Translate this page into:
Bioconversion of corn straw to ethanol by cellulolytic yeasts immobilized in Mucuna urens matrix
⁎Corresponding author at: Department of Microbiology, Federal University of Agriculture, Abeokuta, Ogun State, Nigeria. bola.blessing@yahoo.com (Blessing Adebola Adelabu),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Production of bioethanol from corn straw by cellulolytic yeasts immobilized on Mucuna urens was investigated. Yeast isolates were screened for amylase, cellulase and ethanol production. Effect of bead size, inoculum load, substrate concentration, pH and bead reusability were studied. Bioethanol production was optimum with 4 mm bead size, 10% substrate concentration, pH 4.5 and 10% inoculum load. Maximum ethanol production (55.27 g/L) was achieved by immobilized Saccharomyces diaststicus. Immobilized yeast cells were re-used repeatedly without obvious loss of activity. This study showed that yeasts immobilized on Mucuna urens can effectively utilize lignocellulolytic materials and produce ethanol from it.
Keywords
Bioethanol
Yeasts
Immobilization
Mucuna urens
Saccharomyces diastaticus
1 Introduction
Bioethanol production is receiving attention as an alternative source of energy due to the predictable exhaustion of fuel energy supply (Ariyajaroenwong et al., 2012). Bioethanol is commercially produced on a moderate scale from sugar cane, wheat, corn and other starchy biomass sources (Brooks, 2008). However, this first generation bio-ethanol has been blamed for causing food insecurity. Therefore, attention is being shifted from edible to inedible biomass or lignocellulosic biomass for sustainable ethanol production (Wakil et al., 2013).
Microbial diversity from various habitats such as soil, river water, hypersaline lakes, and insects offers vast opportunities for exploration, as these habitats are the source of useful biomolecules which include enzymes, fatty acids, antibiotics, etc. (Butinar et al. 2005). The development of biotechnology has raised much interest in using cellulase producing microorganisms to convert lignocellulosic biomass from agro-industrial wastes to glucose that can be used in applications such as production of bioethanol (Amita et al., 2006).
Lignocelluloses are major components of biomasses which come from different industries, forestry, agriculture and municipalities. The biodegradation of this lignocellulosic biomass is limited by several factors like crystallinity of cellulose, available surface area, and lignin content (Valcheva et al., 2016). Among these lignocellulosic biomasses, agricultural wastes are more important as this can be converted into products that are of commercial interest such as ethanol, glucose, and single cell protein. Industrial production of ethanol from lignocellulosisic hydrolysates requires the use of microorganisms capable of utilizing the different types of sugar present in it (Balat, 2007; Bettiga et al., 2009). Yeasts have higher ethanol tolerance than bacteria. It is easier to harvest and recycle yeast cells than bacteria cells from the fermentation broth and yeast fermentation is resistant to contaminant from bacteria and viruses. Many yeasts are known to be potential source of extracellular enzymes to produce ethanol and they are Pichia stipitis, Candida shehatae, Candida tropicalis and Pachysolan tannophilus (Saravanakumar et al., 2013), P. salicaria (Kathiresan and Saravanakumar, 2011). These yeasts possess enzymes such as xylose reductase (XR), xylitol dehydrogenase (XDH) and xylulokinase (XK) for direct bioconversion of lignocellulosic materials to ethanol (Khan and Dwivedi, 2013).
Immobilization in biotechnology is the technique used for the physical or chemical fixation of cells, enzymes, or other proteins onto a solid support (Ahmed et al., 2008). Use of natural support materials for cross-linking of cell has added another dimension to a variety of immobilization matrices (Osho et al., 2014). The advantages from such bio-structures are reusability, freedom from toxicity, mechanical strength for necessary support and open spaces within the matrix for growing of cells (Akhtar et al., 2004). Some of natural support matrices reported so far includes Irvingia gabonensis, Detarium microcarpum (Kareem et al., 2014) and Vegetable Sponge (Osho et al., 2014). Mucuna urens is a native West Africa tree which belongs to the genus Mucuna, in the family Fabaceae. Common name of the fruit is ukpo. It is an important tree species in Africa as it bears nuts which are used as thickening agents for dishes such as ogbono soup due to their high content of mucilage (Umoren et al., 2007). It has been observed that Mucuna urens seed flour would be very effective in many industrial food applications because of its stability, gelling, ticking and storage property (Fathima et al., 2010).
Many methods namely entrapment, encapsulation, cross-linking and adsorption method are used for immobilization. Adsorption, entrapment and encapsulation had been extensively studied in cell immobilization (Martins et al., 2013). Although these methods are effective for cell immobilization, they have some disadvantages such as high rate of cell leakage, diffusion limitation, high cost of immobilization and low cell loading. Cross-linking method is effective and durable to cells and enzymes, it is cost effective, cheap and effective (Ali and Khan, 2014). This study reports production of ethanol from corn straw by cellulolytic yeast immobilized in Mucuna urens matrix.
2 Materials and methods
2.1 Substrates
Corn straw was collected from a farm site at Osiele in Abeokuta, Ogun State. The samples were dried and ground to a powder form using an electric blender (Philips INO23) and was sieved using 4 mm mesh. Mucuna urens (Ukpo seed) was purchased from a local market in Abeokuta. The seeds of Mucuna urens were dehulled and blended into powder and defatted using Soxhlet extractor and dried in a hot air oven (Kareem et al., 2013).
2.2 Isolation and characterization of yeast
Soil from 500 m depth of compost piles was collected from the herbarium of Federal University of Agriculture, Abeokuta, Ogun State, Nigeria. The sample was serially diluted and dilutions of 10−3 and 10−5 were plated on Yeast Extract Peptone Dextrose (YEPD) agar. The plates were incubated at 30 °C for 72 h (Wakil et al., 2013). After incubation, yeast colonies which grew on agar plates were characterized based on size, shape and colour. Colonies from different agar plates were subcultured on YEPDA by streak plate technique. The agar plates were incubated at 30 °C for 48 h. Subsequent pure cultures were maintained on agar slant for further characterization and identification. Purified yeast isolates were subjected to standard test and classification schemes as described by Barnett et al. (2000). The tests include those for colony and cell morphology and fermentation tests.
