Translate this page into:
Biochemical evaluation with symptoms of gastrointestinal tract manifestations – A systemic review
⁎Corresponding authors. Rawan.alsomali@hotmail.com (Rawan A. Alsomali), arwa.mwadaan@gmail.com (Arwa M. Wadaan)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Upper Gastrointestinal Bleeding (UGIB) is one of the leading causes of morbidity and mortality. Gastrointestinal tract diseases lead to several reasons, the biochemical test playing an important role to predict the intestinal disease. The drug manifestations specifically, Nonsteroidal anti-inflammatory drugs (NSAID) bacteria Helicobacter pylori infection, alcohol abuse, smoking, obesity, anxiety, and depression identified as risk factors. This review aimed to assess major causes of upper gastrointestinal bleeding. All relevant English-language articles addressing the epidemiology, severity, frequency, risk factors, medications, and incidence of upper gastrointestinal bleeding were included in the study. Two researchers screened and reviewed relevant full-text papers for inclusion using the exclusion and inclusion criteria and 22 articles were included. One investigator extracted data about study sample size, patients' age, risk factors, causes, presentation, comorbidities, drug intake and addiction in patients with upper GI bleeding. Another investigator independently reviewed data accuracy. UGIB was found to be more common between the fifth and eighth decades of life, and it is extremely rare between the ages of 16 and 80. Upper gastrointestinal bleeding is most commonly caused by non-variceal bleeding, with duodenal ulcers, a subtype of peptic ulcer, being the most common cause. The main risk factors were NSAID use and Helicobacter pylori infection. The most common symptoms were hematemesis, melena, and, less frequently, stomach discomfort. The main risk factors of UGIT bleeding were the use of NSAID and Helicobacter pylori infection. Health education about NSAID side effects and proper use and application of proper screening tool for at-risk patients may aid in the prevention of UGIB and reduce the burden of this health problem.
Keywords
Helicobacter pylori
Anti-inflammatory drugs
Gastrointestinal bleeding
Prevalence
Biochemical analysis
1 Introduction
Helicobacter pylori a bacterium is a main cause of chronic gastritis which leads to severe gastrointestinal infections (de Brito et al., 2019; FitzGerald and Smith, 2021). An intraluminal bleed known as upper gastrointestinal bleeding (UGIB) can happen anywhere between the esophagus and the Treitz ligament (DiGregorio and Alvey, 2023). Due to its recurrence, UGIB is four times more common than lower gastrointestinal bleeding (LGIB) and is twice as common in men than in women. This bleeding is regarded as a global burden, with an estimated 80–150 cases out of every 100,000 people and a mortality rate of about 2–15 %. According to the clinical presentation, doctors must categories the kind of GI bleeding (Romcea et al., 2013).
Biochemical analysis adheres the amount of blood loss, GI bleeding may be overt, occult, or cryptic. Acute hematemesis, hematochezia, or chronic melena are possible symptoms (Abdelshaheed and Goldberg, 1997; Hreinsson et al., 2013). This bleeding is a potentially fatal medical emergency that needs to be treated right away by a surgical team. The causes of UGIB can range from nonvariceal, such as peptic ulcer disease (PUD), to variceal, such as esophageal and gastric varices, to many other types, such as chronic liver disease, drug-induced gastrointestinal bleeding, portal hypertension, and malignancy (Ugiagbe and Omuemu, 2016; Masoodi et al., 2019).
For roughly 10 % of the population each year, UGIB continues to be one of the leading causes of morbidity and mortality (Alruzug et al., 2021). However, PUD continues to be the primary cause of UGIB (Youssouf et al., 2020). In older, comorbidly ill patients and NSAIDS users, risk factors for PUD led to an increase in UGIB, which also turned out to be dose-related (Youssouf et al., 2020; Sibiany, 2012). Endoscopy is the preferred method of investigation to pinpoint the underlying cause of acute bleeding and ultimately create a successful management strategy (Ugiagbe and Omuemu., 2016; Alatawi et al., 2020; Strate et al., 2016). Despite the high percentage of UGIB incidences yearly, endoscopic therapy has contributed in the reduction of re-bleeding threat, thus a major drop in mortality incidences over the past 30 years (Youssouf et al., 2020).
Patients underestimate and misuse medication to treat their underlying diseases. Unfortunately, due to the currently adapted hospital policies and pharmacy practices to patients' use of these medication supplements is complicated by a lack of knowledge of regulations and safety data for these products. Health care professionals are aware of these adverse effects and drug interactions that can occur (Almadi et al., 2021). Medicines known to be associated with an increased risk of GI bleeding low-dose aspirin (LDA), low-molecular-weight heparin (LMWH), non-steroidal anti-inflammatory drugs (NSAIDs), proton pump inhibitors (PPIs), selective serotonin receptor antagonists (SSRIs) and platelet inhibitors (Hreinsson et al., 2013; Almadi et al., 2021; Kamboj et al., 2019).
The reasons for using these medicines are lack of knowledge of the amount they take without consulting a physician these drugs sometimes may be acquired over the counter. Other causes of GITB could alcohol abuse, smoking that can lead to digestive problem, obesity as well as anxiety and depressions which leads to stress and different type of disease are risk factors for erosive complications (Masoodi et al., 2019; Kamboj et al., 2019).
Upper gastrointestinal bleeding is a common yet serious medical presentation that is seen in Emergency department or even in surgical words (Singh and Panigrahi, 2013; Blackbourne, 2018). This presentation requires the physicians to be fully oriented and knowledgeable about this bleeding since it could be life threatening condition (Dewan et al., 2014; Suchartlikitwong et al., 2015). The aim of this review was to identify the common causes of upper gastrointestinal bleeding and to investigate the role of certain medication and diseases in causing upper gastrointestinal bleeding.
2 Inclusion and exclusion criteria
This study is a systematic review research approach of the Etiology of upper gastrointestinal bleeding. To identify primary papers relevant to our review, we conducted a systematic and comprehensive search of numerous electronic databases, including PubMed and Google Scholar, with each database searched separately. Upper gastrointestinal bleeding keywords are based on Medical Subject Headings of Variceal and non-variceal upper gastrointestinal bleeding, epidemiology, prevalence, and risk factors were thoroughly applied line by line and reproduced in every source database.
