Translate this page into:
Biochemical and hematological markers as brucellosis indicators in the Najran region of Saudi Arabia
⁎Corresponding author. mmubaraki@ksu.edu.sa (Murad A. Mubaraki)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Background
Human brucellosis is the most common zoonotic disease in the world. It is common infectious disease in Najran region, south western of Saudi Arabia. Several studies emphasized on the role of biochemical and hematological markers in clinical diagnosis of brucellosis, however to the best of our knowledge this is the first study that has been carried out in Najran city.
Aim
This study aimed to assess the utility of peripheral blood biochemical and hematological in early diagnosis of brucellosis in infected patients. As well as, determine the contributing value of biochemical and hematological markers in differential diagnosis of brucellosis.
Materials and methods
A cross sectional study (October 2019 to May 2020) had been carried out. The study was designed to compare between two groups (100 each), control group (healthy) and patient group (brucellosis). We considered here the liver function Test (Alanine aminotransferase, Aspartate aminotransferase, and Alkaline phosphatase), the Renal Function Test (Urea, Creatinine and Albumin) and the Full Blood Count. Both brucellosis and healthy groups compared together using different settings of statistical analysis.
Results
Significant variations in hematology and biochemistry parameters were observed in patients infected with brucellosis. There was a significantly increased in Alanine aminotransferase and Monocytes level compared to healthy group while other indices of Albumin, Eosinophils, Platelet distribution width, and Mean platelet volume were significantly decreased compared to healthy group. Furthermore, demographically age factor was not significantly differed in both groups. However, there was priority for gender while, males were most vulnerable.
Conclusion
Brucella infection may be associated with monocytosis, eosinopenia, thrombocytopenia and low level of albumin and elevated level of alanine aminotransferase. The study emphasized the importance of Biochemical and hematological parameters as simple, feasible and rapid tests as well as they are very indicative particularly in differential diagnosis.
Keywords
Biochemical
Hematological markers
Brucellosis
1 Introduction
Brucellosis is highly contagious and zoonotic worldwide disease and is considered one of the most seven neglected disease. There are approximately 500,000 cases reported annually yet the true incidence is estimated to be 500,000 to 12,500,000 cases each year (WHO, 1997; Berger, 2019). Brucellosis has negative significant impact on animal production economy because it causes abortion, fertility impairment, milk and meat production losses (Rossetti et al., 2017). Despite Brucellosis has been eliminated from many developed nations, it still constitutes an endemic in several countries such as Mediterranean area, Africa and other countries particularly with low income, inadequate resource and consistent contact between human and animals (Musallam et al., 2016; Ducrotoy et al., 2017; Vhoko et al., 2018). Brucellosis is a main health concern in developing countries including Saudi Arabia especially rural area where the constant contact with livestock. Historically, the first case of brucellosis was identified almost 35 years ago (Kambal et al., 1983). A High incidence of brucellosis (18 cases per 100,000 individuals) was estimated in 2011 according Ministry of Health reported (MOH) (Aloufi et al., 2016). However, the prevalence rate decreased in years between 2004 and 2012 (Aloufi et al., 2016). This is considered higher than those of other industry and non-industry nations. Though, it returned to hit again in 2015 (Alkahtani et al., 2020) the curve of incidence showed drop in the following three years until 2018. Demographically, the highest prevalence was at Al-Qassim and Aseer in the South followed by Hail and the Northern borders of Saudi Arabia (Aloufi et al., 2016). Brucellosis is routinely diagnosed by serology (slide agglutination test) which is considered the first line test yet it may cross react with IgM of different organism such as Salmonella enterica serotype Urbana, Franisella tularensis and other (Alkahtani et al., 2020), in addition to its low sensitivity and specificity in endemic area and chronic cases. Moreover, serologically positive cases refer to exposure but it is not evidence for presence of infection. On the other hand, the clinical signs are very significant but sometimes interfere with malaria mainly in endemic area (Dean et al., 2012). Therefore, laboratory investigation are often supportive including erythrocyte sedimentation rate high, c-reactive protein positive, sometimes anaemia as well as elevated liver function enzymes (alanine and aspartate aminotransferase) (Hull and Schumaker, 2018).
There are a variety of brucellosis treatment regimens available, which vary depending on the organ involved, complications, cost, and availability to care (see in Edathodu et al., 2021). Rifampicin and doxycycline are the World Health Organization's approved treatments for brucellosis in adults (Corbel 2006). Another widely prescribed combination is doxycycline and an aminoglycoside (Alavi and Alavi 2013). In Saudi Arabia, the treatment regimen is chosen based on the patient's features and the severity of the ailment (Edathodu et al., 2021).