2.3 Screening for amylolytic yeasts
Yeast isolates were qualitatively screened for using Gram iodine solution. Purified yeast isolates were grown on agar plates containing 1% starch agar which were inoculated with pure yeast isolates and were incubated at 30 °C for 3 days. The plates were flooded with grams iodine solution, colonies forming clear zones were selected for quantitative screening (Kareem et al., 2009). Quantitative screening was carried out using YEPD broth containing MgSO4.7H2O, 0.03 g; FeSO4.7H2O, 0.5 g; MnSO4.H2O, 0.16 g; ZnSO4.7H2O, 0.14 g. Culture medium were inoculated with pure yeast isolates and incubated under shaking condition (150 rpm) at 30 °C for 3 days, amylase production was quantified using the method of Kareem et al. (2009).
2.4 Screening for cellulolytic yeast
Yeast isolates were screened for cellulose qualitatively using congo red test. Purified yeast isolates were grown on agar plates containing 1% Carboxyl Methly Cellulose (CMC). Plates were inoculated with pure yeast isolates and were incubated at 30 °C for 3 days and flooded with 1% Congo red solution for 30 min and de-stained with 1 M NaCl solution for 20 min (Saliu, 2012). Quantitative screening was carried out using modified YEPD which consist of 1% CMC, NH4NO3, 0.2 g; KH2PO4, 0.5 g; CaCl2.2H2O, 0.03 g; MgSO4.7H2O, 0.03 g; FeSO4.7H2O, 0.5 g; MnSO4.H2O, 0.16 g; ZnSO4.7H2O, 0.14 g; Tween-80, 0.1 g. Culture media were inoculated with pure yeast isolates and were incubated under shaking condition (150 rpm) at 30 °C for 3 days and cellulase production was quantified according to the method of Saliu (2012).
2.5 Screening for ethanol producing yeast
Purified yeast isolate were screened for fermentative ability using YEPD broth prepared in test tubes containing inverted Durham tube (Wakil et al., 2013). Test tubes were inoculated and incubated at 30 °C for 3 days, isolates were selected based on the volume of gas in Durham tube during the incubation period (Brooks, 2008). Quantitative screening was carried out by distillation using 5% starch according to the method of Wakil et al. (2013).
2.6 Selection of starters
Four yeasts with best amylolytic, cellulolytic and ethanol producing abilities were subjected to optimization studies and were used for amylase, cellulase and ethanol production in submerged fermentation. These yeasts were stored in glycerol; the glycerol (15 ml) in screw-cap vials was autoclaved and allowed to cool. These were added to suspension of the yeasts and were mixed together and stored in freezer at −20 °C.
2.7 Fermentation of corn straw
Yeast strains were grown in 250 mL Erlenmeyer flask that contained 100 mL of fermentation medium containing corn straw (10%). The flask was inoculated with 5% yeast suspension and incubated at 30 °C for 96 h (Hashem et al., 2013).
2.8 Fractional distillation
Distillation of the fermented medium was carried out using 100 mL of each fermented medium which was dispensed into round-bottom flasks fixed to a distillation column enclosed in running tap water. A conical flask was fixed to the other end of the distillation column to collect the distillate. A heating mantle with temperature adjusted to 78 °C was used to heat the round-bottom flask containing the fermented sample (Wakil et al., 2013).
2.9 Determination of quantity of ethanol produced
The distillate collected over a slow heat was multiplied by the density of ethanol (0.8033 g/ml). g/l is equivalent to the yield of 100 g of dried substrate (Wakil et al., 2013).
2.10 Immobilization of yeasts
Yeast suspension was immobilized with Mucuna urens matrix using the method of Kareem et al. (2013). Mucuna urens (10 g) was cross-linked with glutaraldehyde (2.5%) v/v and stirred for 10 min. Yeast suspension (5 ml) was properly mixed with the slurry obtained from cross-linked Mucuna urens. Spherical beads were formed using a syringe. The slurry was dropped into ethanolic formaldehyde (50:50 v/v) for 24 h.
2.11 Optimization studies of ethanol production
Ethanol production from immobilized yeasts was carried out using YEPD broth containing MgSO4.7H2O, 0.03 g/l; urea 0.3 g/l FeSO4.7H2O, 0.5 g/l; MnSO4.H2O, 0.16 g/l; ZnSO4.7H2O, 0.14 g/l and 10% corn straw. Medium was incubated at 30 °C for 72 h (Hashem et al., 2013). Ethanol production from immobilized yeasts was optimized using different parameters such as bead size, substrate concentration, inoculum load, pH and bead reusability. Comparative study was also carried out to compare ethanol production from free and immobilized yeast.
2.12 Statistical analysis
All the experiments were performed in triplicates, results were presented as mean ± standard deviation and were also analyzed by ANOVA using statistical software SPSS version 17. 0.
3 Results and discussion
3.1 Isolation of yeast
A total of twelve (12) yeasts were isolated. All the isolates grew well at 10% NaCl combined with 5% glucose while only two grew at 50% glucose. Sugar fermentation test showed that all the isolates ferment glucose and fructose, two isolates ferment maltose, arabinose and raffinose. Two isolates do not ferment sorbose. Physiological and biochemical tests identified the yeasts as Candida shehatae, Candida tropicalis, Debaryomyces hansenii. Wikerhamomyces chambardii, Saccharomyces cerevisiae, S. diastaticus, Trichosporon beemeri and T aquatile. Saccharomyces cerevisiae were the predominant yeast isolated, they have been reported to be ubiquitous and utilize a wide range of nutrients due to its ability to secrete a large number of digestive enzymes (Saliu, 2012). Abah et al. (2010) identified S. cerevisiae as cellulolytic yeast isolated from rotten iris potato.