The inclusion criteria were original research articles published in reviewed journals that primarily focused on the epidemiology, severity, frequency, risk factors, medications, and incidence of upper gastrointestinal bleeding. Articles that were conducted All descriptive-analytical cross-sectional, and epidemiological studies, as well as cohort studies with appropriate methods that were included, observational, retrospective, and prospective studies were published between 2012 and 2022 as well as previous studies that focused on worldwide populations, were included. This study excludes enclosed case reports, case series research designs, and any pediatric-related research in Fig. 1.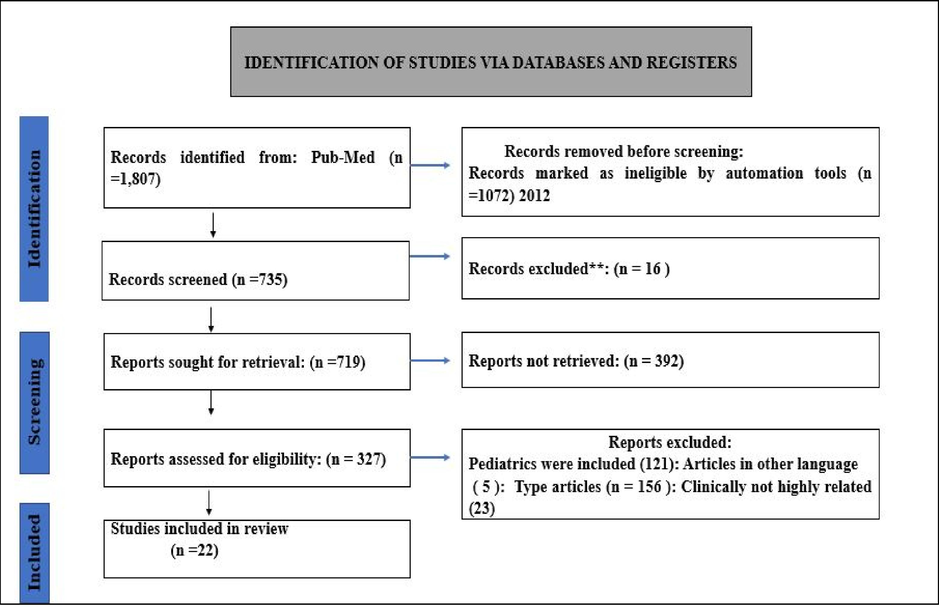
Identification of upper gastrointestinal bleeding related studies from the database.
2.1 Study selection
This article of the search results was reviewed, and requested and appraised. Relevant full-text papers were screened, and reviewed for inclusion by two researchers based on the exclusion and inclusion criteria. Any disputes between writers were settled by a conversation with the other four authors. This assured that only publications related to the study topics were included. Both reviewers found these papers acceptable for analysis and relevant for inclusion in our documented review. Around 45 publications were chosen as the most clinically relevant out of 125 articles indexed between 2012 and 2022, and their full texts were evaluated. A total of 22 of the 45 were included after a thorough examination. Additional referenced articles were used in the introduction section of the research shown in Fig. 1.
2.2 Upper gastrointestinal bleeding
This study reviewed numerous studies on upper gastrointestinal bleeding that revealed a range of results. First, 42 ± 18 years is the common mean age commonly cited as being in the fifth decade's years (Romcea et al., 2013; Ugiagbe and Omuemu., 2016) (Table 1). Only one study (Masoodi et al., 2019) revealed the extremely extreme ages of adult patients, who ranged from 16 to 80 years old. The study, men made up more than 50 % of the cases of upper gastrointestinal bleeding, making them the more prevalent gender (Alruzug et al., 2021; Youssouf et al., 2020) (Table 1). N.B.: NA = not applicable.
Name of article
Total of patients
Average age
Female patients
Male
Patients
1
Spectrum of upper gastrointestinal hemorrhage in coastal Odisha ((Singh and Panigrahi., 2013)
608
42 ± 18.2
NA
NA
2
The etiology of upper gastrointestinal bleeding in cirrhotic patients ((Romcea et al., 2013)
1,284
Average 56.76
16–86489
38.09 %795
61.91 %
3
Epidemiology of upper gastrointestinal bleeding and helicobacter pylori infection (Suchartlikitwong et al.,2015)
3,488
63.3 ± 15.94 years,
13–103 years1,446
41.5 %2,042
58.5 %
4
Spectrum of upper gastrointestinal bleed ((Parvez et al., 2016).
337
55.11 ± 14.8 years
14–85 years
65
19.3 %272
80.7 %
5
A prospective cohort study of patients presenting to the emergency department (Shenoy et al., 2021)
210
51 ± 16.8
50
25.8 %160
76.2 %
6
Predictors of variceal or nonvariceal source of upper GI bleeding (Matei et al., 2013)
533
16 were excluded
517NA
NA
NA
7
Presentation and endoscopic findings (M. Sibiany., 2012)
1149
49.74 ± 1 year
270
23.5 %879
76.5 %
8
A study of clinical and endoscopic profile of acute upper GI bleeding (Dewan et al., 2014)
120
14–88
48.76 ± 17.19
30
25 %90
75 %
9
Etiology of upper GI bleeding in the university of Benin ((Ugiagbe and Omuemu., 2016).
1084
14–90 years
51.48 ± 17.5102
32.8 %209
67.2 %
10
Etiology and endoscopic profile of middle aged and elderly ((Mahajan and Chandail, 2017)
1270
40 – 72
489
38.50 %781
61.50 %
11
Time trends of causes of upper gastrointestinal bleeding and endoscopic findings ((Alruzug et al., 2021)
2075
56.8
(18–113) years667
32.1 %1408
67.9 %
12
Upper gastrointestinal bleeding: Incidence, etiology and outcomes in a population-based setting ((Hreinsson et al., 2013)
1731
NA
NA
NA
13
Findings of Esophagogastroduodenoscopy in Patients Suspected of Upper Gastrointestinal Bleeding Referred to the Main Endoscopy Unit at King Fahad Specialist Hospital ((Alatawi et al., 2020).
327
18–67
Minimum = 18
Median = 45
Maximum = 6726
35.6 %47
64.4 %
14
Upper gastrointestinal bleeding: Causes and patient outcomes ((Almadi et al., 2021)
259
Mean age 57.1
18.01 %NA
66.8 %
15
Variceal hemorrhage: Saudi tertiary center experience of clinical presentations, complications and mortality ((Fallatah et al., 2012).
125
NA
NA
NA
16
Risk factors for mortality among patients admitted with upper gastrointestinal bleeding at a tertiary hospital: A prospective cohort study ((Moledina and Komba., 2017)
170
Median age
40
31–56.349
28.8 %121
71.2 %
17
Retrospective study of etiology of non-variceal acute gastrointestinal bleeding in Eastern Himalayan region of India in Sikkim ((Bhutia & Lamtha et al., 2019)
127
70 were excluded16–72
Mean age
48.5
NA
NA
18
Current Trends in Etiological Profile of Acute Upper Gastrointestinal Bleeding in Northern India: A Retrospective Analysis of 5-Year Endoscopic Data (Bodh et al., 2021).
1513
NA
384
(25.4)1129
(74.6)
19
Causes of Upper Gastrointestinal Bleeding Among Pilgrims During the Hajj Period in the Islamic Years 1437–1439 (2016–2018) ((Youssouf et al., 2020).
