Translate this page into:
Bioethanol production by mangrove-derived marine yeast, Sacchromyces cerevisiae
*Corresponding author. Tel.: +91 4144243223x210 kathirsum@rediffmail.com (K. Kathiresan)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The present work tested the bioethanol production by two strains of yeast, Saccharomyces cerevisiae. The marine strain displayed higher bioethanol production than the terrestrial strain did and hence, it was selected for optimizing the fermentation conditions for ethanol production by using the center composite design of response surface methodology. The R2 value of 0.92 of statistical model revealed its acceptability. The factors such as temperature, incubation period, acid processed sawdust and agitation were optimized on bioethanol production by S. cerevisiae. The marine strain showed the maximum ethanol production of 69.58% of total distillate under the optimal conditions: temperature 30 °C, sawdust concentration of 6.84 mg/l under the agitation speed of 360 rpm at 89 h of incubation. This work revealed that mangrove-derived marine yeast strain of S. cerevisiae was potential for bioethanol production over the terrestrial strain.
Keywords
Yeast
Sacchromyces cerevisiae
Mangroves
Bioethanol
Marine yeasts
1 Introduction
Bioethanol production has gained prominence as an alternative source of energy due to the predictable exhaustion of fuel energy supply (Zaldivar et al., 2001; Ariyajaroenwong et al., 2012). The bioethanol production through microbial fermentation provides an economically competitive source of energy (Mcaloon et al., 2000; Yasuyuki et al., 2011). Plant-derived lignocellulose can be used for the production of biofuels after undertaking some pretreatments (Zhang et al., 2007; Minhee et al., 2011). Biological and acid treatments of the lignocellulosic waste materials improve the bioethanol production by Saccharomyces sp. (Saritha et al., 2011; Yasuyuki et al., 2011; Lei et al., 2012). Cellulose can be effectively hydrolyzed and depolymerized into sugars which are then fermented to produce ethanol (Mitsunori et al., 2011). Crude enzymes are also used for biological conversion of plant biomass into fuels and high value chemicals, which find a wide range of applications in food, animal feed, textile, fuel and chemical industries (Mitsunori et al., 2011). The pretreatment of lignocellulosic waste materials produces cellulose, glucose and other mono sugars which favor the high yield of ethanol (Jorgensen et al., 2007; Claudio et al., 2011).
Cellulosic materials such as sawdust are renewable natural biological resources and generation of bio-based products and bioenergy from such substances is highly necessary. Various industries across the world generate huge volumes of cellulosic wastes which have an immense potential to be utilized for the production of several bio-products (Das and Singh, 2004). These substances provide a low-cost and uniquely sustainable resource for production of many organic fuels and chemicals that can reduce greenhouse gas emissions, enhance energy security, improve the economy, dispose problematic solid wastes, and improve air quality (Das and Singh, 2004).
The yeast Saccharomyces cerevisiae is the potential yeast for the ethanol production from lignocellulose against toxic chemicals present in the substrate (Tomas et al., 2010). Many yeasts are also known to be the potential source of extracellular enzymes to produce ethanol and they are Pichia stipitis, Candida shehatae and Pachysolan tannophilus (Kirk et al., 2002), P. salicaria (Kathiresan and Saravanakumar, 2011), Zymomonas mobilis (Laura et al., 2011), S. cerevisiae (Tomas et al., 2010). Similarly marine algae such as Undaria pinnatifida, Chlorella vulgaris, Chlamydomonas reinhardtii and Kappaphycus alvarezii have also been reported as the efficient substrate for ethanol production with bacterial fermentation (Maria et al., 2011, 2012; Soojin et al., 2011; Lin Tanaka, 2006; Tian et al., 2009; Bing et al., 2010; Jin et al., 2011; Maria et al., 2012). However, marine strains of the yeast are less known for their potential in ethanol production. Hence, the present study was conducted to compare the efficacy of marine and terrestrial strains of S. cerevisiae on ethanol production and to optimize the fermentation conditions, required for the ethanol production.