3.2 Screening for amylase, cellulase and ethanol producing yeast
All the yeast tested positive for amylase production by showing clear zones on starch agar. The yeast isolates produced zones of clearance from 8.0 to 21.0 mm in diameter. Wikerhamomyces chambardii had the highest halo zones (21.0 mm) followed by D. hansenii (20.0 mm) while T. aquatile had the lowest (8.0 mm) (Data not shown). Quantitative screening showed that Wikerhamomyces chambardii had the highest amylase activity (551.54 U/ml), the ability of this yeast to hydrolyze this agro-waste faster than others could also be that the yeasts possess genes that code for rapid cellulase production (Rai et al., 2012). This is followed by S. diastaticus (527.21 U/ml) while the lowest was recorded for T. aquatile (100.78 U/ml) (Table 1). All isolates also tested positive for cellulase production by showing clear zones on CMC agar. Wikerhamomyces chambardii produced the highest halo zone (35.0 mm), Wickerhamomyces spp. had been described by Virginia et al. (2010) as novel yeast from natural environments, which are related to Candida spp. Candida spp. also produced cellulase enzyme, this is in agreement with the work of Sadaf and Abdul (2013), where C. tropicalis produced cellulase from environmental samples fermented in mineral salt medium. Result of the quantitative screening shows that the highest cellulase activity was produced by W. chambardii (174.67 U/ml) followed by S. diastaticus (161.38 U/ml) while the least cellulase activity was observed in T. aquatile (72.35 U/ml) (Table 1). Among the isolates, eight showed fermentative ability (Data not shown) with S. diastaticus showing the best fermentative ability, this could be due to presence of pyruvate decarboxylase (PDC) and alcohol dehydrogenase (ADH) enzymes in the yeast (Wakil et al., 2013). Abah et al. (2010) had described S. diastaticus as amylolytic and cellulolytic yeast that can produce ethanol). Quantitative screening for ethanol production showed that S. diaststicus had the best ethanol producing ability (31.96 g/l), followed by W. chambardii (26.13 g/l), this yeast had just been recently added to group of yeasts that produce ethanol from lignocellulosic materials (Kurtzman, 2011). Candida species also produced ethanol, C. shehatae had been reported by Ayumi et al. (2015) as ethanol producing yeast. Trichosporon beemeri produced the least (13.62 g/l) (Table 1). Each value is a mean of 3 readings ± standard deviation. Values in the same column followed by the same letter (or subscripts) are not significantly different (p ≤ 0.05) according to Duncan’s Multiple Range Test.
Yeast Isolates
Enzyme activity (U/ml)
Ethanol (g/l)
Amylase
Cellulase
C. shehatae
346.63 ± 11.76
161.38 ± 23.21
20.13 ± 6.27
C. tropicalis
319.50 ± 34.63
164.67 ± 24.54
22.96 ± 10.58.
S. cerevisiae
171.63 ± 20.28
100.38 ± 9.67
13.59 ± 2.37
D. hansenii
411.84 ± 18.36
142.50 ± 18.03
14.40 ± 0.96
W. chambardii
551.54 ± 32.54
174.67 ± 23.21
26.13 ± 6.27
T. beemeri
219.50 ± 22.21
135.66 ± 10.34
13.62 ± 2.87
S. cerevisiae
158.78 ± 20.62
94.86 ± 6.23
20.53 ± 3.44
W. chambardii
276.06 ± 34.63
139.21 ± 20.67
Nill
S. cerevisiae
264.51 ± 33.81
125.78 ± 22.54
Nill
S.diastaticus
527.21 ± 25.80
161.22 ± 20.21
31.96 ± 10.58
T. aquatile
100.78 ± 4.48
72.35 ± 7.61
Nill
D. hansenii
229.65 ± 33.32
104.81 ± 9.44