93
63.37 ± 12.83
32
34.41 %61
65.59 %
20
in acute GI bleeding a single center study in the western region of Saudi Arabia ((Masoodi et al., 2019)
120
58.4 ± 18.7
76
21
Primary non-variceal upper gastrointestinal bleeding in NSAID and low-dose aspirin users: Development and validation of risk scores for either medication in two large Dutch cohorts (de Groot et al., 2014)
421
NA
NA
NA
22
Risk of upper and lower gastrointestinal bleeding in patients taking nonsteroidal anti-inflammatory drugs, antiplatelet agents, or anticoagulants (Lanas et al., 2015)
1008
66.6 ± 16.0
NA
NA
Hematemesis is a common initial symptom in these cases, and its severity varies depending on the underlying causes (Ugiagbe and Omuemu., 2016; Sibiany, 2012). Melena (Alatawi et al., 2020). was the second most typical presentation despite the fact that hematemesis and melena have been reported together in some cases (Almadi et al., 2021; Singh and Panigrahi, 2013). Abdominal pain was the least frequent presenting symptom (Youssouf et al., 2020) (See Table 2).
Study
Country
Study design
Inclusion criteria
Study outcome
Lanas et al., 2015
Spain
case-control study
hospitalized patients for gastrointestinal bleeding 20–90 years old non-variceal GI bleeding with no HX of liver disease, coagulation disorders, or malignancy.
Anticoagulants, low-dose aspirin, NSAIDs, and other non-aspirin-APA drugs are associated with increased risk of upper and lower gastrointestinal bleeding. Use of anticoagulants appears to be the strongest risk factor for gastrointestinal bleeding.
de Groot et al., 2014
Netherlands
Retrospective study
Patients aged 18 & above Patients newly using NSAID and ASA
Risk factors for primary upper GI bleeding are to a large extent similar for NSAID and ASA users.
Masoodi et al., 2019
KSA
Retrospective study
Patients with confirmed upper GI bleeding (variceal and non-variceal) were enrolled in this study.
The variceal bleeding due to portal hypertension included both cirrhotic & non-cirrhotic patients.Portal hypertension due to HCV related CLD was the most frequent cause of UGI bleeding in this study.
Youssouf et al., 2020
KSA
Retrospective study
All patients who had UGIB and attended the endoscopy department of the King Abdulaziz Hospital, Mecca, during the AL-Hajj (2016–2018).
The most common cause of UGIB was the presence of gastric origin (erosive gastritis/gastric ulcer/gastric tumor). The most common factors were medications, especially non-steroidal anti-inflammatory drugs (NSAIDs) and blood thinners.
Bodh et al., 2021
India
Retrospective study
All patients who had presented with AUGIB in the form of hematemesis, melena, and hematochezia with hemodynamic instability from May 2015 to December 2019 and underwent EGD in the Department of Gastroenterology, IGMC, Shimla
Peptic ulcer disease was the commonest cause of AUGIB followed by portal hypertension. Other less common.
Bhutia & Lamtha, 2019
India
Retrospective study
Patients endoscopically proven variceal bleed (between 15 January 2015 and 14 February 2016)
Duodenal ulcer was the most common lesion followed by gastric ulcer, gastric erosions, gastric ectasia, gastroesophageal reflux, Mallory–Weiss tear, gastric antral vascular ectasia, and gastric hyperplastic pedunculated polyps.
Moledina & Komba, 2017
Tanzania
prospective cohort study
Adults admitted with UGIB period of seven months at a tertiary hospital.
Oesophageal varices was the most common cause of UGIB
Fallatah et al., 2012
KSA
Retrospective study
All patients with AVB due to unde r l y i n g liver cirrhosis who had undergone emergency endoscopic intervention.
Among chirrotic patients with AVB, chronic hepatitis C was the commonest underlying etiology for liver disease and hematemesis was the predominant symptom.
Almadi et al., 2021
KSA
Retrospective study
Patients undergoing EGDs for the indication of UGIB, hematemesis, coffee-ground emesis, melena, or hematochezia from January 2006 to January 2015(9 years) were included in the study.
Causes of UGIB were predominantly nonvariceal (esophagitis/gastroesophageal reflux disease (GERD), gastric erosions, duodenal ulcers, and gastric ulcers.
Alatawi et al., 2020
KSA
Retrospective study
All adult patients above the age of 18 years who were suspected of UGIB and referred for (EGD) were included January1,2017 and December31,2019
Non-variceal causes showed higher prevalence as causes of UGIB than variceal causes in the Tabuk area. Chronic duodenal and gastric ulcers were the most common culprits of bleeding, whereas duodenitis, gastritis, esophagitis, and Mallory-Weiss syndrome were the least common non-variceal causes.
Hreinsson et al., 2013
Iceland
Prospective study
all patients who underwent upper gastrointestinal endoscopy (UGE), during the year of 2010 at the National University Hospital of Iceland
LDA, NSAIDs and warfarin play an important role in AUGIB etiology and particularly combinations of drugs.
Alruzug et al., 2021
KSA
Retrospective study
patients who underwent an esophagogastroduodenoscopy with an indication of UGIB or presented with hematemesis, melena, or both, as well as those who had hematochezia, from January 2004 to December 2016(13 years).
The majority of UGIB were from a NVUGIB source (80.5 %). The most common endoscopic diagnosis was gastroduodenal erosions, duodenal ulcers, reflux esophagitis, esophageal varices, and gastric ulcers.
Mahajan and Chandail, 2017
India
Retrospective study
patients who presented to the hospital from May 2015 to August 2017 with upper GI bleed, underwent upper GI endoscopy and are above 40 of age
Portal hypertension was the most common cause of upper GI bleeding, while the most common endoscopic lesions were esophageal varices, followed by gastric erosion/gastritis, and duodenal ulcer
Ugiagbe and Omuemu, 2016
Nigeria
Retrospective study
Records of all patients referred with upper GI bleeding to the endoscopy unit of the University of Benin Teaching Hospital from February 2006 to January 2013 were reviewed.
The most common cause of upper GI bleeding was peptic ulcer disease (PUD),, followed by gastritis.
Dewanet al., 2014
Nepal
prospective
All patients who presented with features of acute upper gastrointestinal bleeding i.e. hematemesis, malena, syncope or hospitalized
Upper Gastrointestinal Bleeding was caused by esophageal varices (47.5 %), peptic ulcer disease (33.3 %).
Sibiany, 2012
KSA
Retrospective study
even-year analysis of all patients who underwent an emergency endoscopy for upper GI bleeding at KAUH.
Bleeding esophageal varies was the most common cause (37.6 %), followed by peptic ulcer disease (23.7 %) and gastritis (11.3 %).
Matei et al., 2013
Romania
Prospective study
Patients who are above 18 and presenting to the emergency department with hematemesis and/or melena, and underwent upper gastrointestinal endoscop for a 12-month period (August 2012 - July 2013).
