Translate this page into:
Biochemical characterization of citrus bent leaf viroid infecting citrus cultivars
⁎Corresponding authors. wangxiukang@yau.edu.cn (Xiukang Wang), yasir.iftikhar@uos.edu.pk (Yasir Iftikhar)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Citrus bent leaf viroid (CBLVd) is an emerging and widely distributed viroid along with its variants in citrus growing areas of world. Activation of defense mechanism is associated with different enzymes and inhibitors, accumulated in the infected host. Limited studies were found on biochemical characterization of citrus viroids. Therefore, study was focused on the biochemical activities such as determination of chlorophyll contents, total soluble phenolics (TSP), phenylalanine ammonia lyase (PAL) and polyphenol oxidase (PPO) activity in three citrus cultivars kinnow (C. reticulate), feutrell’s early (C. reticulate) and mosambi (C. sinensis) infected with CBLVd from Sargodha, Pakistan. The results showed that chlorophyll contents were significantly lower in the diseased leaves samples of all the citrus cultivars as compared to healthy ones. Whereas, TSP was found in higher concentration in the CBLVd infected samples of citrus cultivars. Similarly, activities of PPO and PAL was increased significantly in leaves of citrus cultivars infected with CBLVd as compared to healthy. These findings were in confirmation that chlorophyll contents, enzymes and inhibitors were associated to response of plants towards pathogen infection. To the best of our knowledge, these biochemical alterations in host plants against CBLVd were studied for the first time not only in the Asian region but also in Pakistan. The results will lead to investigate the biochemical alterations in other citrus viroids infection.
Keywords
CBLVd
Chlorophyll
PPO
PAL
TSP
Cultivars
1 Introduction
Citrus is fruit crop with full of nutrition cultivating all over the tropical and sub-tropical areas of the world. Virus and virus-like pathogens have a vital role in limiting the citrus production in Pakistan (Roistacher, 1991). These pathogens not only debilitate the citrus trees but also deteriorate the quality (Tariq et al., 2007). Citrus viroids are emerging threat along with other virus and virus-like diseases in citrus growing areas (Iftikhar et al., 2021a,b). New frontiers in virology were opened with the discovery of viroids as an infectious agent smaller than virus (Diener, 1971). Most of the citrus viroids belong to pospiviroidae have been found in citrus plantation of the world. Among them citrus bent leaf viroid (CBLVd) has been detected in symptomatic and asymptomatic citrus samples causing reduction in size of fruit including low yield (Duran-Vila, 2000; Ito et al., 2000, 2002).
After the discovery of CBLVd for the first time in Japan, the presence of this viroid has been confirmed in Pakistan (Ito et al. 2000; Cao et al., 2009; Bakhtawar et al., 2021). The plant response towards the CBLVd infection is not associated to particular symptoms production (Hutton et al., 2000). Molecular detection is a sensitive and quick way to detect and characterize the CBLVd (Garnsey et al., 2002). Other than biological and molecular indexing, biochemical alterations are also important changes to be studied. Different biochemical changes reflect the infection of virus and virus like pathogens. These biochemical alterations are change in phenolics and plant growth regulators reflect the virus infection (Verma and Verma, 2003, Iftikhar et al., 2011). Biochemical alterations are supportive in understanding the interaction between pathogen and plant host during pathogenesis.
Symptoms development in plants due to virus infection also depend on physiological changes (Qamar et al., 2016). Loss of chlorophyll contents leads to leaves yellowing (Bos, 1999; Iftikhar et al., 2011). Biochemical alterations like increase in amino acid contents has been reported in virus infection (Tu and Ford 1970; Qamar et al., 2016). The alteration in polyphenol oxidase (PPO) activity resulting the tissue browning (Vaughn et al., 1988), but the genes involving in PPO activity activated the defense mechanism of plants against virus infection (Li and Steffens, 2002). Biochemical characterization will lead to understanding the exhibition of symptoms and change in physiology of virus infected plants.