2 Materials and methods
2.1 Yeast cultures
Marine strain of S. cerevisiae (JN387604) was isolated from mangrove soil. Mangrove soil samples collected from Pichavaram mangrove forest, southeast coast of India were transferred at 4 °C and analyzed for marine yeasts within 4–6 h of sampling. The soil samples were serially diluted and Yeast Malt Agar medium was used for isolation of yeasts (Yeast malt Agar) (Fell, 2005). In this method, sterile media were poured into Petri dishes aseptically and allowed to solidify. One milliliter of the serially diluted sample was pipetted out into sterile Petri-dishes. It was spread in the plate first by rotating it in clockwise and then in anti-clockwise direction for three times and then spread with the help of a “L” rod. The plates were incubated in an inverted position at 28 ± 2 °C. After 7 to 10 days of incubation the yeasts were identified up to the molecular level as S. cerevisiae and deposited in National Center for Biotechnology Information (NCBI). Another terrestrial strain of S. cerevisiae was obtained from the commercially available baker's yeast. Both strains were maintained at below 4 °C in agar slants and sub cultured for further experimental studies.
2.2 Preparation of sawdust
Sawdust containing cellulose, hemicelluloses and polysugars, was used for the bioethanol production as a substrate after pretreatment. Sawdust was obtained from local wood mills, sieved through a 1.5 mm sieve for maintaining uniform particle size, and washed with tap water for removing the impurities from sawdust and finally dried at 60 °C overnight. The sawdust was pre-hydrolyzed by using 0.8% phosphoric acid by using the method of Kathiresan and Saravanakumar (2011). The processed sawdust was incorporated at different concentrations into the medium based on the experimental design given in Table 1.
Std run
Temperature
Incubation period (h)
Sawdust (mg/l)
Agitation (rpm)
Bioethanol production (%)
Actual
Predicted
1
20
0
1
100
0.60
5.35
2
40
0
1
100
0.50
5.66
3
20
120
1
100
29.35
20.54
4
40
120
1
100
16.59
12.56
5
20
0
10
100
0.20
2.19
6
40
0
10
100
0.30
0.37
7
20
120
10
100
56.59
48.87
8
40
120
10
100
42.26
38.76
9
20
0
1
500
0.30
6.40
10
40
0
1
500
0.56
12.16
11
20
120
1
500
25.69
29.49
12
40
120
1
500
26.35
26.96
13
20
0
10
500
0.56
8.47
14
40
0
10
500
0.69
12.10
15
20
120
10
500
65.60
63.04
16
40
120
10
500
59.26
58.38
17
10
60
5.5
300
32.56
33.06
18
50
60
5.5
300
35.69
28.71
19
30
60
5.5
300
0.26
21.00
20
30
180
5.5
300
25.69
40.47
21
30
60
3.5
300
0.23
6.12
22
30
60
14.5
300
22.27
22.14
23
30
60
5.5
100
19.56
28.85
24
30
60
5.5
700
65.29
49.53
25
30
60
5.5
300
69.25
69.42
26
30
60
5.5
300
69.20
69.42
27
30
60
5.5
300
69.56
69.42
28
30
60
5.5
300
69.58
69.42
29
30
60
5.5
300
69.58
69.42
30
30
60
5.5
300
69.36
69.42
2.3 Selection of potential strain for production of bioethanol
In order to select the potential strain for bioethanol production, a primary experiment was done by using two different strains of yeasts following the method of Caputi et al. (1968). In this method, an inoculum of 1 ml of the yeast (23 × 103 CFU ml−1) was enriched in Yeast Malt Broth production medium (Dextrose-5.0 g, Peptone-5.0 g, Yeast extract-3.0 g and Malt extract-3.0 g in 1000 mL distilled water added with 50% seawater for marine derived strain and distilled water for terrestrial strain). The fermentation was carried out in 500 mL Erlenmeyer flasks using 100 mL of medium. It was kept for fermentation at 28 °C on a shaker at 120 rpm in triplicate. The level of ethanol in the distilled culture filtrate was tested in a gas chromatographically after 120 h of incubation.
2.4 Determination of bioethanol
Concentration of ethanol in the distillate of culture filtrate was estimated by using a Hewlett Packard 5890 Series II gas chromatography with chromosorb 105 column and nitrogen as a carrier gas. The temperature of the injection port, oven and detection port was 250, 120, and 250 °C, respectively. For the analysis, 1 μl of liquid samples was injected into gas chromatography. The ethanol concentration was determined by using an ethanol standard plot and is expressed in percentage.
2.5 Experimental setup by response surface methodology (RSM) for bioethanol production
In this experiment the potential strain of S. cerevisiae (JN387604) was selected for optimization. The testing of individual and interaction effect of the temperature (20 to 40 °C), incubation period (h) (0 to 120 h), sawdust (1–10 mg l−1) and agitation (100–500 rpm) on ethanol production was carried out. The maximum yield of ethanol production was tested by using 30 experimental setups derived from a statistical model – central composite design of response surface methodology. The coded values of the fermentation factors were determined by the following Eq. (1).