Nill
Wikerhamomyces chambardii, Candida shehatae, Candida tropicalis and Saccharomyces diastaticus were selected for ethanol production. These yeasts were deposited in the Culture Collection Centre of the Federal University of Agriculture, Abeokuta, Ogun -State, Nigeria as W. chambardii ABMC1, C. shehatae FUMC1, C. tropicalis FUMC2 and S. diastaticus ABMC2.
3.3 Effect of bead size on bioethanol production from corn straw
Production of ethanol from corn straw by yeasts immobilized in 3, 4, 5 and 6 mm diameter beads is shown in Fig. 1. There was gradual increase in volume of ethanol produced with bead size from 3 mm to 4 mm and thereafter decrease with increase in bead size (Fig. 1). As illustrated in Fig. 1, highest volume of ethanol was obtained with bead size 4 mm, which indicate that at this size, the number of pore spaces made available is highest and the number of cell occupying each space is maximum for substrate utilization (Kareem et al., 2013). Bigger sizes showed decrease in bead size, this showed that smaller beads have more surface area per unit volume and hence more productivity (Kareem et al., 2014). Cells in bigger beads do not have easy access to the substrate, thus it reacts with the molecules and forms product (Sevda and Rodrigues, 2011). Immobilized S. diastaticus produced highest volume of ethanol (52.1 g/L) while immobilized C. shehatae produced 26.3 g/L which is d least ethanol from hydrolysis of corn straw (Fig. 1). This could have been due to presence of pyruvate decarboxylase present in Saccharomyces spp. which is an enzyme responsible for ethanol production. Wakil et al. (2013) had described Saccharomyces spp. as amylolytic and cellulolytic yeast that can produce ethanol.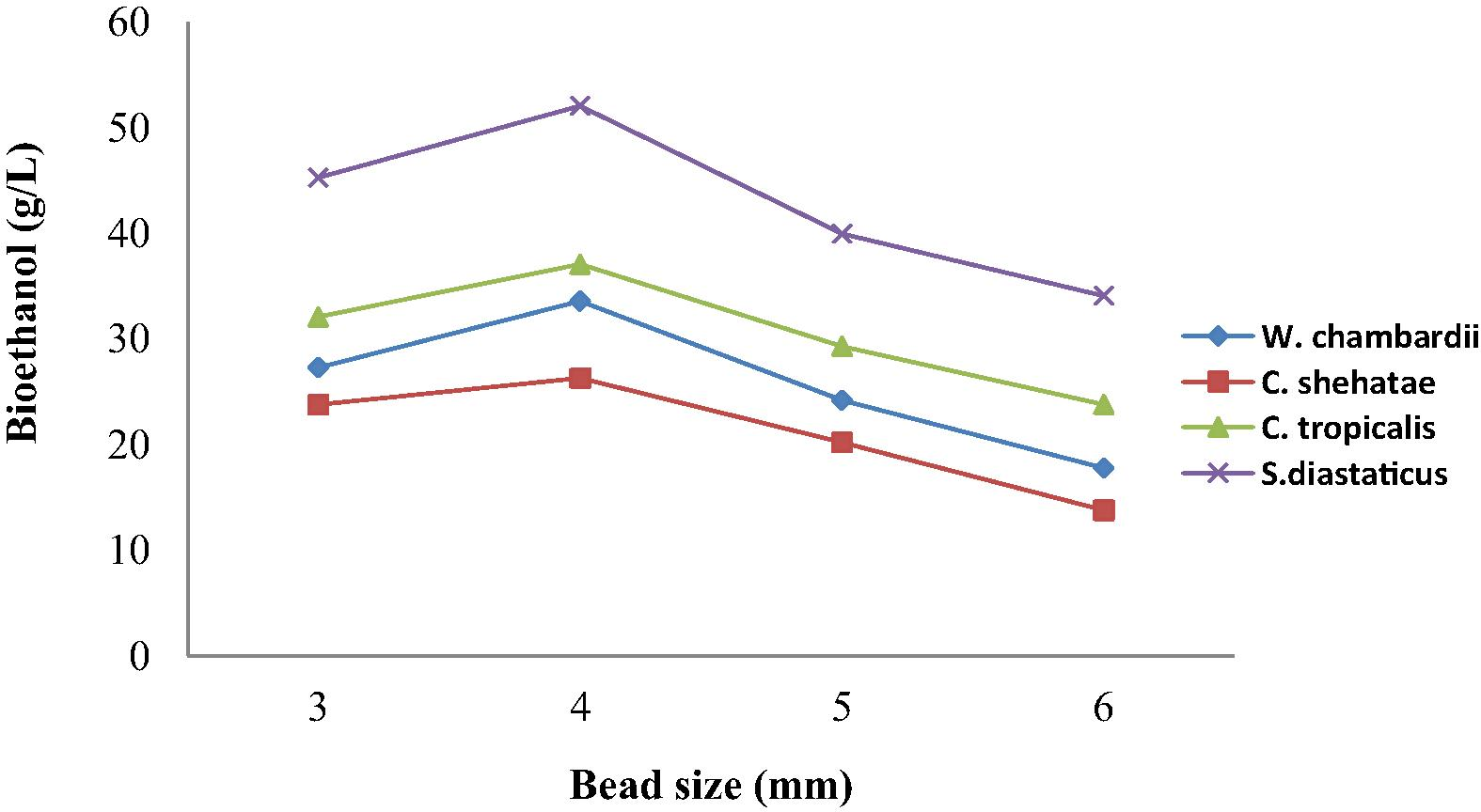
Effect of bead size on bioethanol production from corn straw.
3.4 Effect of corn straw concentration on bioethanol production
The result presented in Fig. 2 showed effect of corn straw concentration on ethanol production by immobilized yeast cells. Optimum substrate concentration of corn straw for ethanol production was observed to be 10%, while 15% produced the least ethanol (Fig. 2). Productivity decreased by increasing substrate concentration above 10%. Increase in corn straw concentration could have led to high concentration of complex sugars in the fermentation medium and this could have had high inhibitory effect on yeast growth and their capability to produce ethanol (Kumar and Murthy, 2011). Iqbal et al. (2010) reported that increase in substrate concentration has a dynamic influence on cellulose hydrolysis, hence affect ethanol production. Maximum volume of ethanol was produced by immobilized S. diastaticus (55.27 g/L), followed by C. tropicalis (40.32 g/L), while immobilized C. shehatae produced the least volume of ethanol (30.19 g/L) from hydrolysis of corn straw (Fig. 2). Savvharomyces diastaticus has been reported by Prasad et al. (2007) as yeast that has an efficient anaerobic sugar metabolism, tolerates inhibitory industrial substrates better than other microorganisms and ferment hexose abundantly present in lignocelluosic hydrolysates, such as glucose, mannose and galactose with high yield and productivity.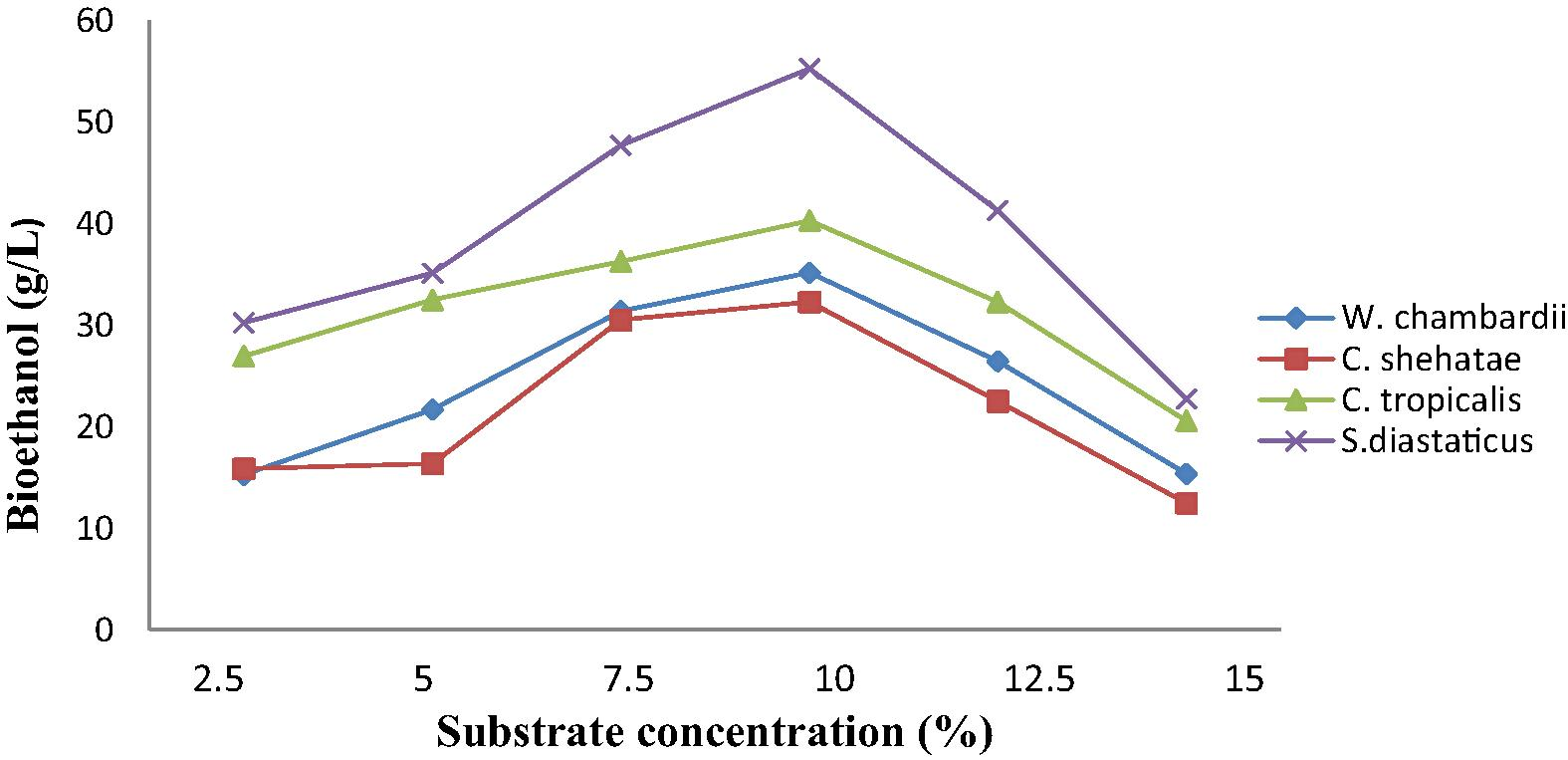
Effect of corn straw concentration on ethanol production by immobilized yeasts.