Therefore, this study came to our attention to evaluate how the hematological such as complete blood count (CBC) and biochemical parameters such as (liver and renal function tests) have utility in diagnosis of brucellosis, surely together with clinical and serological diagnosis.
2 Material and methods
2.1 Study design and setting and participants
This cross-sectional descriptive comparative study, conducted among cases who serologically positive of brucellosis from October 2019 to May 2020, at King Khalid Hospital in Najran, Saudi Arabia (See map in Fig. 1). Exclusion criteria included use of antibiotics, patients with fever, but negative for Brucella testing. Cases laboratory records were collected for complete blood count, plasma liver, renal function test and serum Brucella testing. Patients with a 1/160 titer and higher were deemed as serologically positive.
Map of Najran, Saudi Arabia (Modified from maphil.com).
2.2 Data collection
Patients were classified as infected with Brucella if the result of serological test for Brucella IgM and IgG was positive. IgM and IgG antibodies were detected in all subjects with Brucella infection by serum agglutination test (SAT). In the SAT, the serum samples were diluted serially with 0.5% saline and equivalent dilution volumes (1:10 to 1:1280) and B. abortus and B. melitensis antigens (Omega Diagnostic Ltd, UK) were mixed in test tubes and incubated for 24 hrs in 37° C incubator. A 1/160 titer and higher were considered as serologically positive. Moreover, all patients who tested positive for Brucellosis were included in this study; patients with fever, but tested negative for brucellosis were excluded from this study. Samples of 5 ml of venous blood from each individual from each category were collected in three kinds of blood tubes (Plain tube, Li-Heparin and EDTA). Ethyl diamine tetra acidic acid (EDTA) anti-coagulated peripheral blood samples were collected in microtainer tube or vacutainer tubes and stored at room temperature to be ready for CBC test and leucocytes differential count (WBC, RBC, Hgb, HCT, MCV, MCH, MCHC, PLT, MPV, PDW, neutrophil, lymphocyte, eosinophil, monocyte and basophil). CBC tests were performed and analysed using full automated hematological analyzer (Unicel DxH800 analyzer) (Beckman coulter Inc.) 3.0 Version software. On the other hand, Li-Heparin anti-coagulated peripheral blood samples were collected in microtainer tube or vacutainer tubes. Samples were spun down analysed in the primary tube then stored at (4–8 °C) for 72 hrs in refrigerator. The biochemical profile of liver such as (ALT, AST, and ALP) and renal profile such as (Urea, Creatinine, and Albumin) were analysed using a full automated biochemistry analyzer (Unicel DxC800 analyzer) (Beckman coulter Inc.) 3.0 Version software. The procedure according to the work institute of running the samples is the maintenance of machines according to the reference manual. The samples should be identified, giving lab number and put in a shaker for at least 5 min depending on the sample type. The interpretation of the results is done by specialists of biochemistry and hematology and technicians in the departments of biochemistry and hematology. The results have been published in accordance with the general policy.
2.3 Ethical consideration
The study received ethical approval from the Institutional Review Board, Ministry of Health, General Directorate of Health Affaire Najran (Ref. No. 2019–22 E). All data were kept confidential and were available only to the research team.
2.4 Statistical analysis
Statistical analysis was performed using (SPSS, version 22.0 for windows) and (GraphPad prism version 8.0 for windows). The data of analysis was recorded as mean ± standard error of mean (SEM) of the constant variables according their group. Categorical variables with the corresponding percentages were identified as frequencies. Independent sample T-test (unpaired t test) was used to expose variations and differences in the variables category between all groups. A P value of <0.05 was considered significant for all statistical parameters.
3 Results
3.1 Socio-demographic characteristics of the brucellosis group and healthy group
A total of 100 brucellosis patients and 100 healthy participants as a control group were enrolled in this study. The majority of participants for both healthy and infected groups were mainly male (58%, 83%), in compare to female (42%, 17%) respectively. However, in the term of age, there was no statistical difference between the healthy group (43.58 ± 19.60) and the infected group (43.33 ± 18.19). On the contrary, there was priority for gender; the incidence of brucellosis in males was higher than of those of healthy (Fig. 2).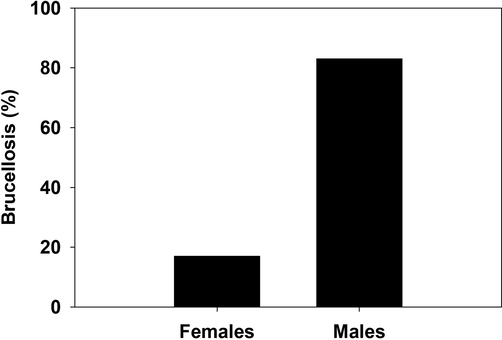
Percentage of brucellosis in infected females and males.