A statistical program package Design Expert 8.0.6, was used for regression and model fit analysis of the data obtained and to estimate the coefficient of the regression equation, and analyzed the variance of selected factors and model significance.
3 Results
3.1 Selection of the potential strain for the ethanol production
The terrestrial and marine strains of S. cerevisiae were tested for their efficiency on ethanol production. The percentage of the ethanol production was calculated with instrumental default standard value of 65 g l−1 which is equivalent to 100%. Among the two strains the mangrove-derived marine strain of S. cerevisiae produced 38.56% yield as against 21.25% by the terrestrial strain. In other words, the marine strain produced 17.31% higher ethanol than the terrestrial strain did. The marine strain was then selected for further optimization of fermentation conditions for bioethanol production.
3.2 Optimization of bioethanol production by marine strain of S. cerevisiae
The experimental design fitness was tested by the quadratic model along with the contour error plot that are presented in Fig. 1a,b. The bioethanol production for each cycle was performed as per the experimental design. Experimental responses along with predicted response are given in Table 1. The application of the response surface methodology based on the estimates of the parameters indicated an experimental relationship between the response and input variables expressed by the following quadratic model Eq. (3)
Statistically significant, NS Non-significant.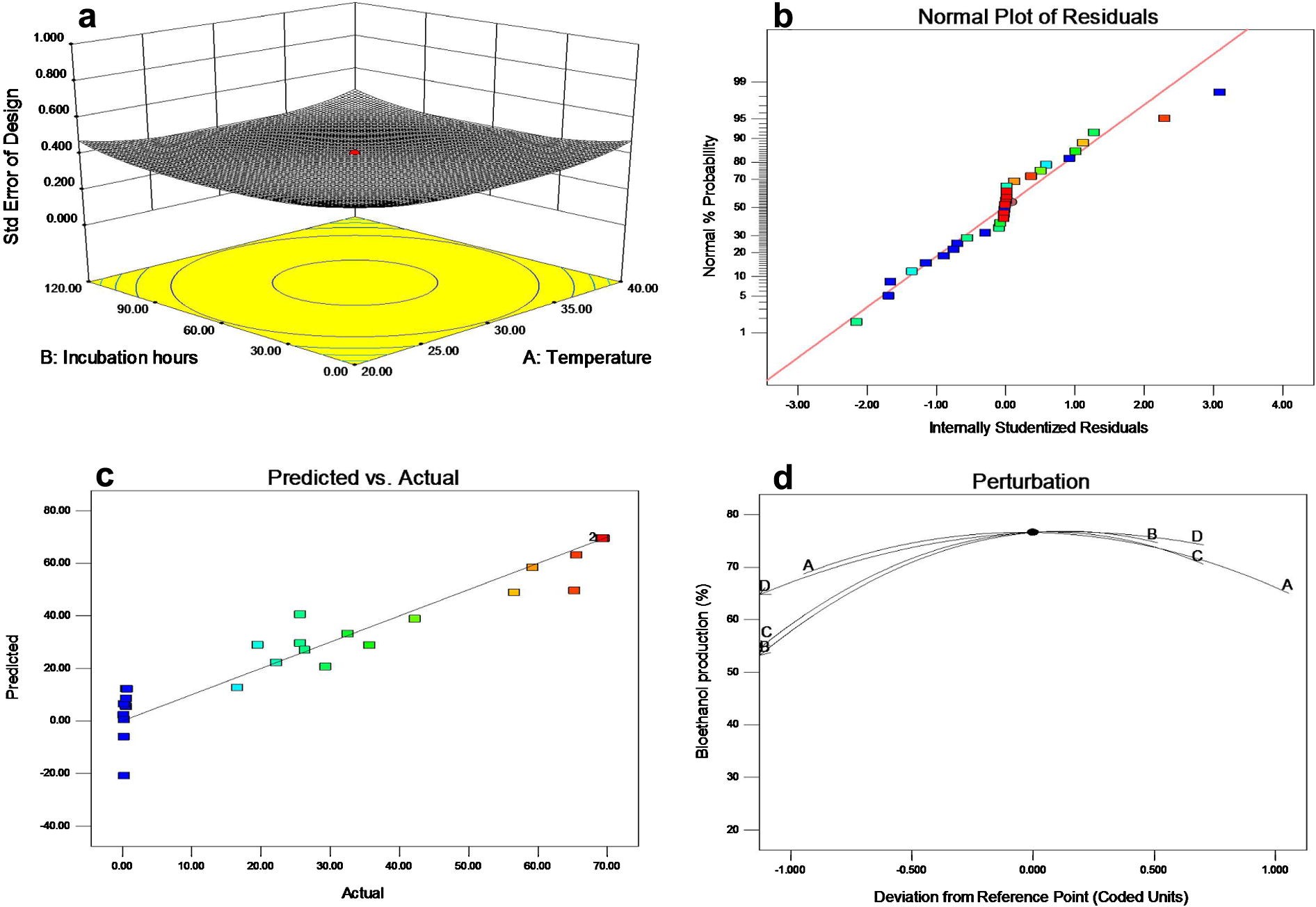
(a) Three-dimensional standard error plot, (b) Normal plot for the residuals and normal percentage of probability for the response of predicted and experimental values, (c) Predicted and actual experimental response for the bioethanol production and (d) perturbation plot for bioethanol production by mangrove derived S. cerevisiae.
