silver nanoparticles from Neurada procumbens and its antibacterial activity against multi-drug resistant microbial pathogens. J. King Saud Univ. – Sci.. 2020;32(2):1346-1352.
- [CrossRef] [Google Scholar]
- Advancements in plant and microbe-based synthesis of metallic nanoparticles and their antimicrobial activity against plant pathogen. Nanomater.. 2020;10:1146-1169.
- [Google Scholar]
- Biogenic nanosilver against multidrug-resistant bacteria (MDRB) Antibiot.. 2018;7:69-97.
- [Google Scholar]
- Synthesis paradigm and applications of silver nanoparticles (AgNPs), a review. Sustainable Mater.Technol.. 2017;13:18-23.
- [CrossRef] [Google Scholar]
- Callus extract mediated green synthesis of silver nanoparticles, their characterization and cytotoxicity evaluation against MDA-MB-231 and PC-3 Cells. BioNanoSci.. 2020;10(1):11-22.
- [CrossRef] [Google Scholar]
- Biomedical applications of silver nanoparticles: an up-to-date overview. Nanomaterials. 2018;8(9):681.
- [CrossRef] [Google Scholar]
- A review of bark-extract-mediated green synthesis of metallic nanoparticles and their applications. Molecules. 2019;24:4354-4371.
- [CrossRef] [Google Scholar]
- Plant-mediated synthesis of silver nanoparticles: their characteristic properties and therapeutic applications. Nanoscale Res. Lett.. 2016;2016(11):40.
- [CrossRef] [Google Scholar]
- Green synthesis and characterization of monodispersed silver nanoparticles using root bark aqueous extract of Annona muricata Linn and their antimicrobial activity. Appl. Nanosci.. 2017;7(8):905-911.
- [CrossRef] [Google Scholar]
- Green synthesis of silver nanoparticles using terrestrial fern (Gleichenia Pectinata (Willd.) C. Presl.): characterization and antimicrobial studies. Heliyon. 2019;5(4):e01543.
- [CrossRef] [Google Scholar]
- Green synthesis of silver nanoparticles using Datura stramonium leaf extract and assessment of their antibacterial activity. Resour.-Effic. Technol.. 2017;3(3):280-284.
- [CrossRef] [Google Scholar]
- Synthesis of silver nanoparticles by chemical and biological methods and their antimicrobial properties. J. Exp. Nanosci.. 2016;11(9):714-721.
- [CrossRef] [Google Scholar]
- Biogenic synthesis of silver nanoparticles and its synergistic antimicrobial potency: an overview. J. Appl. Biotechnol. Bioeng.. 2019;6(1):22-28.
- [CrossRef] [Google Scholar]
- Effective inhibition of phytopathogenic microbes by eco-friendly leaf extract mediated silver nanoparticles (AgNPs) Indian J. Microbiol.. 2019;59(3):273-287.
- [CrossRef] [Google Scholar]
- Biosynthesis of silver nanoparticles using Cucumis prophetarum aqueous leaf extract and their antibacterial and antiproliferative activity against cancer cell lines. ACS Omega. 2020;5(10):5520-5528.
- [CrossRef] [Google Scholar]
- Synthesis of silver nanoparticles: chemical, physical and biological methods. Res. Pharm. Sci.. 2014;9(6):385-406. PMCID: PMC4326978
- [Google Scholar]
- Antimicrobial effect of silver nanoparticles (AgNPs) and their mechanism – a mini review. Micro & Nano Letters. 2018;13(3):277-280.
- [CrossRef] [Google Scholar]
- Biosynthesis of nanoparticles and silver nanoparticles. Bioresour. Bioprocess.. 2015;2:47-57.
- [CrossRef] [Google Scholar]
- Characterization and antibacterial activity of biosynthesized silver nanoparticles using the ethanolic extract of Pelargonium sidoides DC. J. Nanomater.. 2019;2019:1-10.
- [CrossRef] [Google Scholar]
- Plant mediated synthesis of silver nanoparticles using Punica granatum aqueous leaf extract. J. Microbiol. Exp.. 2018;6(4):175-178.