Of patients with UGIB, 29.8 % had variceal and 70.2 % non-variceal bleeding. Six factors were associated with variceal hemorrhage: cirrhosis, history of variceal hemorrhage, ascites, thrombocytopenia, elevated, and elevated bilirubin. Two factors were associated with non-variceal bleeding: the use of NSAIDs and of anticoagulants.
Shenoy et al., 2021
India
Prospective study
included all patients presenting with hematemesis or melena, between June 2016 and January 2017 to the ED
One third (35.7 %) had variceal bleed, 21 % had peptic ulcer disease (PUD), and 43.3 %bled due to other etiology.
Parvez et al., 2016
India
Retrospective study
Patients included were aged > 12 years. A diagnosis of acute UGIB was based on the presence of hematemesis and/or melena.
The most common etiology of UGIB was peptic ulcer (40.05 %) followed by varices (33 %).
Suchartlikitwong et al., 2015
Thailand
Retrospective study
Data retrieved were only regarding patients presented with UGIB In the period of June 2007 to January 2013
The three most common causes of UGIB were peptic ulcer (38.2 %), nonulcer-mucosal lesions (23.4 %), and esophageal-related causes (20.4 %).
Romcea et al., 2013
Romania
Prospective study
Data retrieved from November 2004 to December 2006 of Patients who were already diagnosed with liver cirrhosis.
Almost 27 % of cirrhotic patients with upper gastrointestinal hemorrhage had bleeding from a non-variceal source, the most common etiology being peptic ulcer. Duodenal ulcer was the main cause of non-variceal upper gastrointestinal bleeding followed by gastric ulcer.
Singh and Panigrahi, 2013
India
Retrospective study
data analyzed between (2007–2010) of all patients presented with UGIB
The commonest cause of UGIB was duodenal ulcer (DU) (57.57 %). Portal hypertension was responsible for bleed in only 12.83 %.
Physicians may be guided and helped to classify the severity of the bleeding and determine the underlying causes of this bleeding by initial physical examinations, laboratory work-ups (CBC, coagulation profile, and LFTs), as well as the presentation (Dewan et al., 2014). Upper gastrointestinal bleeding is caused by a number of significant comorbidities, including hypertension, diabetes mellitus, ischemic heart disease, liver disease, and less frequently, gastrointestinal malignancies (Suchartlikitwong et al., 2015).
2.3 Risk factors of upper gastrointestinal
This review revealed that anticoagulants, low-dose aspirin, NSAIDs, and other non-aspirin-APA drugs were the main causes of both UGITB and LGITB. With anticoagulants the strongest risk factor for GIT bleeding (Lanas et al., 2015). NSAID use were also the main risk factors for primary UGITB according to (de Groot et al., 2014; Youssouf et al., 2020 (Youssouf et al., 2020; Hreinsson et al., 2013; Hreinsson et al., 2013; Matei et al., 2013; Matei et al., 2013).
It was discovered that Helicobacter pylori was a significant risk factor for UGITB (Romcea et al., 2013; Hreinsson et al., 2013). When H. pylori and additional NSAID use coexist, the risk of peptic ulcers and bleeding significantly rises (Shrestha and Sapkota, 2014). Duodenal ulcers were more frequently than gastric ulcers linked to H. pylori (Shrestha and Sapkota, 2014). It has been demonstrated that attempts to eradicate the H. pylori infection have reduced the frequency of upper gastrointestinal bleeding, which emphasizes the link between the two conditions (Dewan et al., 2014).
Smoking, which was regarded as a well-established risk factor, was another significant risk factor for UGITB, along with drinking alcohol (Hreinsson et al., 2013; Singh and Panigrahi, 2013). Both the variceal and non-variceal major aetiologias are influenced by these factors. Compared to duodenal ulcers, smoking is significantly more likely to cause gastric ulcers (Masoodi et al., 2019; Shenoy et al., 2021). With variceal forms of bleeding, alcohol was seen more frequently (Masoodi et al., 2019).
2.4 Causes of upper gastrointestinal bleeding
From the possible etiology of UGIB in Fig. 2, peptic ulcer disease (PUD) accounts for 43 % to 58 % of the cases. Of those, the majority is secondary to gastritis or duodenitis (27.62 %). Moreover, oesophageal varices account for 8.03 %, polyps for 4.34 %, malignant tumour for 3.18 %, gastric varices for 1.56 %, oesophagitis for 5.64 %, Mallory Weiss for 3.23 %, vascular abnormalities for 2.07 %, and others for 0.75 % (Stanley and Laine, 2019; Cooper, 2019).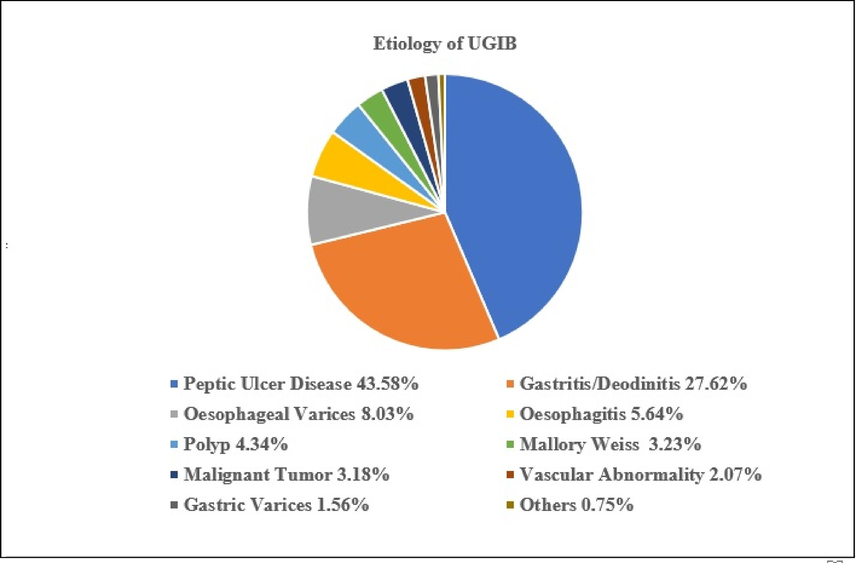
Etiology of upper gastrointestinal bleeding from literatures.
Of the non-variceal causes was peptic ulcer. A study found that peptic ulcer disease was the commonest cause of AUGIB followed by portal hypertension (Bodh et al., 2021). While Ugiagbe & Omuemu found that the most common cause of UGI bleeding was peptic ulcer disease (PUD) followed by gastritis (Ugiagbe and Omuemu., 2016). The same was revealed by Parvez et al., reported that the peptic ulcer was the most common cause (40.05 %) followed by varices (33 %) (Parvez et al., 2016).
Another study has reported that peptic ulcer was the commonest cause (38.2 %) followed by nonulcer-mucosal lesions (23.4 %), and esophageal-related causes (20.4 %) (Suchartlikitwong et al., 2015). Even among cirrhotic patients, Romcea et al found that 27 % of with UGB had a non-variceal source with peptic ulcer the commonest causes. Duodenal ulcer was the main cause followed by gastric ulcer (Romcea et al., 2013).