Source
Sum of squares
Df
Mean square
F Value
p-Value Prob > F
Model
20975.75
14
1498.26
13.21
<0.0001***
A-Temperature
28.42
1
28.42
0.25
0.6238NS
B-Incubation period (h)
5668.45
1
5668.45
50.00
<0.0001***
C-Sawdust (mg/l)
1198.27
1
1198.27
10.57
0.0054**
D-Agitation (rpm)
641.49
1
641.49
5.65
0.0311*
AB
68.72
1
68.72
0.60
0.4483NS
AC
4.51
1
4.51
0.03
0.8445NS
AD
29.70
1
29.70
0.26
0.6162NS
BC
991.30
1
991.30
8.74
0.0098**
BD
62.41
1
62.41
0.55
0.4695NS
CD
27.30
1
27.30
0.24
0.6307NS
A2
2545.49
1
2545.49
22.45
0.0003***
B2
6106.59
1
6106.59
53.87
<0.0001***
C2
6464.87
1
6464.87
57.03
<0.0001***
D2
1567.02
1
1567.02
13.82
0.0021**
Residual
1700.36
15
113.35
Lack of fit
1700.21
10
70.02
4.492
0.23NS
Pure error
0.15
5
0.03
Core total
22676.12
29
The individual and interaction effects of incubation period and temperature on the bioethanol production were tested. Incubation period was significant but temperature and its interactions were not significant on bioethanol production (Table 2). The maximum bioethanol yield of 69.58% was obtained at a temperature of 30 °C in 89 h of incubation (Fig. 2a).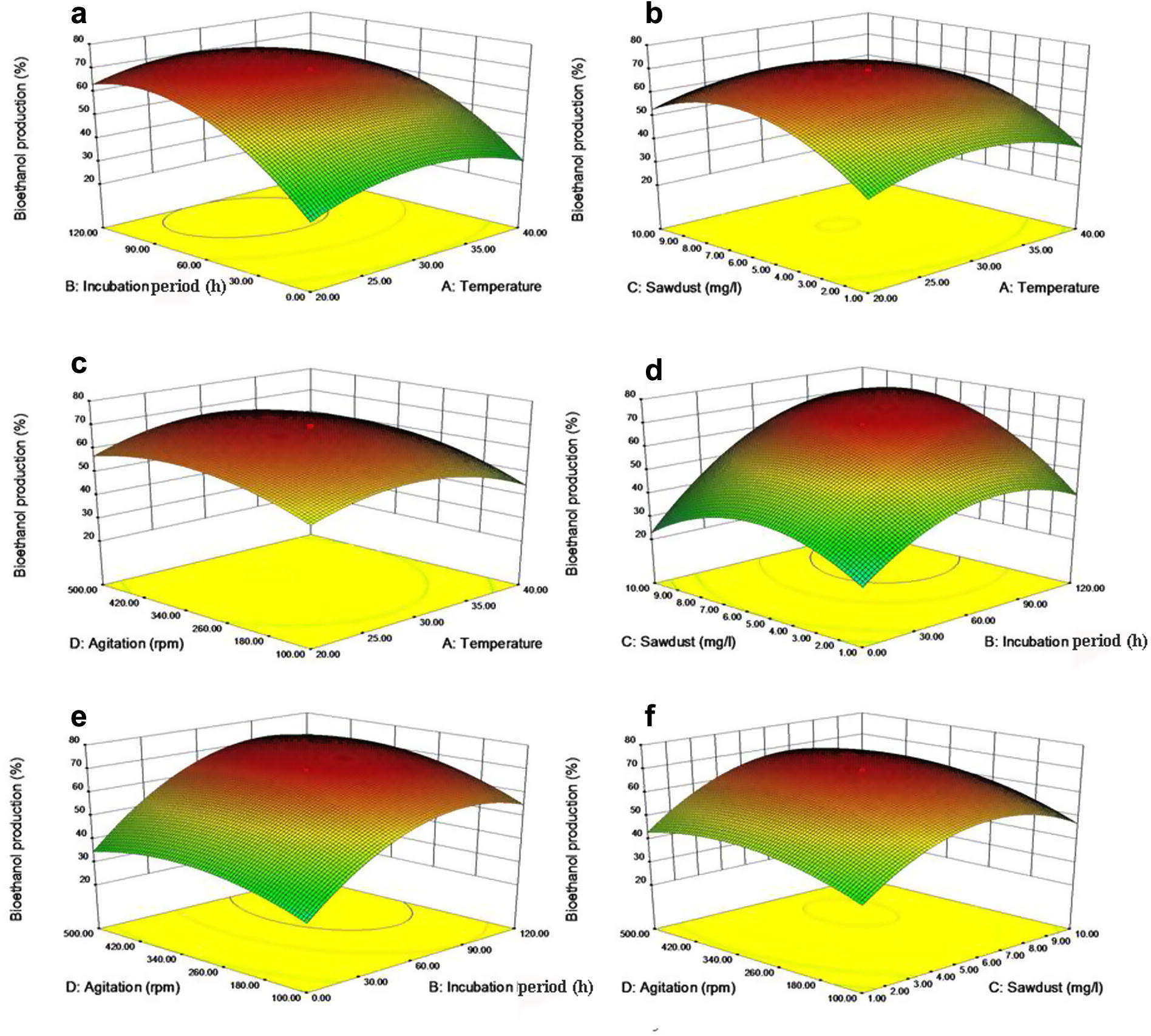
Three-dimensional response surface plot for (a) Effect of incubation period and temperature, (b) Effect of sawdust concentration and temperature, (c) Effect of agitation speed and temperature, (d) Effect of sawdust concentration and incubation period, (e) Effect of agitation and incubation period and (f) Effect of agitation and sawdust concentration, on bioethanol production by S. cerevisiae.
The sawdust concentration was significant but the interaction effect of the temperature and sawdust was not significant on bioethanol production (Table 2). The maximum yield of 69.58% bioethanol was obtained with the sawdust concentration of 6.84 mg l−1 at 30 °C (Fig. 2b).