3.2 Linkage between alterations in hematological profile (CBC) and positive brucellosis cases
Comparing the complete blood count (Table 1) and leucocyte differential count (Table 2) from each group, we observed no remarkable differences in CBC parameters except the Platelet Distribution Width (PDW), Mean Platelet Volume (MPV), monocyte and eosinophile percentage. There were no significance between white blood cells count (WBC), Red blood cells count (RBC), Hemoglobin (Hb), Hematocrit (Hct), Mean Cell Value (MCV), Mean Corpuscular Hemoglobin (MCH), Mean Corpuscular Hemoglobin Concentration (MCHC) and platelet count (PLT) in brucellosis and healthy group. On the contrary, the parameters of PDW and MPV of diseased group displayed the mean ± SEM values of (11.05 ± 0.22, 9.36 ± 0.15), which referred to a significant reduction compared to healthy group that showed the mean ± SEM values of (11.93 ± 0.278, 9.99 ± 0.16) respectively. The other two parameters were relating to differential of WBC count where, monocyte level (MONO%) was considerably increased (10.34 ± 0.50) in patients than those of healthy (8.664 ± 0.287) Moreover, eosinophil level (EOS%) significantly decreased (2.03 ± 0.26) in brucellosis patients compared to the control group (3.224 ± 0.262). The other components of differential WBC count indicated no notable significance between groups. (*), Significance at p ≤ 0.05. (*), Significance at p ≤ 0.05.
Characteristic
Description
Healthy (n = 100)
Brucellosis (n = 100)
p value
White blood cells
Mean ± SEM
6.649 ± 0.356
6.740 ± 0.472
(×103/µL)
min – max
1.37–32.31
1.53–42.27
0.877
Red blood cells
Mean ± SEM
5.096 ± 0.071
4.995 ± 0.076
(×1012/µL)
min – max
3.39–7.23
2.43–7.13
0.333
Hemoglobin (g/L)
Mean ± SEM
13.654 ± 0.209
13.470 ± 0.216
min – max
7.1–19.7
6.3–17.1
0.541
Hematocrit (%)
Mean ± SEM
41.715 ± 0.601
41.002 ± 0.674
min – max
22.30–53.50
11.78–51.70
0.431
MCV.FL
Mean ± SEM
82.106 ± 0.760
82.987 ± 0.625
min – max
61.5–100.9
61.2–99.3
0.371
MCH.PG
Mean ± SEM
26.876 ± 0.295
27.146 ± 0.267
min – max
19.3–34.3
18.3–32.5
0.498
MCHC %
Mean ± SEM
32.462 ± 0.344
33.154 ± 0.494
min – max
3.0–36.0
27.1–77.8
0.252
PDW.FL
Mean ± SEM
11.925 ± 0.278
11.045 ± 0.218
min – max
7.2–23.6
6.9–17.9
0.014*
MPV.FL
Mean ± SEM
9.994 ± 0.155
9.360 ± 0.150
min – max
6.5–14.6
6.20–12.60
0.004
PLT 10^3/μl
Mean ± SEM
285.090 ± 8.748
262.620 ± 9.735
min – max
77–558
50–625
0.088
Characteristic
Description
Healthy (n = 100)
Brucellosis (n = 100)
p value
NEUTS%
Mean ± SEM
47.549 ± 1.730
46.315 ± 1.544
min–max
21.8–91.2
8.5–84.0
0.595
LYMPH%
Mean ± SEM
40.035 ± 1.484
41.298 ± 1.395
min–max
2.6–66.3
10.5–74.4
0.536
MONO%
Mean ± SEM
8.664 ± 0.287
10.341 ± 0.496
min–max
1.0–17.6
0.9–43.6
0.004*
EOS%
Mean ± SEM
3.224 ± 0.262
2.026 ± 0.255
min–max
0.0–16.7
0.0–17.6
0.001*
BASO%
Mean ± SEM
0.650 ± 0.059
0.537 ± 0.038
min – max
0.0–5.1
0.0–2.5
0.109
3.3 Linkage between alterations in biochemical parameters and positive brucellosis cases
Hepatic biomarkers and renal panels were laboratory investigated for both healthy and diseased groups. Hepatic biomarkers include alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP) and albumin (ALB) while the renal panels include creatinine (CREAT) and urea (BUN). The activity of ALT enzyme revealed a significant increase (43.871 ± 3.624) in infected group when it was compared to healthy one (31.075 ± 2.059). In contrast, the activity of other two hepatic enzymes AST and ALP showed no any distinguished significance between both groups. Moreover, albumin level revealed a remarkable decrease in brucellosis patients (34.976 ± 0.702) compared to the healthy group (38.003 ± 0.595). On the other hand, the renal panels whether creatine or urea levels showed insignificance levels when they were analyzed in both groups (Table3). (*), Significance at p ≤ 0.05.