The study was conducted to determine Phenolic compounds has been recognized as a vital factor in defense mechanism in pathogenesis due to wide range of pathogens (Bennett and Wallsgrove, 1994). During the pathogen infection plant showed the increase in PAL, PPO activity (Deborah et al., 2001; Kumari and Vengadaramana, 2017). Biochemical alterations leading to the symptoms development and defense responses against virus and virus-like pathogen has been studied but still needed to be characterized. Viroid infection is involved in changes in certain microRNAs derived from rRNA (Flores et al., 2020).
Biochemical alterations like; Chlorophyll contents, phenolic compounds, PPO and PAL activity in citrus due to viroid infections has not been studied categorically and pathogenesis is supported by the mechanisms studied in host plants infected by virus and virus-like pathogens. Scanty of literature is available in regarding the biochemical alteration in response of citrus viroid. Therefore, the study was aimed to characterize the CBLVd infection biochemically in different citrus cultivars which has not been studied yet. The findings will also definitely lead to biochemical characterization of asymptomatic viroid infection in citrus.
2 Materials and methods
2.1 Biochemical parameters
The Citrus bent leaf viroid (CBLVd) infected leaves samples of three citrus cultivars Kinnow and Feutrell’s early (Citrus reticulate Blanco.) from mandarin and Mosambi (C. sinensis L. Osbeck) from sweet orange were collected for biochemical characterization. These varieties were targeted based on the wide distribution and commercial availability in all the citrus growing areas of Sargodha, Pakistan. The infected leaves samples were PCR confirmed from the previous study (Bakhtawar et al., 2021). The CBLVd infected samples were compared with healthy having no symptoms and confirmed through PCR. Fifteen CBLVd infected and five healthy leaves samples of each cultivar were collected for biochemical parameters. Each assay was carried out with CRD experimental design and repeated three times. Followings were the biochemical parameters to characterize the CBLVd in citrus growing areas of Sargodha, Punjab, Pakistan.
2.2 Estimation of chlorophyll contents
Total chlorophyll was determined in infected and healthy leaves followed DMSO method reported by Hiscox and Israelstam (1979). The discs of leaves of about 500 mg each were collected in test tubes followed by adding the 10 ml of dimethyl sulphoxide (DMSO). The tubes were incubated at room temperature overnight with a dark period of 1–2 hrs. The absorbance was recorded at 663 and 645 nm in a spectrophotometer. All the chlorophyll contents were calculated using the equation given by Arnon (1949).
2.3 Estimation of total soluble phenolic (TSP)
Total soluble phenolic (TSP) were determined from CBLVd infected leaves and compared with healthy leaves samples of citrus trees followed the method reported by Julkunen-Tiitto (1985). Forty-five mg of infected leaves samples of weighed about 45 mg were slogged using acetone of concentration 80% and incubated in water bath for one hour at 50 °C. After the incubation the slurry was centrifuged in a refrigerated centrifuge machine @ 10,000 rpm at 4 °C for 10 min. The supernatant was collected in microfuge tubes. The dilution of 100 µl aliquot with 2 ml distilled water was made followed by adding Folin ciocalteus phenol reagent (1 ml) prior shaken vigorously. The 20% Na2CO3 (5 ml) was used to make the volume up to 10 ml by adding 5 ml and vortexed for 5–10 s. Readings were recorded at 750 nm after the incubation of 20 min in a spectrophotometer. The reference standard curve of 20, 40, 60, 80 and 100 µg Galic acid was prepared. The TSP were measured and compared between infected and healthy citrus samples.
2.4 Polyphenol oxidase activity (PPO)
Enzymatic activity of PPO was measured in CBLVd infected and healthy citrus samples (Jockusch, 1966). Infected leaves were pulverized in liquid nitrogen. The 2.5 g homogenate was mixed with 5 ml phosphate buffer of having 0.05 M sodium phosphate and 5% Poly vinyl polypyrrolidone (PVP) (wt/vol) and pH was adjusted to 6.0. The filtration was performed through muslin cloth followed by refrigerated centrifugation for 5 min at 13,000 rpm. Supernatant (1 ml) was added with 2.9 ml of 0.05 M phosphate buffer containing 0.1 M catechol (1 ml) in a new falcon tube. Three aliquots were made of mixture to determine the PPO activity at 546 nm for four minutes with the intervals of 20 s and presented as μl−1 min−1.