The individual and interaction effects of the agitation and temperature on the bioethanol production were tested. The individual effect of the agitation was significant but its interaction was not significant on the bioethanol production. Statistically, bioethanol production was significantly increased with increasing rotations per minute (Table 2; Fig. 2c).
Bioethanol production varied significantly between the sawdust concentrations or incubation period (h) and also their interaction and combined effects (Table 2). The maximum yield of 69.58% bioethanol was obtained with the sawdust concentration of 6.84 mg l−1 at 89 h of incubation (Fig. 2d).
Bioethanol production varied significantly between the sawdust concentration and incubation period (h) and also their interaction effects (Table 2). The maximum yield of 69.58% bioethanol was recorded under the agitation speed of 360 rpm at 89 h of incubation (Fig. 2e).
Bioethanol production varied significantly with sawdust concentration and agitation speed but not significantly with interactive effect (Table 2). Statistically optimized condition for the maximum yield of bioethanol production was analyzed by the method of contour plotting (Fig. 2f). The maximum yield of the bioethanol production reached to 69.58% under the optimal conditions of temperature at 30 °C, sawdust concentration of 6.84 mg l−1 under the agitation speed of 360 rpm in 89 h of incubation (Fig. 1d). The bioethanol productions were experimentally performed by using statistically optimized fermentation conditions. In that 72.5% of bioethanol was produced by marine yeasts. It was 2.98% higher than that in the statistically optimized condition. It revealed that the statistical optimization was acceptable for the large scale production of the bioethanol.