- [CrossRef] [Google Scholar]
- Plant-mediated synthesis of silver nanoparticles using fruit extract of Cleome viscosa L.: assessment of their antibacterial and anticancer activity. Karbala Int. J. Mod. Sci.. 2017;4(1):61-68.
- [CrossRef] [Google Scholar]
- Enhanced anti-bacterial activity of biogenic silver nanoparticles synthesized from Terminalia mantaly extracts. Int. J. Nanomed.. 2019;14:9031-9046.
- [CrossRef] [Google Scholar]
- Biogenic synthesis of silver nanoparticles using phyllanthus emblica fruit extract and its inhibitory action against the pathogen acidovorax oryzae strain rs-2 of rice bacterial brown stripe. Front. Microbiol.. 2019;10:820-837.
- [CrossRef] [Google Scholar]
- Green synthesis of silver nanoparticles from Moringa oleifera leaf extracts and its antimicrobial potential. Adv. Nat. Sci.: Nanosci. Nanotechnol.. 2018;9(1):015011.
- [CrossRef] [Google Scholar]
- Seed extract-mediated synthesis of silver nanoparticles from Putranjiva roxburghii Wall.: phytochemical characterization, antibacterial activity and anticancer activity against MCF-7 cell line. Indian J. Pharm. Sci.. 2020;82(2):260-269.
- [CrossRef] [Google Scholar]
- Synthesis of biogenic silver nanoparticles using Zanthoxylum rhetsa (Roxb.) DC seed coat extract as reducing agent and in-vitro assessment of anticancer effect on A549 lung cancer cell line. Int. J. Pharm. Res.. 2020;12(3):302-314.
- [Google Scholar]
- Biogenic synthesis and characterization of silver nanoparticles using aqueous leaf extract of Scoparia dulcis L. and assessment of their antimicrobial property. Drug Chem. Toxicol.. 2019;43(3):307-321.
- [CrossRef] [Google Scholar]
- Biosynthesis, characterization, and anticancer effect of plant-mediated silver nanoparticles using Coptis chinensis. Int. J. Nanomed.. 2019;14:1969-1978.
- [CrossRef] [Google Scholar]
- Mechanism of plant-mediated synthesis of silver nanoparticles – a review on biomolecules involved, characterisation and antibacterial activity. Chem. Biol. Interact.. 2017;273:219-227.
- [CrossRef] [Google Scholar]
- Green synthesis, characterization, antibacterial, antioxidant and photocatalytic activity of Parkia speciosa leaves extract mediated silver nanoparticles. Results Phys.. 2019;15:102565.
- [CrossRef] [Google Scholar]
- Garcinia indica mediated biogenic synthesis of silver nanoparticles with antibacterial and antioxidant activities. Colloids Surf., B. 2018;164:210-217.
- [CrossRef] [Google Scholar]
- Cocos nucifera leaf extract mediated green synthesis of silver nanoparticles for enhanced antibacterial activity. J. Inorg. Organomet. Polym.. 2020;30(9):3305-3316.
- [CrossRef] [Google Scholar]
- Rapid biosynthesis and characterization of silver nanoparticles from the leaf extract of Tropaeolum majus L. and its enhanced in-vitro antibacterial, antifungal, antioxidant and anticancer properties. J. Photochem. Photobiol., B. 2019;191:65-74.
- [CrossRef] [Google Scholar]
- Bioengineering of Piper longum L. extract mediated silver nanoparticles and their potential biomedical applications. Mater. Sci. Eng., C. 2019;104:109984.
- [CrossRef] [Google Scholar]
- Biosynthesis, characterization and antimicrobial studies of green synthesized silver nanoparticles from fruit extract of Syzygium alternifolium (Wt.) Walp. an endemic, endangered medicinal tree taxon. Appl. Nanosci.. 2016;6(2):223-233.
- [CrossRef] [Google Scholar]
- Silver nanoparticles: synthesis, characterization, properties, applications, and therapeutic approaches. Int. J. Mol. Sci.. 2016;17:1534-1567.
- [Google Scholar]