2.5 Phenylalanine ammonia lyase activity (PAL)
The enzymatic activity of PAL was determined through the method reported by Okey et al., (1997). The activity was monitored at the time intervals of 0, 2 and 8 h. Infected and healthy leaves were crushed in liquid nitrogen. The 2.5 g homogenized slurry was mixed with 5 ml of solution contains Tris (50 mM), 2-mercaptoethanol (15 mM) and Poly vinyl polypyrrolidone (5%) followed by filtration through muslin cloth. The filtered solution was centrifuged @ 13,000 rpm for 5 min at 4 °C. The supernatant (1 ml) was suspended with 2 ml 0.05 M borate buffer (pH 8.8) with the addition of 0.02 M L-phenylalanine (1 ml). The incubation was at 30 °C for the period of 1 h. 0.2 ml of 6 M trichloroacetic acid (TCA) was used as stopping solution. Three aliquots of solution in separate tubes were prepared to measure the activity of PAL (μg g−1) at 290 nm.
3 Results and discussion
3.1 Estimation of chlorophyll contents
Chlorophyll contents such as; Chlorophyll A, Chlorophyll B and Total chlorophyll in CBLVd infected leaves samples of three citrus cultivars; Kinnow, Feutrell’s early and Mosambi were determined. It was found that chlorophyll A in CBLVd infected samples of all the three varieties were significantly lower as compared to healthy ones. Among the cultivars, the infected samples of Feutrell’s early had higher chlorophyll A as compared to those in Kinnow and Mosambi (Fig. 1). The chlorophyll A in kinnow, mosambi and feutrell’s early were 78.70 mg/ml, 106.33 mg/ml and 154.28 mg/ml respectively as compared to healthy samples of the same varieties, which were not significantly different (Fig. 1).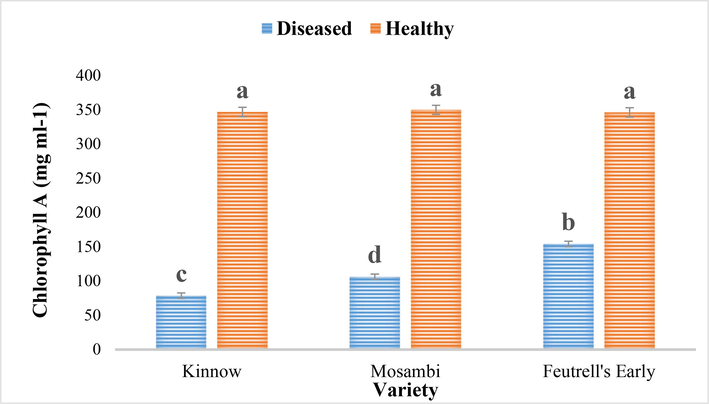
Chlorophyll A in the diseased and healthy leaves of Kinnow (C. reticulate), mosambi (C. sinensis) and feutrell’s early (C. reticulate).
Similarly, the same trend was observed in chlorophyll B. The chlorophyll B were not only significantly different among the cultivars but also with those of healthy leaves samples of same cultivars (Fig. 2). Among the citrus cultivars, infected leaves of feutrell’s early had higher chlorophyll B with the mean value of 114.06 mg/ml followed by mosambi (106.33 mg/ml) and kinnow (78.70 mg/ml). The chlorophyll B was significantly low in CBLVd infected citrus leaves of all the three varieties as compared to that in healthy citrus sample (Fig. 2).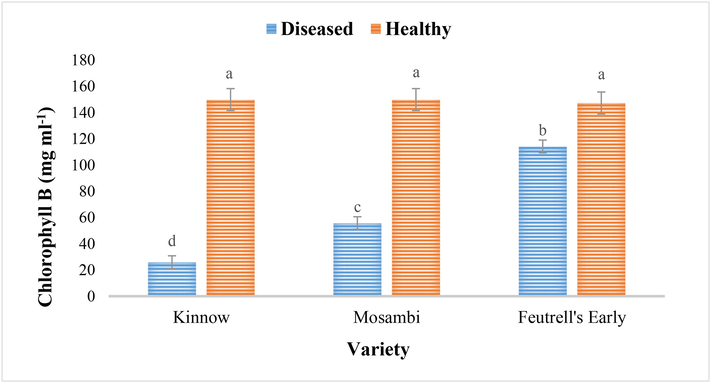
Chlorophyll B in the diseased and healthy leaves of Kinnow (C. reticulate), mosambi (C. sinensis) and feutrell’s early (C. reticulate).