4 Discussion
Researchers have analyzed bioethanol production by microorganisms of terrestrial or marine origin (Sun and Cheng, 2002; Lin Tanaka, 2006; Kathiresan and Saravanakumar, 2011; Senthilraja et al., 2011). However, the marine and terrestrial strains have not been compared experimentally, but only theoretically for their bioethanol production (Senthilraja et al., 2011). The present work perhaps for the first time compared experimentally the marine and terrestrial strains of S. cerevisiae on the bioethanol production. The present experiment proved that both strains of S. cerevisiae produced the bioethanol but the marine strain produced 17.31% higher bioethanol than the terrestrial strain, indicating the efficiency of marine yeast over terrestrial strain. This is in accordance with Kathiresan and Saravanakumar (2011) who theoretically compared terrestrial and marine yeasts.
Optimization of fermentation conditions on bioethanol production was carried out with the statistical model of response surface methodology. The important fermentation factors such as temperature, incubation period (h), phosphoric acid processed sawdust and agitation speed on bioethanol production were tested and also their individual and interaction effects. The experimental design fitness was tested by the quadratic model along with the contour error plot that are presented in Fig. 1a,b and the value of adjusted R2 0.85 was close to predicted R2 value 0.92 that revealed a high correlation between the observed values and the predicted values and also lack of the fit was not significant. It revealed that the present model was acceptable. Hence the present study used sawdust as a cellulose biomass. Among the various types of processing of the sawdust used for alcohol production, McCain (2003) suggested the use of corn for making huge profits from ethanol production. However, the wood cellulose system requires slightly more energy to produce the ethanol than using other sources (Arkenol, 2004). (Not clear). Arkenol (2004) has reported that 2 kg of wood produces 1 l of ethanol. The 2 kg wood materials produce 1 l of ethanol whereas 2.69 kg of corn grain is required to produce the same volume of ethanol (Kidd and Pimentel, 1992). Based on the previous reports, the wood materials such as saw dust are a potential source for the fuel production than the corn grain and other glucose derivatives. Therefore the present study used sawdust as substrate for the bioethanol production.
Marine strain of S. cerevisiae showed the maximum bioethanol production of 69.58% under the optimal conditions of temperature (30 °C), sawdust concentration of (6.84 mg l−1) under the agitation speed of (360 rpm) in 89 h of incubation (Fig. 1d). Thus the present study suggested that the statistical tool of central composite design of response surface methodology was suitable to find out the reliable values of fermentation conditions for enhanced ethanol production, as supported by the work of Ratnam et al. (2005). The present study concluded that mangrove-derived marine S. cereviseae was promising over terrestrial strain for bioethanol production.
Acknowledgement
The authors are thankful to the authorities of Annamalai University for providing facilities.
References
- Repeated-batch ethanol production from sweet sorghum juice by Saccharomyces cerevisiae immobilized on sweet sorghum stalks. Energies. 2012;5:1215-1228.
- [CrossRef] [Google Scholar]
- Arkenol, 2004. Our technology: concentrated acid hydrolysis. <www.arkenol.com/Arkenol%20Inc/tech01.html> (accessed 8.02.04).
- Genome-wide transcriptional analysis of Saccharomyces cerevisiae during industrial bioethanol fermentation. J. Ind. Microbiol. Biotechnol.. 2010;37:43-55.
- [Google Scholar]
- Spectrophotometric determination of chromic complex formed during oxidation of alcohol. Am. J. Enol. Viticult.. 1968;19:160-165.
- [Google Scholar]
- Mendonca bioethanol production from tension and opposite wood of Eucalyptus globulus using organosolv pretreatment. J. Ind. Microbiol. Biotechnol.. 2011;38:1861-1866.
- [Google Scholar]
- Useful byproducts from cellulosic wastes of agriculture and food industry—a critical appraisal. Crit. Rev. Food Sci. Nutr.. 2004;44(2):77-89.
- [Google Scholar]
- The role of nucleotide analysis in the systematics of the yeast genera Cryptococcus sp. and Rhodotorula sp. Stud. Mycol.. 2005;38:129-146.
- [Google Scholar]
- Heterologous expression of transaldolase gene tal from Saccharomyces cerevisiae in Fusarium oxysporum for enhanced bioethanol production. Appl. Biochem. Biotechnol.. 2011;164:1023-1036.
- [Google Scholar]
- Enzymatic conversion of lignocellulose into fermentable sugars: challenges and opportunities. Biofuel Bioprod. Bior.. 2007;1:119-134.