Also, duodenal ulcer was the most common lesion followed by gastric ulcer, gastric erosions, gastric ectasia, gastroesophageal reflux, Mallory–Weiss tear, gastric antral vascular ectasia, and gastric hyperplastic pedunculated polyps (Fischbach et al., 2018). Another study revealed that nonvariceal causes (esophagitis/gastroesophageal reflux disease (GERD), gastric erosions, duodenal ulcers, and gastric ulcers) were the most common causes (Almadi et al., 2021).
In Saudi Arabia, especially in Tabuk area, revealed the same results, where the non-variceal causes were the most common. Chronic duodenal and gastric ulcers were the most common culprits of bleeding, whereas duodenitis, gastritis, esophagitis, and Mallory-Weiss syndrome were the least common non-variceal causes (Alatawi et al., 2020).
The Prevalence and epidemiology of UGIB is show in Fig. 3. It distresses 80 to 150 people out of every 100,000 people annually. The projected death rates assortment from 2 to 15 %. In comparison to placebo, patients on long-term as well as low-dose aspirin are more likely to develop overt UGIB. There is a two- to three-fold rise in UGIB instances when aspirin is coupled with P2Y12 inhibitors like clopidogrel. The risk of UGIB increases when given to patients with triple therapy, which includes aspirin, a P2Y12 inhibitor, and a vitamin K antagonist [Sehested et al., 2019]. A high prevalence of non-variceal cause was also observed (80.5 %), where the most common endoscopic diagnosis of UGIB causes were gastroduodenal erosions, duodenal ulcers, reflux esophagitis, esophageal varices, and gastric ulcers (Alruzug et al., 2021). Duodenal ulcer also accounted for 57.57 % of UGITB (Singh and Panigrahi, 2013).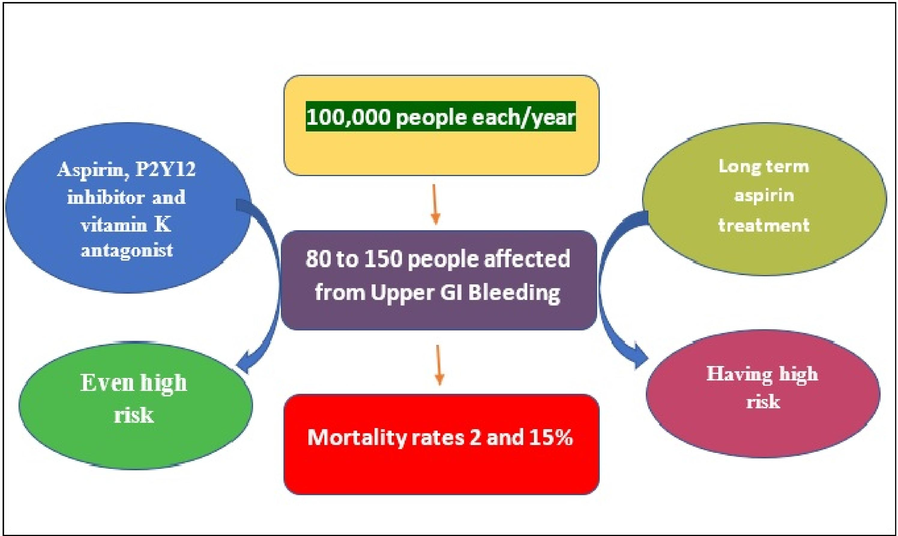
Prevalence and epidemiology rate of gastrointestinal bleeding from database.
As for variceal causes, result was found that 35.7 % of cases had variceal bleed, while 21 % had peptic ulcer disease (PUD), and 43.3 % bled due to other etiology (Shenoy et al., 2021). Portal hypertension due to HCV related CLD was the most frequent cause of UGITB (Masoodi et al., 2019).
Numerous studies were reported to UGIB these studies are concluded most frequent cause of UGIB was esophageal varices (Moledina and Komba, 2017; Dewan et al., 2014; Sibiany, 2012). Fallatah et al., hematemesis was the most common symptom among chirrotic patients with AVB, and chronic hepatitis C was the most common underlying cause of liver disease (Fallatah et al., 2012). Mahajan et al., disclosed, portal hypertension was found to be the most frequent cause of UGITB, while esophageal varices, gastric erosion/gastritis, and duodenal ulcers were found to be the most frequent endoscopic lesions (Mahajan and Chandail., 2017).
This review found that the common mean age of studied patients was 42 ± 18 years and it was commonly reported among the years of the fifth decade (Romcea et al., 2013; Ugiagbe and Omuemu., 2016). Similar findings from earlier studies, where UGITB was prevalent in the age range of 41 to 60 years, were reported (Ugiagbe and Omuemu, 2016). 54 % of the patients, according to research by Parvez et al., were between the ages of 20 and 60 (Bodh et al., 2021). The majority of patients (52.3 %) who presented with acute UGIB, according to Prasad et al. from South India, were also between the ages of 41 and 60 (Prasad et al., 2016). Other studies (Marmo et al., 2010; Romagnuolo et al., 2007). Also found the same outcome. This is consistent with studies that indicated that UGITB was prevalent in patients over the age of 40 (Mahajan and Chandail., 2017; Elghuel, 2011).
Male patients made up more than half of the patients with UGITB in this review. Previous literature also revealed this gender disparity. Even at a young age, males were found to have a higher incidence of UGIB than females, with a male-to-female ratio in some studies reaching 6:1 (Elghuel, 2011; Zaltman et al., 2002). Additionally, the male predominance was noted in earlier studies (Dewan et al., 2014; Hearnshaw et al., 2011; Malghani et al., 2019). This gender disparity was previously attributed to males' higher rates of esophageal varices and higher alcohol consumption (Malghani et al., 2019).
The higher prevalence of underlying diseases in men, such as liver disease, and the higher propensity of alcohol consumption in men also contributed to this male predominance (Roerecke et al., 2019). People who work as peasants are more likely to contract illnesses like schistosomiasis, which can cause portal hypertension. In a study by Elliott (1996), it was discovered that the types of activities people engaged in and the local patterns of production influenced how exposed they were to schistosome-infested water sources (Elliott, 1996). Additionally, other studies have noted the same higher prevalence in men and have explained it by the fact that women tend to conceal the symptoms of their diseases and are less likely to visit the barbershop or the hospital than men (Almadi et al., 2021; Malghani et al., 2019). Another cause was that one of the environmental causes of peptic ulcer is smoking which is more common in males (Sayehmiri et al., 2018).