3.5 Effect of initial pH on bioethanol production from corn straw
Effect of pH on ethanol production from corn straw by immobilized yeast cells are presented in Fig. 3. The result reveals that 4.5 was the optimum pH for ethanol production except for S. diaststicus, which was 5.0. This result showed that cells entrapped in Mucuna urens matrix are protected from environmental conditions (Nishant et al., 2014). Least ethanol was produced with pH 7, this might be due to the fact alkaline pH has an inhibitory effect on growth of yeasts. Data illustrated in Fig. 3 clearly indicated that immobilized cells of C. tropicalis had highest volume of ethanol (40.5 g/L), followed by immobilized cells of W. chambardii and C. shehatae (34.8 g/L), while immobilized S. diastaticus produced least ethanol (31.5 g/L) (Fig. 3). Adriana et al. (2015) reported that ethanol fermentation from discarded carrots using immobilized S. cerevisiae had its optimum at pH 5.5.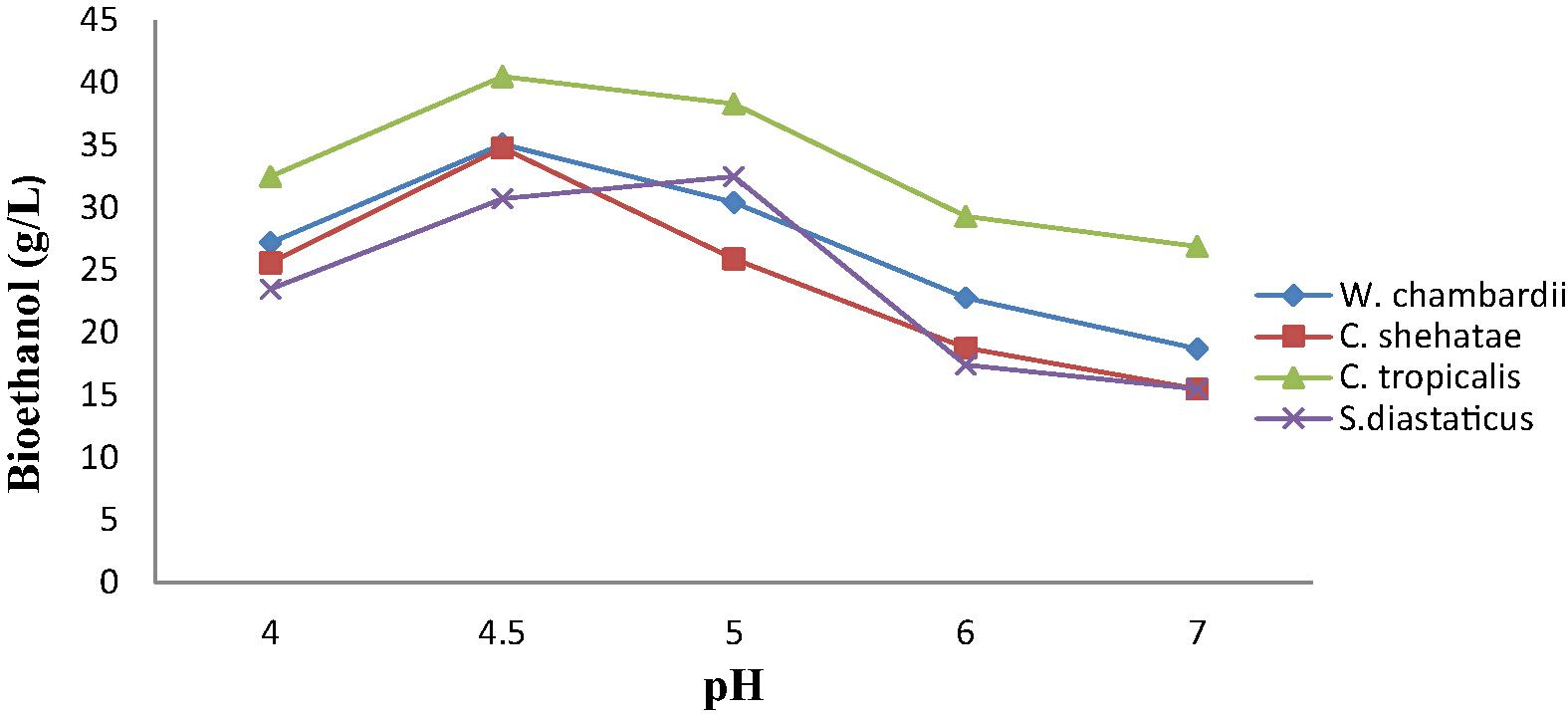
Effect of initial pH on ethanol production from corn straw by immobilized yeasts.