Total chlorophyll was also estimated in all the three citrus cultivars and found the same trend as in chlorophyll A and B (Fig. 3). Based on estimation of chlorophyll contents there was significant difference of chlorophyll not only among the infected samples of citrus varieties but also with the healthy citrus samples as controls.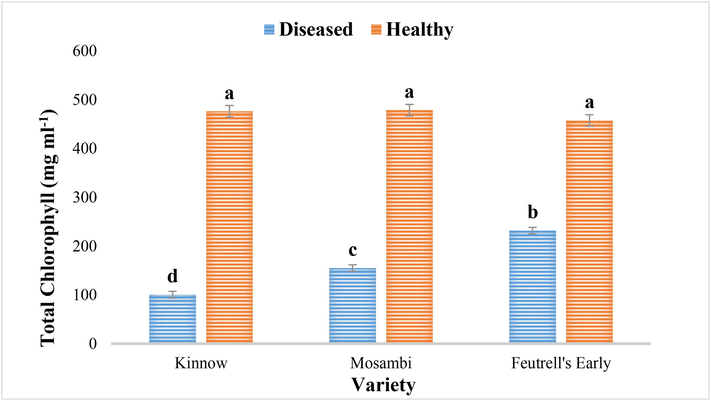
Total chlorophyll in the diseased and healthy leaves of Kinnow (C. reticulate), mosambi (C. sinensis) and feutrell’s early (C. reticulate).
Chlorosis is generally caused by virus pathogens of plants (Bar-Joseph and Lee 2000). Chlorophyll contents were also significantly lower in CBLVd infected citrus samples as compared to healthy. Yellowing and chlorosis has also been observed in citrus infected with CBLVd (Ito et al., 2002). Our results were also in conformity with Ito et al., (2002). Previous study also observed the significant decrease in chlorophyll contents during the citrus tristeza virus infection in citrus (Iftikhar et al., 2011, Iftikhar et al., 2021a, Iftikhar et al., 2021b). Chlorosis or yellowing is also one of the exhibited symptoms associated to citrus viroids (Tangkanchanapas et al., 2018). Another virus disease caused by Chilli veinal mottle virus also reduced the chlorophyll contents in diseased samples (Ali et al., 2020). The loss of chlorophyll contents or chlorosis is one of the most common symptoms in all type of plant pathogens.
3.2 Estimation of total soluble phenolic (TSP), polyphenol oxidase activity (PPO) and phenylalanine ammonia lyase activity (PAL)
The amount of TSP was at significantly higher rate in diseased leaves samples of three citrus cultivars as compared to healthy samples. It was observed that diseased samples of kinnow had significantly higher TSP (2.95) as compared to those of other two cultivars; mosambi (2.28) and feutrell’s early (1.94). The TSP in healthy samples of citrus cultivars were not significantly different with each other (Fig. 4).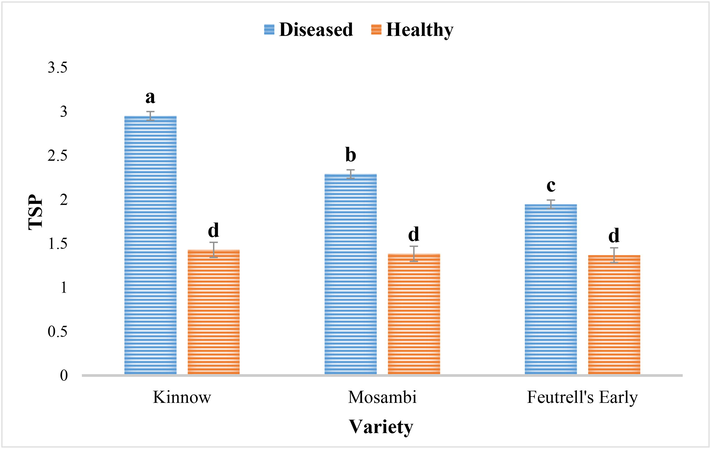
Total soluble phenolics in the diseased and healthy leaves of Kinnow (C. reticulate), mosambi (C. sinensis) and feutrell’s early (C. reticulate).