The current review revealed that hematemesis was the most frequently reported presentation, followed by melena, and abdominal pain was the least frequently reported (Salazar et al., 2023). The two most prevalent symptoms of GIB that have been documented in prior studies are hematemesis and melena (Gaiani et al., 2018; Walker et al., 1990; Li et al., 2019). According to a previous study, hematemesis and melena together account for 44 % of cases of upper GIT bleeding, while hematemesis alone accounts for 36 % and melena alone accounts for 20 % (Hafez et al., 2019). This was consistent with the research by (Sankar et al., 2017; Hafez et al., 2013). Therefore, it was advised that before performing endoscopy, the diagnosis be pointed towards a variceal or non-variceal bleeding. According to some authors, variceal haemorrhage is indicated by the presence of cirrhosis clinical signs, hematochezia, hematemesis of fresh blood, and alcohol consumption (Alharbi et al., 2012).
The results of the current review demonstrated that the primary risk factors for UGITB were anticoagulants, low-dose aspirin, NSAIDs, and other non-aspirin-APA medications. In the current review, medications, especially NSAIDs, were a significant contributor to UGITB. Similar to our current findings, numerous studies have shown that drugs like NSAIDs and blood thinners are linked to a higher risk of UGIB. The main causes of this risk are increased mucosal damage and bleeding susceptibility, which increase the likelihood of UGIB and rebleeding (Dinçer et al., 2019; Goldstein and Cryer, 2015).
The most significant risk factors in the pathogenesis of peptic ulcer disease and ulcer bleeding are Helicobacter pylori and nonsteroidal anti-inflammatory drug (NSAID) or low-dose aspirin consumption (Malfertheiner et al., 2009). Patients with H. pylori infection have a slightly increased risk of peptic ulcer bleeding, and those taking NSAIDs have a roughly fivefold increased risk (Huang et al., 2002). According to reports, patients taking low doses of aspirin have a risk of upper gastrointestinal bleeding that is almost twice as high as that of controls, and that risk rises even further in those who are H. pylori positive (Sostres et al., 2015).
It is well known that H. pylori poses a significant risk for developing peptic ulcer disease (PUD), which may eventually result in UGIB (Lau et al., 2011). The link between H. pylori and PUD was suggested as a possible explanation for this risk. Through a number of virulence factors that affect PUD's colonization and severity, H. pylori raises the risk of developing it. Cytotoxin-associated gene A (cagA), in particular, encodes a type IV secretion apparatus that is used to deliver CagA, which is thought to interact with a number of host proteins, into the cytoplasm of the host cell to induce inflammation and an elevated risk of ulcers. Similar to this, all strains of H. Pylori have vacuolating toxin A (VacA), which generates vacA genes. The strains of H. pylori harboring the s1m1 allele and i1 allele are thought to also play a substantial role in the development of ulcers, due to their high toxicity (Testerman and Morris, 2014).
Gamma-glutamyl transpeptidase (GGT) from H. pylori also promotes the development of peptic ulcers by increasing the release of IL-8 and hydrogen peroxide from epithelial cells. These mechanisms enable H. pylori to contribute to the development of PUD, but the prevalence of ulcers not connected to H. pylori is increasing as a result of rising NSAID by (Testerman and Morris, 2014).
In this review, smoking was a significant risk factor for UGIT. It has been determined that smoking is a significant risk factor for GIT disorders, including peptic ulcers. Smoking was found to be positively associated with the pathogenesis of peptic ulcers and the postponement of ulcer healing in a previous systematic review. It was discovered that cigarette smoke and its active components can impair the mucosal immune system, inhibit cell renewal, reduce blood flow in the GI mucosa, and kill mucosal cells. The activation of nicotinic acetylcholine receptors, the formation of DNA adducts, the stimulation of tumor angiogenesis, and the modulation of immune responses in the GI mucosa are some of the mechanisms by which smoking causes tumorigenesis and encourages the growth of cancer (Li et al., 2014). An increase in smoking intensity was linked to an increased risk of major bleeding, according to a different study (Langsted and Nordestgaard, 2019). Current smokers were also found to have an increased risk of any major bleeding, including GIT bleeding.
As tobacco is found in cigarettes, cigars, cheroots, and pipe tobacco, which contains thousands of chemicals including a significant amount of toxins, it is conceivable that smoking tobacco can cause fragile vessels that are prone to rupture and as a result, bleeding episodes (Ding et al., 2008). By fostering the growth of free radicals, these toxins have the potential to damage the structure of the vessel wall over the long term as well as acutely. Furthermore, smoking has been shown to impair the endothelium's ability to produce nitric oxide (Powell, 1998). and nitric oxide is a key regulator of the smooth muscle tone in the vessel wall. It is produced in response to increased blood flow and other physiological stimuli. Therefore, smoking may impair endothelium dilation, which may result in more rigid arteries and vessels. Increased shear stress at the endothelial surface as a result causes the endothelium to become leaky, allowing various cells and proteins to pass through. Such modifications may occasionally result in endothelium damage, rupturing the vessel and resulting in bleeding (Langsted and Nordestgaard, 2019). Upper gastrointestinal bleeding (UGIB) secondary to peptic ulcer disease (PUD) continues to be a common medical emergency with significant morbidity, mortality, and healthcare costs (Khamaysi and Gralnek, 2013) despite advances in management.
According to the most recent review, peptic ulcers are the most typical cause of UGITB. This concurs with earlier literature (Tielleman et al., 2015; Kashyap et al., 2005). In the past, 30 % or so of people with peptic ulcers experienced recurrent gastric bleeding. However, the rate of recurrent bleeding has dropped to about 10 % due to the rapid advancement in endoscopic treatment techniques (Eisner et al., 2017). According to research by De Groot and his team (de Groot et al., 2014), peptic ulcers were more accurately predicted by the Forrest classification than duodenal ulcers.
3 Conclusion
According to this study, upper gastrointestinal bleeding is more common between the fifth decades of life and extremely uncommon in the ages of below 16 and above 80. Upper gastrointestinal bleeding is most frequently caused by a non-variceal form of bleeding, with duodenal ulcers as a subtype of peptic ulcer is the leading cause. NSAID use and Helicobacter pylori infection were the main precipitating risk factors. Hematemesis, melena, and, less frequently, stomach discomfort were the typical presentations.
Data and materials availability
All data associated with this study are present in the paper.
Authors’ contribution
Omar Al-Aidaroos: Conceptualization, Data collection, Investigation, Funding acquisition, Writing – original draft. Rawan A. Alsomali: Conceptualization, Data collection, Investigation, Funding acquisition, Writing – original draft. Arwa M. Wadaan: Conceptualization, Data collection, Investigation, Funding acquisition, Writing – original draft. Ghuzlan A. Zubaidi: Conceptualization, Data collection, Investigation, Funding acquisition, Writing – original draft. Roaa A. Alsanea: Investigation, Methodology, Project administration, Resources, Funding and review and editing. Hanan S. Alkhelaiwi: Investigation, Methodology, Project administration, Resources, Funding and review and editing. Dana N. Alsayed: Investigation, Methodology, Project administration, Resources, Funding and review and editing.