3.6 Effect of inoculum load of on bioethanol production from corn straw
Fig. 4 showed that inoculum load of the immobilized yeasts have an important effect on ethanol production. Different amount of standard inocula (5, 7.5, 10, 12.5 and 15% v/v) showed varying effect on ethanol. The Figure showed that immobilized inoculum concentration of 10% v/v gave the maximum ethanol production, followed by 7.5% while 15% produced the least ethanol during corn and sorghum straw hydrolysis (Fig. 4). The reason behind the low production rate with 5 and 7.5% inoculum concentration can be attributed to lower cell density in the bead at low cell loadings (Majolagbe et al., 2010). The positive effect of increasing cell loading from 5 to 10%, which led to improve ethanol production, was the same results obtained for the production of gluconic acid by Aspergillus niger immobilized in Calcium alginate beads (Dong et al., 2013). Higher concentration of cell did not lead to improved ethanol yield. This may be attributed to a decrease in mechanical strength of gel particles as cell loading increases (Dong et al., 2013). Immobilized S. diastaticus produced the highest volume of ethanol of 49.10 g/L, followed by W. chambardii and C. tropicalis which had the same volume of ethanol (44.2 g/L), while the least ethanol was produced by C. shehatae (40.1 g/L) (Fig. 4). Among all the yeast S. diastaticus was proved more successful for ethanol production as compared to other species, this is due to the fact that some species adopt different metabolic pathways by having special genes or special enzymes such as invertase genes and invertase enzymes respectively for the conversion of hydrolyzed sugars to ethanol or other metabolites (Fregonesi et al., 2007).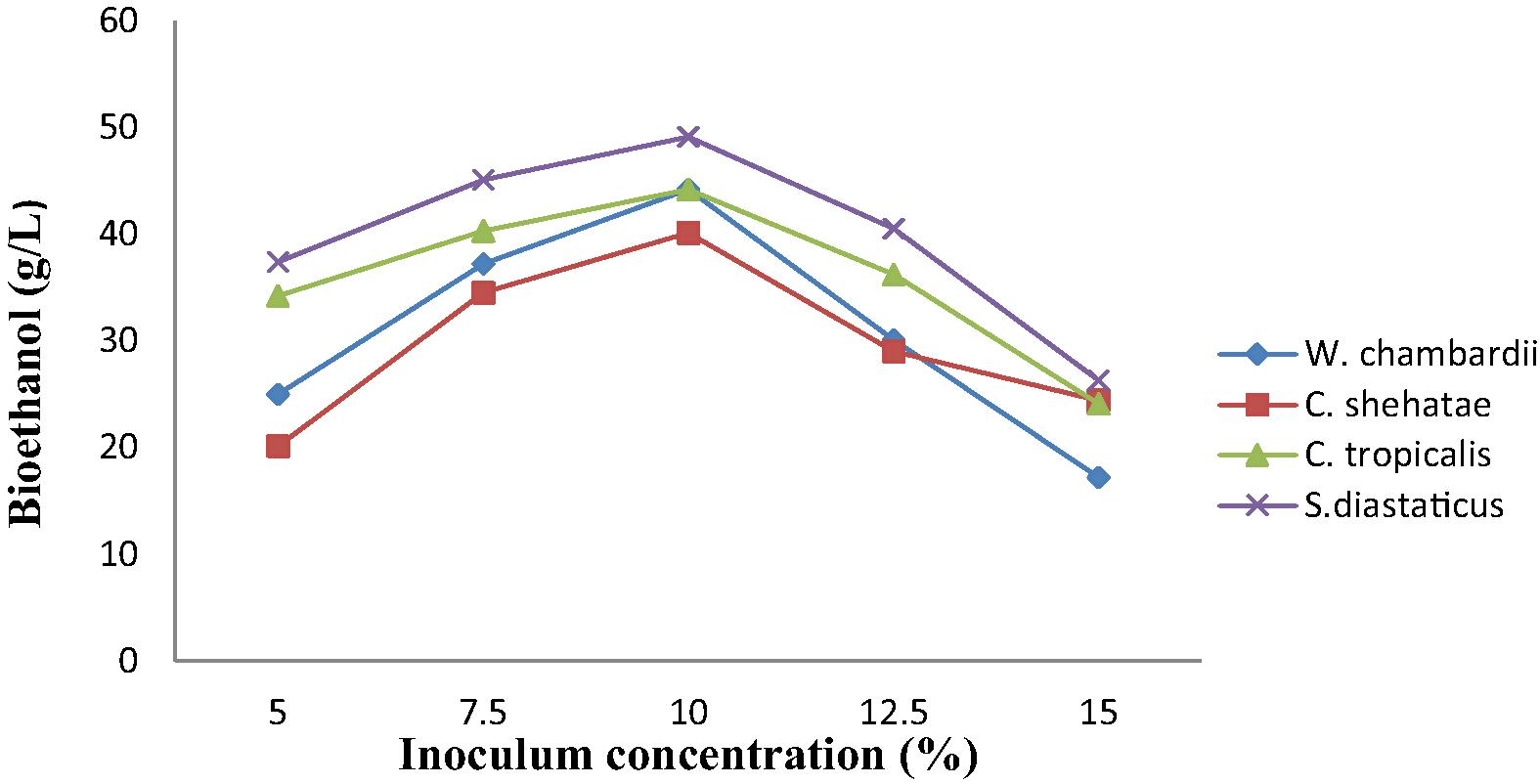
Effect of inoculum load on bioethanol production from corn straw.