The PPO activity in diseased leaves samples from three citrus cultivars were significantly higher than in the healthy leaves of respective citrus varieties. Among the cultivars, the PPO activity was significantly increased in diseased samples of kinnow (0.88 μl−1 min−1), mosambi (0.38 μl−1 min−1) and feutrell’s early (0.31 μl−1 min−1) (Fig. 5).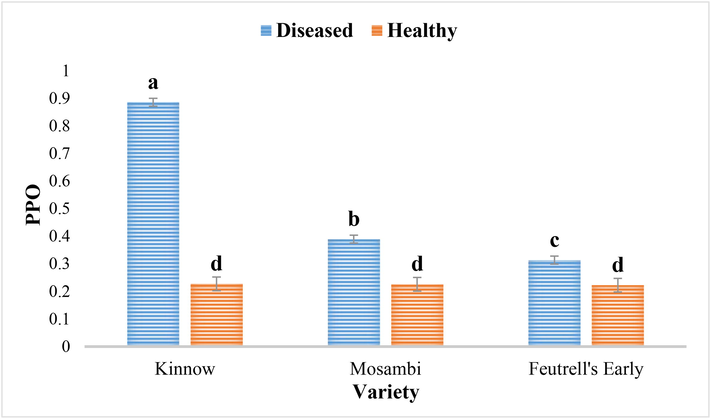
Polyphenol oxidase activity in the diseased and healthy leaves of Kinnow (C. reticulate), mosambi (C. sinensis) and feutrell’s early (C. reticulate).
During the determination of PAL activity in diseased and healthy leaves samples of kinnow, mosambi and feutrell’s early, results showed that PAL activity was significantly increased in diseased samples as compared to healthy samples of all the citrus varieties. Comparison among diseased samples of citrus varieties showed the significantly higher PAL activity in kinnow followed by mosambi and feutrell’s early with the mean values of 0.82 μg g−1, 0.20 μg g−1 and 0.07 μg g−1 Respectively (Fig. 6).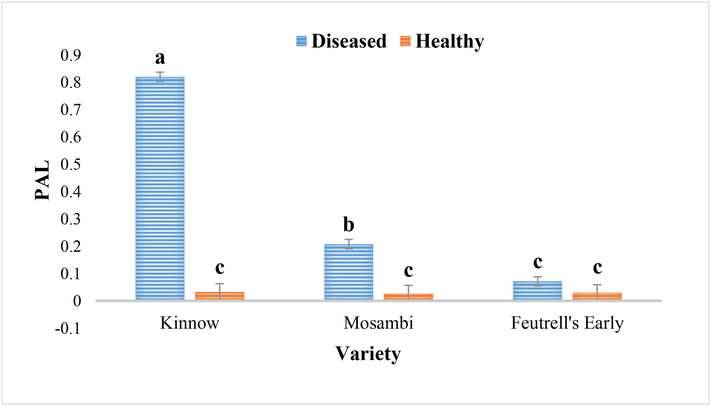
Phenylalanine ammonia lyase activity in the diseased and healthy leaves of Kinnow (C. reticulate), mosambi (C. sinensis) and feutrell’s early (C. reticulate).
The variety of secondary metabolites play a key role in plant defense mechanism against pathogen attack (Baldwin et al., 2010; Graham and Graham, 1991). Secondary metabolites in plants during pathogen infection are the responsible for the expression of symptoms and biochemical changes (Nicholson and Hammerschmidt 1992). There is a little information available regarding the biochemical alteration in TSP, PPO and PAL activities in citrus infected with citrus viroid infection.
The phenolic compounds were higher in leaves samples of citrus cultivars infected with CBLVd. The activity of phenolic compounds in plants is associated with defense mechanism during the diseases caused by different plant pathogens (Saini et al., 1988). Phenolic compounds are involved in strengthening the host cell wall (Graham and Graham, 1991). The total soluble phenols were higher after the yellow vein mosaic virus infection in Okra (Seth et al., 2017; Yadav et al., 2018). After the pathogen infection plant activate the defense mechanism associated with Phenylalanine ammonia lyase (PALase) activity (Daayf et al., 1997; Prasannath 2017). Therefore, higher amount of phenols and increase in PPO and PAL activity is attributed with the pathogen infection (Tomás-Barberán and Espín, 2001).