Acknowledgments
The Authors extended their appreciation to Almaarefa University for supporting this project.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Biochemical tests in diseases of the intestinal tract: their contributions to diagnosis, management, and understanding the pathophysiology of specific disease states. Crit. Rev. Clin. Lab. Sci.. 1997;34(2):141-223.
- [CrossRef] [Google Scholar]
- Findings of esophagogastroduodenoscopy in patients suspected of upper gastrointestinal bleeding referred to the main endoscopy unit at King Fahad Specialist Hospital. Cureus. 2020;12(12):e11862.
- [Google Scholar]
- Predictors of a variceal source among patients presenting with upper gastrointestinal bleeding. Can. J. Gastroenterol. Hepatol.. 2012;26(4):187-192. (REASON Investigators)
- [CrossRef] [Google Scholar]
- Upper gastrointestinal bleeding: Causes and patient outcomes. Saudi J. Gastroenterol.. 2021;27(1):20-27.
- [CrossRef] [Google Scholar]
- Time trends of causes of upper gastrointestinal bleeding and endoscopic findings. Saudi J. Gastroenterol.. 2021;27(1):28-34.
- [CrossRef] [Google Scholar]
- Blackbourne, L.H., 2018. Surgical recall: Recall Series Editor and Senior Editor, eighth ed. https://abu.edu.iq/sites/default/files/lbrary/surgical_recall_6th_e1.pdf.
- Retrospective study of etiology of non variceal acute gastrointestinal bleeding in Eastern Himalayan region of india in Sikkim. J Family Med Prim Care. 2019;8(2):573-575.
- [CrossRef] [Google Scholar]
- Current trends in etiological profile of acuteupper gastrointestinal bleeding in Northern India: a retrospective analysis of 5-Year endoscopic data. J. Digestive Endoscopy. 2021;12(1):31-35.
- [CrossRef] [Google Scholar]
- Interventions for preventing upper gastrointestinal bleeding in people admitted to intensive care units. Critical Care Nurse. 2019;39(2):102-103.
- [CrossRef] [Google Scholar]
- Pathogenesis and clinical management of Helicobacter pylori gastric infection. World J. Gastroenterol.. 2019;25(37):5578-5589.
- [CrossRef] [Google Scholar]
- Primary non-variceal upper gastrointestinal bleeding in NSAID and low-dose aspirin users: development and validation of risk scores for either medication in two large Dutch cohorts. J. Gastroenterol.. 2014;49(2):245-253.
- [CrossRef] [Google Scholar]
- A study of clinical and endoscopic profile of acute upper, gastrointestinal bleeding. Kathmandu Univ. Med. J. (KUMJ). 2014;12(45):21-25.
- [CrossRef] [Google Scholar]
- DiGregorio, AM, Alvey, H, June. Gastrointestinal Bleeding. In: StatPearls. Treasure Island (FL), 5. StatPearls Publishing, p. 2023.
- NSAID, antiaggregant, and/or anticoagulant-related upper gastrointestinal bleeding: is there any change in prophylaxis rate after a 10-year period? Turk. J. Gastroenterol.. 2019;30(6):505-510.
- [CrossRef] [Google Scholar]
- Levels of tobacco-specific nitrosamines and polycyclic aromatic hydrocarbons in mainstream smoke from different tobacco varieties. Cancer Epidemiol. Biomark. Prev.. 2008;17(12):3366-4337.
- [CrossRef] [Google Scholar]
- Gastric ulcer complications after the introduction of proton pump inhibitors into clinical routine: 20-Year experience. Visc. Med.. 2017;33(3):221-226.
- [CrossRef] [Google Scholar]
- The characteristics of adults with upper gastrointestinal bleeding admitted to Tripoli Medical Center: a retrospective case-series analysis. Libyan J. Med.. 2011;6:1-5.
- [CrossRef] [Google Scholar]
- Schistosomiasis. Pathophysiology, diagnosis, and treatment. Gastroenterol. Clin. North Am.. 1996;25:599-625.
- [CrossRef] [Google Scholar]
- Variceal hemorrhage: Saudi tertiary center experience of clinical presentations, complications and mortality. World J. Hepatol.. 2012;4(9):268-273.
- [CrossRef] [Google Scholar]
- Helicobacter pylori infection. Dtsch. Arztebl. Int.. 2018;115(25):429-436.
- [CrossRef] [Google Scholar]
- An overview of helicobacter pylori infection. Methods Mol. Biol.. 2021;2283:1-14.
- [CrossRef] [Google Scholar]
- Clinical approach to the patient with acute gastrointestinal bleeding. Acta Biomed. 2018;89(8-S):12-19.
- [CrossRef] [Google Scholar]
- Gastrointestinal injury associated with NSAID use: a case study and review of risk factors and preventative strategies. Drug Healthc. Patient Saf.. 2015;7:31-41.
- [CrossRef] [Google Scholar]
- Assessment of the causes and outcomes of upper gastrointestinal tract bleeding patients in Aswan University Hospital. Egypt. J. Hosp. Med.. 2019;74(6):1359-1364.
- [Google Scholar]
- Quality of life in peptic ulcer patients referring to Al-Zahra hospital of Isfahan, Iran. Gastroenterol. Hepatol. Bed. Bench. 2013;6(Suppl 1):S87-S92.
- [Google Scholar]
- Acute upper gastrointestinal bleeding in the UK: patient characteristics, diagnoses and outcomes in the 2007 UK audit. Gut. 2011;60(10):1327-1335.
- [CrossRef] [Google Scholar]
- Upper gastrointestinal bleeding: Incidence, etiology and outcomes in a population-based setting. Scand. J. Gastroenterol.. 2013;48(4):439-447.
- [CrossRef] [Google Scholar]
- Role of Helicobacter pylori infection and non-steroidal anti-inflammatory drugs in peptic-ulcer disease, a meta-analysis. Lancet. 2002;359:14-22.
- [CrossRef] [Google Scholar]
- Upper Gastrointestinal Bleeding: Etiologies and Management. Mayo Clin. Proc.. 2019;94(4):697-703.
- [CrossRef] [Google Scholar]
- A clinical profile of acute upper gastrointestinal bleeding at moderate altitude. JIACM. 2005;6(224–228)
- [CrossRef] [Google Scholar]
- Acute upper gastrointestinal bleeding (UGIB) - initial evaluation and management. Best Pract. Res. Clin. Gastroenterol.. 2013;27:633-638.
- [CrossRef] [Google Scholar]
- Risk of upper and lower gastrointestinal bleeding in patients taking nonsteroidal anti-inflammatory drugs, antiplatelet agents, or anticoagulants. Clin. Gastroenterol. Hepato.. 2015;13(5):906-912.
- [CrossRef] [Google Scholar]
- Smoking is associated with increased risk of major bleeding: a prospective cohort study. Thromb. Haemost.. 2019;119(1):39-47.
- [CrossRef] [Google Scholar]
- Systematic review of the epidemiology of complicated peptic ulcer disease: incidence, recurrence, risk factors and mortality. Digestion. 2011;84(2):102-113.