3.7 Effect of reusability of immobilized yeasts on bioethanol production from corn straw
Fig. 5 showed effect of repeated use of immobilized yeasts on ethanol production from corn straw. Results showed that immobilized yeast cells could be used repeatedly without obvious loss of activity. It was observed that immobilized W. chambardii produced highest ethanol from corn straw during cell re-use. It retained 68, 44 and 37% of ethanol yield for second, third and fourth cycles respectively (Fig. 5). This result is slightly better than those reported by Anwar et al. (2009) who found that immobilized cells retained 30% of its initial activity After the fifth cycles, ethanol yield was 12%, this may be as a result of clogging of cells inside the matrix (Kareem et al., 2013). Candida tropicalis had least ethanol after cell reusability (Fig. 5). Candida shehatae had 53, 42 and 31% of ethanol yield for second, third and fourth cycles respectively while C. tropicalis had least ethanol after cell reusability.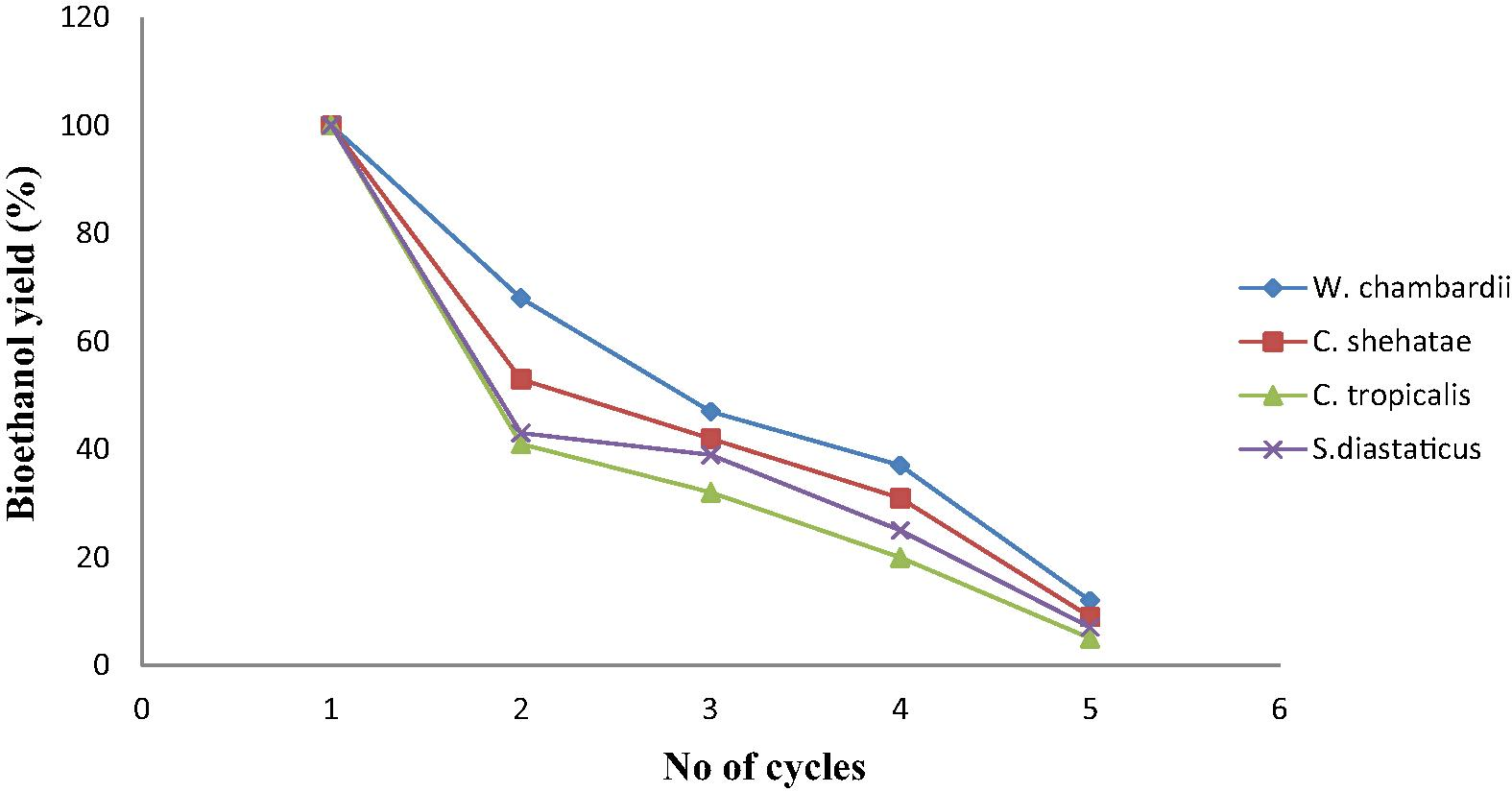
Reusability of immobilized yeasts on bioethanol production from corn straw.
3.8 Comparative ethanol production by free and immobilized cells of S. Diaststicus
Table 2 presents ethanol production by free and immobilized cells of S. diastaticus. Immobilized cells produced higher volume of ethanol (24.90 g/L) while free cells had lower ethanol production of 15.40 g/L. The result agreed with Chandel et al. (2009) that cells of S. cerevisiae immobilized on sugarcane pieces produced ethanol faster than free cells. According to Rattananpan et al. (2011), immobilization protected microbial cells against the possible toxic effects in the fermentation. As a result, the fermentation performance of the immobilized yeast was improved in comparison with that of the free yeast. Immobilized yeast cells had been reported to have advantages such as higher productivity and decreased contamination over free cell (Kareem et al., 2013). Note: each value is a mean of 3 readings ± standard deviation. Various superscripts in table indicate significant differences (p < 0.05).
Fermentation time
Immobilized cells
Free cells
0
0.00 ± 0.000a
0.00 ± 0.000a
24
11.70 ± 1.184b
8.50 ± 0.564b
48
15.40 ± 1.125c
9.40 ± 1.212c
72
24.90 ± 1.068e
15.40 ± 1.125e
96
15.40 ± 1.125d
12.81 ± 1.124d
4 Conclusion
From the study, it was concluded that cellulolytic yeasts (Wikermomyces chambardii, Candida shehatae, Candida tropicalis and Saccharomyces diastaticus) were successfully immobilized on Mucuna urens by cross-linking method with glutaraldehyde and these yeasts can directly produce bioethanol from corn straw through a single-step process.
References
- Potential of wild strain Saccharomyces cerevisiae in Ethanol production. American-Eurasian J. Sci. Res.. 2010;5(30):187-191.
- [Google Scholar]
- Optimization of ethanol fermentation from discarded carrots using immobilized Saccharomyces cerevisiae. Int. J. Energy Environ. Eng.. 2015;6:129-135.
- [Google Scholar]
- Optimization, immobilization of extracellular alkaline protease and characterization of its enzymatic properties. Res. J. Agric. Biol. Sci.. 2008;4(5):434-446.
- [Google Scholar]
- Removal and recovery of nickel (II) from aqueous solution by loofa sponge-immobilized biomass of Chlorella sorokiniana: characterization studies. J. Hazard Mater.. 2004;108:85-94.
- [Google Scholar]
- Comparative studies on bioethanol production with immobilized cells of Saccharomyces cerevisiae local strains and Saccharomyces cerevisiae MTCC 170 by stationary and shaking fermentation methods. Int. J. Curr. Microbiol. Appl. Sci.. 2014;3(1):380-390.
- [Google Scholar]
- Improvement of the quality of whole wheat bread by supplementation of xylanase from Aspergillus foetidus. Bioresour. Technol.. 2006;97:2047-2053.
- [Google Scholar]
- Calcium Alginate: a support material for immobilization of proteases from newly isolated strain of Bacillus subtilis KIBGE-HAS. World Appl. Sci. J.. 2009;7(10):1281-1286.
- [Google Scholar]
- Repeated-batch ethanol production from sweet sorghum juice by Saccharomyces cerevisiae immobilized on sweet sorghum stalks. Energies. 2012;5:1215-1228.