The physiological response of plants to virus, bacterial and fungal infection showed higher phenols and increased activity of PO and PPO enzymes (Gäumann, 1956; Goodman et al., 1967; Dasgupta, 1988). It is opined that increase in PPO and PAL activities and changes in phenolic compounds are the normal responses of plants after the pathogen infection (Harbourne, 1964; Seth et al., 2017; Yadav et al., 2018). Our results were in conformity with the previous literature mentioned above. Although they discussed the general behavior of phenols, PAL and PPO activity after the infection of phyto-pathogen. Moreover, these biochemical alterations in the result of CBLVd infection in citrus varieties has been discussed for the first time. This information will also lead to the characterization of asymptomatic infection of citrus viroids.
4 Conclusions
Biochemical alterations in plants after the infection of pathogens is associated with the defense activity in host. The conclusions have been drawn from the above study that citrus viroids, like other pathogen, induce the biochemical changes in host upon infection. It is also concluded that phenols, PAL and PPO activities are always increased in infected plants as compared to healthy. Our study also demonstrated that viroid infection in plant induce the defense response which is similar to that of induced by other pathogens.
Acknowledgements
The authors extend their appreciation to the Deanship of Scientific Research, King Khalid University for funding this work through research groups program under grant number R.G.P. 2/11/42.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Role of Plant extracts and salicylic acid for the management of chili veinal mottle virus disease. Pakistan J. Phytopathol.. 2020;32(2):147-157.
- [Google Scholar]
- Detection of Citrus Bent Leaf Viroid in Citrus Orchards of Sargodha. Pakistan. Arab J. Plant Protect.. 2021;39(2):159-163.
- [Google Scholar]
- Secondary metabolites in plant defence mechanisms. New Phytol.. 1994;127(4):617-633.
- [Google Scholar]
- Plant Viruses, Unique and Intriguing Pathogens: A Textbook of Plant Virology. Backhuys Publishers; 1999.
- First Report of Citrus bent leaf viroid and Citrus dwarfing viroid from Citrus in Punjab, Pakistan. Plant Dis.. 2009;93(8) 840-840
- [Google Scholar]
- Methyl ester of p-coumaric acid: a phytoalexin-like compound from long English cucumber leaves. J. Chem. Ecol.. 1997;23(6):1517-1526.
- [Google Scholar]
- Principles of Plant Pathology. Allied Publishers; 1988.
- Time-course study of the induction of defense enzymes, phenolics and lignin in rice in response to infection by pathogen and non-pathogen/Zeitlicher Verlauf der Induktion von Abwehrsystemen, phenolischen Verbindungen und Lignin als Reaktion auf die Infektion mit einem Pathogen oder Nichtpathogen. Zeitschrift für Pflanzenkrankheiten und Pflanzenschutz/J. Plant Dis. Protect. 2001:204-216.
- [Google Scholar]
- Potato spindle tuber “virus”: IV. A replicating, low molecular weight RNA. Virology. 1971;45(2):411-428.
- [Google Scholar]
- Viroid pathogenesis: a critical appraisal of the role of RNA silencing in triggering the initial molecular lesion. FEMS Microbiol. Rev.. 2020;44(3):386-398.
- [Google Scholar]
- Practical field detection of citrus viroids in Florida by RT-PCR. In: International Organization of Citrus Virologists Conference Proceedings (1957-2010). 2002. No. 15
- [Google Scholar]
- The biochemistry and physiology of infectious plant disease. Biochem. Physiol. Infect. Plant Dis. 1967
- [Google Scholar]
- Rapid accumulation of anionic peroxidases and phenolic polymers in soybean cotyledon tissues following treatment with Phytophthora megasperma f. sp. glycinea wall glucan. Plant Physiol.. 1991;97(4):1445-1455.
- [Google Scholar]
- A method for the extraction of chlorophyll from leaf tissue without maceration. Can. J. Bot.. 1979;57(12):1332-1334.