- [Google Scholar]
- Bio-ethanol production by marine yeasts isolated from coastal mangrove sediment. Int. Multidisciplin. Res. J. Biotechnol.. 2011;1(1):19-24.
- [Google Scholar]
- Integrated Resource Management: A Groforestry for Development: Academic. CA: San Diego; 1992.
- Bioethanol production in batch mode by a native strain of Zymomonas mobilis. World J. Microbiol. Biotechnol.. 2011;27:2521-2528.
- [Google Scholar]
- Dilute acid pretreatment for cellulosic alcohol production. Biomass Conv. Bioref. 2012
- [CrossRef] [Google Scholar]
- Bioethanol production from the acid hydrolysate of the carrageenophyte Kappaphycus alvarezii (cottonii) J. Appl. Phycol. 2011
- [CrossRef] [Google Scholar]
- Detoxification of acidic catalyzed hydrolysate of Kappaphycus alvarezii (cottonii) Bioprocess Biosyst. Eng.. 2012;35:93-98.
- [Google Scholar]
- Kinetics of sugars consumption and ethanol inhibition in carob pulp fermentation by Saccharomyces cerevisiae in batch and fedbatch cultures. J. Ind. Microbiol. Biotechnol.. 2012;39:789-797.
- [Google Scholar]
- Mcaloon, A., Taylor, F., Yee, W., Ibsen, K., Wooley, R., 2000. Determining, the cost of producing ethanol from corn starch and lignocellulosic feedstocks. Report No. NREL/TP-580-28893. Eastern Regional Research Centre, Wyndmoor, PA and National Renewable Energy Laboratory, Golden Co.
- McCain, J., 2003. Statement of Senator McCain on the Energy Bill: Press Release. Wednesday, November 2003.
- Bioethanol production from optimized pretreatment of cassava stem. Korean J. Chem. Eng.. 2011;28(1):119-125.
- [Google Scholar]
- Bioethanol from sea lettuce with the use of crude enzymes derived from waste. J. Mater. Cycles Waste Manag.. 2011;13:321-326.
- [Google Scholar]
- Design and Analysis of Experiments (fifth ed.,). John Wiley & Sons, Inc; 2001.
- Optimization of medium constituents and fermentation conditions for the production of ethanol from palmyra jaggery using response surface methodology. World J. Microbiol. Biotechnol.. 2005;21:399-404.
- [Google Scholar]
- Biological pretreatment of lignocellulosic substrates for enhanced delignification and enzymatic digestibility. Indian J. Microbiol. 2011
- [CrossRef] [Google Scholar]
- Comparative analysis of bioethanol production by different strains of immobilized marine yeast. J. Yeast Fun. Res.. 2011;2(8):113-116.
- [Google Scholar]
- Converting carbohydrates extracted from marine algae into ethanol using various ethanolic Escherichia coli strains. Appl. Biochem. Biotechnol.. 2011;164:878-888.
- [Google Scholar]
- Hydrolysis of lignocellulosic materials for ethanol production: a review. Bioresource. Technol.. 2002;83:1-11.
- [Google Scholar]
- Ethanol fermentation from biomass resources: current state and prospects. Appl. Microbiol. Biotechnol.. 2006;69:627-642.
- [Google Scholar]
- Yeast strains for ethanol production from lignocellulosic hydrolysates during in situ detoxification. Biotechno. Adv. Bioenergy Res.. 2009;27(5):656-660.
- [Google Scholar]
- Adaptation of the xylose fermenting yeast Saccharomyces cerevisiae F12 for improving ethanol production in different fed-batch SSF processes. J. Ind. Microbiol. Biotechnol.. 2010;37:1211-1220.
- [Google Scholar]
- Conversion of sulfuric acid lignin generated during bioethanol production from lignocellulosic materials into polyesters with ɛ-caprolactone. J. Wood Sci.. 2011;57:214-218.
- [Google Scholar]
- Fuel ethanol production from lignocellulose: a challenge for metabolic engineering and process integration. Appl. Microbiol. Biotechnol.. 2001;56:17-34.
- [Google Scholar]
- Zhang, H.J., Fan, S.G., Gu, X.J., 2007. The development of bioenergy and its impacts on global food and agricultural production and trade. China Food and Nutrition. No. 9. <www.ilib.cn/A-zgswyyy200709011.htmlS>.