- [Google Scholar]
- Direct ethanol production from starch using a natural isolate, Scheffersomyces shehatae: toward consolidated bioprocessing. Sci. Rep.. 2015;5:9593.
- [Google Scholar]
- Global bio-fuel processing and production trends. J. Energy Explor. Exploit.. 2007;25:195-218.
- [Google Scholar]
- Yeasts characteristics and identification (third ed.). Cambridge University Press; 2000. pp 11-39
- Arabinose and xylose fermentation by recombinant Saccharomyces cerevisiae expressing a fungal pentose utilization pathway. Bioresour. Technol.. 2009;23:112-121.
- [Google Scholar]
- Ethanol production potential of local yeast strains isolated from ripe banana peels. Afr. J. Biotechnol.. 2008;7(20):3749-3752.
- [Google Scholar]
- Bioconversion of de-oiled rice bran (DORB) hemicellulosic hydrolysate into ethanol by Pichia Stipitis NCM3499 under optimized conditions. Int. J. Food Eng.. 2009;5(1):8.
- [Google Scholar]
- Hydrogel, aerogel and film of cellulose nanofibrils functionalized with silver nanoparticles. Carbohydrate Polysaccharide. 2013;95:760-767.
- [Google Scholar]
- Nutritional and antinutritional assessment of Mucuna pruriens (L.) DC var. pruriens an underutilized tribal pulse. Adv. Biores.. 2010;12:79-89.
- [Google Scholar]
- Continuous fermentation of sugar cane syrup using immobilized yeast cells. Bioresour. Bioeng.. 2007;97:48-52.
- [Google Scholar]
- Optimization of the fermentation conditions for ethanol production by new thermotolerant yeast strains of Kluyveromyces sp. Afr. J. Microbiol. Res.. 2013;7:4550-4561.
- [Google Scholar]
- Media optimization for hyperproduction of carboxymethylcellulase using proximally analysed agro-industrial residues with Trichoderma harzianum under solid state fermentation. Int. J. Agro Veterin. Med. Sci.. 2010;4(2):47-55.
- [Google Scholar]
- Cow pea: A novel substrate for solid state production of amylase by Aspergillus oryzae. Afr. J. Microbiol. Res.. 2009;3(12):974-977.
- [Google Scholar]
- Production of citric acid by Aspergillus niger immobilized in Detarium microcarpum matrix. Malaysian J. Microbiol.. 2013;9(2):161-165.
- [Google Scholar]
- Removal of Mn(Ii) and Fe(Ii) by Aspergillus sp. Tu-Gm14 immobilized on Detarium Microcarpum matrix. Glob. NEST J.. 2014;16(4):597-608.
- [Google Scholar]
- Bio-ethanol production by marine yeasts isolated from coastal mangrove sediment. Int. Multidisciplin. Res. J. Biotechnol.. 2011;1(1):19-24.
- [Google Scholar]
- Fermentation of Biomass for Production of Ethanol. Univ. J. Environ. Res. Technol.. 2013;3(1):1-13.
- [Google Scholar]
- Impact of pretreatment and downstream processing technologies on economics and energy in cellulosic ethanol production. Biotechnol. Biofuels. 2011;4:27.
- [Google Scholar]
- Kurtzman, C.P., 2011. 2008. Wickerhamomyces Kurtzman, Robnett and Basehoar-Powers. The Yeasts, a Taxonomic Study, (fifth ed.), Kurtzman, C. P., Fell J.W., Boekhout, T. (Eds.) Amsterdam, Elsevier, pp. 899–917.
- Biodesulphurization of crude-oil using immobilized spores of Rhizopus Nigricans. Adv. Nat. Appl. Sci.. 2010;4(1):29-32.
- [Google Scholar]
- Immobilization of microbial cells: A promising tool for treatment of toxic pollutants in industrial wastewater. Afr. J. Biotechnol.. 2013;12(28):4412-4418.
- [Google Scholar]
- Deviation in production of bioethanol applying stress tolerant yeast: a current approach. Int. J. Pharm. Sci. Invent.. 2014;3(5):10-19.
- [Google Scholar]
- Transesterification of Jatropha seeds oil by vegetative sponge immobilized lipase of Alternaria sp. MGGP 06 for fatty acid methyl ester production under optimized conditions. Pet. Technol. Dev. J.. 2014;1:56-70.
- [Google Scholar]
- Ethanol production from sweet sorghum syrup for utilization as automotive fuel in India. J. Energy Fuels. 2007;21(4):2415-2420.
- [Google Scholar]
- Optimization of process parameters for cellulose production by novel thermotolerant yeast. Bioresources. 2012;7(4):5401-5414.
- [Google Scholar]
- Ethanol production by repeated batch and continuous fermentations of blackstrap molasses using immobilized yeast cells on thin-shell silk cocoons. J. Appl. Energy. 2011;88:4400-4404.
- [Google Scholar]
- Isolation and characterization of cellulose degrading Candida tropicalis W2 from environmental samples. Pak. J. Zool.. 2013;45(3):809-816.
- [Google Scholar]
- Saliu, B. K., 2012. Production of ethanol from some cellulosic waste biomass hydrolyzed using fungal cellulases. Ph.D Thesis, University of Ilorin, pp. 160.
- Bioethanol production by mangrove-derived marine yeast Sacchromyces cerevisiae. J. King Saud Univ. Sci.. 2013;25:121-127.
- [Google Scholar]
- The making of pomegranate wine using yeast immobilized on sodium alginate. Afr. J. Food Sci.. 2011;5(5):229-304.
- [Google Scholar]
- Assessment of the nutritional quality of processed horse eye bean (Mucuna urens) by in vivo rat bioassay. J. Food Agric. Environ.. 2007;5(2):132-135.
- [Google Scholar]
- Topchemical kinetics mechanism of cellulose hydrolysis on fast-growing tree species. React. Kinet. Catal. L.. 2016;71:231-238.
- [Google Scholar]
- Wickerhamomyces patagonicus sp. nov., an ascomycetous yeast species from Patagonia, Argentina. Int. J. Syst. Evol. Microbiol.. 2010;60:1693-1696.
- [Google Scholar]
- Production of bioethanol from palm oil mill effluent using starter cultures. N.Y. Sci. J.. 2013;6(3):77-85.
- [Google Scholar]