Description
Characteristic
Healthy (n = 100)
Brucellosis (n = 100)
p value
Mean ± SEM
ALT U/L
31.075 ± 2.059
43.871 ± 3.624
min – max
8.9–109.0
8.0–209.0
0.002*
Mean ± SEM
AST U/L
31.423 ± 2.173
37.684 ± 3.640
min – max
8.0–174.0
12.0–327.0
0.141
Mean ± SEM
ALP U/L
73.1222 ± 4.602
82.678 ± 3.467
min – max
33.0–366.0
35.0–255.0
0.099
Mean ± SEM
ALB g/L
38.003 ± 0.595
34.976 ± 0.702
min – max
20.0–49.0
18.0–48.0
0.001*
Mean ± SEM
BUN mmol/L
4.7779 ± 0.213
4.715 ± 0.288
min – max
1.80–17.20
18.0–48.0
0.861
Mean ± SEM
CREAT.μmol/L
67.3800 ± 0.003
83.14 ± 2.664
min – max
12.30–213.00
31.63–632.00
0.0925
4 Discussion
Brucellosis is a highly transmissible worldwide zoonotic disease, transmitted between animals and humans. Brucella melitensis is regarded as the most virulent and prevalent strain in the world, including Saudi Arabia and the infection is spread through contact with animals and animal products (Al Jindan, 2021). Moreover, eradication of disease encounters a very strong challenge where it is distributed all over the world with high prevalence in the developing nations. In Saudi Arabia, it constitutes a health issue this because of presence of some customs and traditions particularly in rural region where the problem of consumption of unpasteurized dairy products and direct contact with livestock. Brucellosis can affect one or more organs throughout the body with manifestation of variable signs. Lack of suitable human vaccine so far along with failure of therapeutic regime to a certain degree (Bosilkovski et al., 2021) as well as increasing global burden of the disease all of them together are considering challenging factors undermine the management strategy of brucellosis. Furthermore, given the signs of brucellosis are nonspecific and presence of some drawbacks of serological tests (still the gold standard diagnosis) to a certain degree, the study tried to find out a laboratory diagnostic aide to predict early brucellosis infection as well as differential diagnosis. It is well known that diagnosis of brucellosis depends on clinical manifestations, serology and bacteriological data. However, in the case of endemicity these diagnostic measures are facing resistance because of persistent elevation of antibodies even after treatment (Ariza et al., 1992) as well as wide range of clinical signs. Thus, the use of adjunctive procedure could help in diagnosis and follow up the disease (Ozturk et al., 2012). Consistent with these ideas, our work studied the contribution of hematological and biochemical markers in diagnosis of brucellosis through comparing the alterations of theses indices between diseased and healthy group. Our results found that all hematological indices (CBC) and leucocytes differential count are almost the same between diseased and control groups except four readings PDW, MPV, monocyte and eosinophil percentage. Brucellosis characterized by case of inflammatory changes including elevation of acute phase reactants (Cift and Yucel, 2018). MPV and PDW are defined as indirect inflammatory markers and platelets activation indicator (Dinc et al., 2015). MPV is used as platelet activation index and it is might be altered by inflammation state (Ozturk et al., 2012; Dinc et al., 2015). However, MPV in many case were not indicative (Sandhaus and Meyer, 2002) it is taken by many physicians as clue of abnormalities of platelet counts as well as it was considered as contributing factor in diagnosis of brucellosis in addition, it is cheap and more feasible test together with other inflammatory markers (Ozturk et al., 2012). This comes in compliance with our study since MPV level was found to be significantly lower than those of control group. Concerning PDW which is another inflammatory index, it highly increased in some disorders such as pulmonary tuberculosis as common inflammatory response (Sahin and Yıldız, 2012). However, the current studied brucellosis cases showed significant reduction. Despite disorders are of bacterial origin, they expressed PDW differently, this might be for unclear reason. Leucocytes differential count played another important role in this study where eosinophiles and monocytes showed significant changes at the level of diseased group. In the present study, significant reduction in eosinophil was observed in brucellosis patients. In the same context it was revealed that typhoid fever, paratyphoid fever, extreme tissue damage after surgery, and the use of adrenal cortex hormone or adrenocorticotrophic hormone all cause eosinopenia Jiang (Jiang et al., 2019). Additionally, neutropenia is a pathognomonic finding in patients suffering from typhoid fever (Mallouh and Sa'di, 1987). In addition, it was found that Brucella antigen share some component with typhoid and paratyphoid fever, thus it became clear that Brucella cause neutropenia as well. This finding also was supported by