- [Google Scholar]
- Viroid dwarfing for high density citrus plantings. Horticult. Rev.. 2000;24:277-317.
- [Google Scholar]
- Iftikhar, Y., Majeed, M. Z., Vadamalai, G., & Sajid, A., 2021. Indexing Virus and Virus-Like Diseases of Citrus.
- Some biochemical changes in tristeza infected citrus trees in Pakistan. Int. J. Sci. Nature. 2011;2:621-624.
- [Google Scholar]
- Overview of strain characterization in relation to serological and molecular detection of Citrus tristeza Closterovirus. Phyton. 2021;90(4):1063.
- [Google Scholar]
- A population of variants of a viroid closely related to citrus viroid-I in citrus plants. Arch. Virol.. 2000;145(10):2105-2114.
- [Google Scholar]
- Simultaneous detection of six citrus viroids and Apple stem grooving virus from citrus plants by multiplex reverse transcription polymerase chain reaction. J. Virol. Methods. 2002;106(2):235-239.
- [Google Scholar]
- The role of host genes, temperature and polyphenoloxidase in the necrotization of TMV infected tobacco tissue. J. Phytopathol.. 1966;55(2):185-192.
- [Google Scholar]
- Phenolic constituents in the leaves of northern willows: methods for the analysis of certain phenolics. J. Agric. Food. Chem.. 1985;33(2):213-217.
- [Google Scholar]
- Stimulation of defense enzymes in tomato (Solanum lycopersicum L.) and chilli (Capsicum annuum L.) in response to exogenous application of different chemical elicitors. Univ. J. Plant Sci.. 2017;5(1):10-15.
- [Google Scholar]
- Overexpression of polyphenol oxidase in transgenic tomato plants results in enhanced bacterial disease resistance. Planta. 2002;215(2):239-247.
- [Google Scholar]
- Phytophthora canker resistance in cacao: Role of peroxidase, polyphenoloxidase and phenylalanine ammonia-lyase. J. Phytopathol.. 1997;145(7):295-299.
- [Google Scholar]
- Plant defense-related enzymes against pathogens: a review. AGRIEAST. J. Agric. Sci.. 2017;11(1):38-48.
- [CrossRef] [Google Scholar]
- Disease severity index as it affects responses in potato virus X-challenged potato varieties. Int. J. Vegetable Sci.. 2016;22(5):471-479.
- [Google Scholar]
- Graft-Transmissible Diseases of Citrus: Handbook for Detection and Diagnosis. Food & Agriculture Org; 1991.
- Total phenols and sugar content in wheat cultivars resistant and susceptible to Ustilago nuda (Jens.) Rostrup. Biochemie und physiologie der Pflanzen. 1988;183(1):89-93.
- [Google Scholar]
- Genetic control of yellow vein mosaic virus disease in okra and its relationship with biochemical parameters. Euphytica. 2017;213(2):30.
- [Google Scholar]
- First reported occurrence of citrus bent leaf viroid and citrus dwarfing viroid on imported oranges from China and lime fruits from Cambodia. Virusdisease. 2018;29(3):416-417.
- [Google Scholar]
- Effect of foliar application of micronutrients on the yield and quality of sweet orange (Citrus sinensis L.) Pak. J. Biol. Sci. 2007;10(11):1823-1828.
- [Google Scholar]
- Phenolic compounds and related enzymes as determinants of quality in fruits and vegetables. J. Sci. Food Agric.. 2001;81(9):853-876.
- [Google Scholar]
- Maize dwarf mosaic virus infection in susceptible and resistant corn: virus multiplication, free amino acid concentrations, and symptom severity. Phytopathology. 1970;60(11):1605-1608.
- [Google Scholar]
- Polyphenol oxidase: the chloroplast oxidase with no established function. Physiol. Plant.. 1988;72(3):659-665.
- [Google Scholar]
- Basics of Plant Virology. Oxford and IBH Publishing Company Pvt. Limited; 2003.
- Enation leaf curl virus (ELCV): a real threat in major okra production belts of India: a review. J. Pharmacogn. Phytochem. 2018;7(2):3795-3802.
- [Google Scholar]