- [CrossRef] [Google Scholar]
- Cigarette smoking and gastrointestinal diseases: the causal relationship and underlying molecular mechanisms (review) Int. J. Mol. Med.. 2014;34(2):372-380.
- [CrossRef] [Google Scholar]
- Effect of acute upper gastrointestinal bleeding manifestations at admission on the in-hospital outcomes of liver cirrhosis: hematemesis versus melena without hematemesis. Eur. J. Gastroenterol. Hepatol.. 2019;31(11):1334-1341.
- [CrossRef] [Google Scholar]
- Etiological and endoscopic profile of middle aged and elderly patients with upper gastrointestinal bleeding in a tertiary care hospital in North India: a retrospective analysis. J. Midlife Health. 2017;8(3):137-141.
- [CrossRef] [Google Scholar]
- Spectrum of endoscopic findings in patients of upper gastrointestinal bleeding at a tertiary care hospital. Cureus. 2019;11(4):e4562
- [CrossRef] [Google Scholar]
- Italian registry on upper gastrointestinal bleeding (Progetto Nazionale Emorragie Digestive–PNED 2). Predicting mortality in non-variceal upper gastrointestinal bleeders: validation of the Italian PNED Score and prospective comparison with the Rockall Score. Am. J. Gastroenterol.. 2010;105(6):1284-1291.
- [CrossRef] [Google Scholar]
- Changing trends in acute upper GI bleeding a single-centre study in the western region of Saudi Arabia. BJMP. 2019;12(3):a019
- [Google Scholar]
- Predictors of variceal or nonvariceal source of upper gastrointestinal bleeding. An etiology predictive score established and validated in a tertiary referral center. J. Gastrointestin. Liver Dis.. 2013;22(4):379-384.
- [Google Scholar]
- Risk factors for mortality among patients admitted with upper gastrointestinal bleeding at a tertiary hospital: a prospective cohort study. BMC Gastroenterol.. 2017;17(1):165-176.
- [CrossRef] [Google Scholar]
- Spectrum of upper gastrointestinal bleed: an experience from Eastern India. J Dig Endosc. 2016;7:55-61.
- [CrossRef] [Google Scholar]
- Vascular damage from smoking: disease mechanisms at the arterial wall. Vasc. Med.. 1998;3(01):21-28.
- [CrossRef] [Google Scholar]
- Acute upper gastrointestinal bleeding in a tertiary care hospital in South India - have we improved the outcomes? Trop. Gastroenterol.. 2016;37(3):168-176.
- [Google Scholar]
- Alcohol consumption and risk of liver cirrhosis: a systematic review and meta-analysis. Am. J. Gastroenterol.. 2019;114(10):1574-1586.
- [CrossRef] [Google Scholar]
- Simple clinical predictors may obviate urgent endoscopy in selected patients with nonvariceal upper gastrointestinal tract bleeding. Arch. Intern. Med.. 2007;167(3):265-270.
- [CrossRef] [Google Scholar]
- The etiology of upper gastrointestinal bleeding in cirrhotic patients. Clujul Med.. 2013;86(1):21-23.
- [Google Scholar]
- Clinical and biochemical footprints of inherited metabolic disorders. XI. Gastrointestinal symptoms. Mol. Genet. Metab.. 2023;138(3):107528
- [CrossRef] [Google Scholar]
- Etiology, risk factors and outcome of acute non-variceal bleed in hospitalised patients-A cross sectional study. Int. J. Curr. Res. Med. Sci.. 2017;3(8):71-77.
- [CrossRef] [Google Scholar]
- Prevalence of peptic ulcer in Iran: systematic review and meta-analysis methods. J. Res. Med. Sci.. 2018;23:8-14.
- [CrossRef] [Google Scholar]
- Reduced risk of gastrointestinal bleeding associated with proton pump inhibitor therapy in patients treated with dual antiplatelet therapy after myocardial infarction. Eur. Heart J.. 2019;40(24):1963-1970.
- [CrossRef] [Google Scholar]
- A prospective cohort study of patients presenting to the emergency department with upper gastrointestinal bleeding. J. Family Med. Prim Care. 2021;10(3):1431-1436.
- [CrossRef] [Google Scholar]
- Etiology and adverse outcome predictors of upper gastrointestinal bleeding in 589 patients in Nepal. Dig. Dis. Sci.. 2014;59(4):814-822.
- [CrossRef] [Google Scholar]
- A. presentation and endoscopic findings of emergency upper gastrointestinal bleeding: a seven-year experience at King Abdulaziz University Hospital, Jeddah, Saudi Arabia. JKAU Med. Sci.. 2012;20(1):57-64.
- [CrossRef] [Google Scholar]
- Spectrum of upper gastrointestinal hemorrhage in coastal Odisha. Trop. Gastroenterol.. 2013;34(1):14-17. PMID: 2392336
- [CrossRef] [Google Scholar]
- Peptic ulcer bleeding risk. The role of Helicobacter pylori infection in NSAID/low-dose aspirin. Am. J. Gastroenterol.. 2015;110:684-689.
- [CrossRef] [Google Scholar]
- Management of acute upper gastrointestinal bleeding. BMJ. 2019;25(364):l536
- [CrossRef] [Google Scholar]
- A prospective study of alcohol consumption and smoking and the risk of major gastrointestinal bleeding in men. PLoS One. 2016;11(11):e0165278
- [Google Scholar]
- Epidemiology of upper gastrointestinal bleeding and Helicobacter pylori infection: review of 3,488 Thai patients. Asian Biomed.. 2015;9(1):87-93.
- [CrossRef] [Google Scholar]
- Beyond the stomach: an updated view of Helicobacter pylori pathogenesis, diagnosis, and treatment. World J. Gastroenterol.. 2014;20(36):12781-111808.
- [CrossRef] [Google Scholar]
- Epidemiology and risk factors for upper gastrointestinal bleeding. Gastrointest. Endosc. Clin. n. Am.. 2015;25:415-428.
- [CrossRef] [Google Scholar]
- Etiology of upper gastrointestinal bleeding in the University of Benin Teaching Hospital, South-Southern Nigeria. Nigerian J. Surg. Sci.. 2016;26(2):29-35.
- [CrossRef] [Google Scholar]
- Walker H.K., Hall W.D., Hurst J.W., eds. Clinical Methods: the History, Physical, and Laboratory Examinations (3rd ed.). Boston: Butterworths; 1990.
- Causes of upper gastrointestinal bleeding among pilgrims during the Hajj period in the Islamic Years 1437–1439 (2016–2018) Cureus. 2020;12(10):e10873.
- [Google Scholar]
- Upper gastrointestinal bleeding in a Brazilian hospital: a retrospective study of endoscopic records. Arq. Gastroenterol.. 2002;39(2):74-80.
- [CrossRef] [Google Scholar]