finding that explained evasion’s pattern of typhoid fever bacteria that help them to avoid immune cells and neutrophil cells (Winter et al., 2014). Furthermore, eosinopenia was considered as a strong diagnostic marker for differentiating between infected and non-infected case, nonetheless, it was a moderate index for distinguishing between Systemic inflammatory response syndrome (SIRS) and infection (Abidi et al., 2008). Monocyte is one of the body defences’ lines and take part of innate immunity. It is activated by chronic infection and diseases and other many disorders. Hence, it is normally triggered by Brucella infection. The current study observed significant increase in monocytes levels in infected patients as well as, it was revealed monocytosis in patients suffered from acute brucellosis which is consistent with study of Tolomeo and his colleagues (Tolomeo et al., 2003). This might be returned to response of high level of resistance to apoptosis because of phagocytic function of the monocyte. Brucellosis is not restricted to only one organ but can distribute to involve various organs particularly digestive tract (García Casallas et al., 2018). Furthermore, acute and chronic brucellosis were incriminated in liver infection as an elevation in ALT and AST value and sometime hepatosplenomegaly can develop (Uluğ et al., 2010). In the same context our laboratory findings revealed deterioration of some liver function parameters such as an elevation in ALT and decreasing of albumin level that is very strong proof of liver involvement. Moreover, acute liver failure is not common complication of brucellosis however it was found to be implicated, in addition, elevation of ALT and falling of albumin values were recorded (García Casallas et al., 2018). Hence, dependence on liver function test is very indicative particularly at level of differential diagnosis. In the current study, the age factor did play a significant role in the incidence of brucellosis in both groups in contrast to the result that showed the populations with an age of ranged from 21 to 60 years are more infected (Alkahtani et al., 2020). In this study, majority of studied cases were males, our results showed that high incidence of brucellosis in males than females. These results come in agreement with studies carried out in southern Saudi Arabia (Alkahtani et al., 2020) and Kiboga District, Central Uganda (Tumwine et al., 2015) but in contrast to those were obtained in Iran (Nematollahi et al., 2017). High prevalence of brucellosis in males could be due to the nature of work of men and this could give explain why brucellosis is dubbed as an occupational disease because it is mainly linked to work infection (Memish, 2001) furthermore, most of Saudi men usually drink fresh raw camel milk particularly when they go for camping for a while (Alkahtani et al., 2020).
5 Strengths and limitations
Several limitations have been encountered. Firstly, we did not recruit some investigation like CRP, ESR, pro-inflammatory cytokine profile, ELISA and blood culture. However the current study concerned to investigate hematological and biochemical indices as early indicators of Brucella infection. This is because of the affordability, rapidity and feasibility of these tests. On contrary we found other tests such as ELISA and blood culture were expensive and need much time. Secondly, the current study did not evaluate the anaemia in Brucella patients which has been associated with brucellosis, and did not collect data on serum iron, vitamin B12, ferritin, and total bilirubin that determine the anaemia. Thirdly, due to the covid19 pandemic that swept the world in 2020, a small sample size was carried out in this study. Therefore, the results and conclusion were not typical of the brucellosis population in Najran city for one year.
6 Conclusion
Based on our laboratory findings including alterations in some hematological inflammatory indices as well as changes in some biochemical markers, we strongly recommend utilize these parameters as a complementary diagnostic tools together with serological and bacteria data particularly in differential diagnosis and follow up diagnosis. The infection was associated with monocytosis, eosinopenia, thrombocytopenia and low level of albumin and elevated level of alanine aminotransferase. Furthermore, such kind of this investigation is more affordable, rapid and need no much experience. Eventually, wide further studies are needed to strengthen our findings and to understand the underlying mechanism for these findings.
Acknowledgement
This study was supported by Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2022R96), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia and also was supported by Researchers Supporting Project (RSP2021/25), King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Eosinopenia is a reliable marker of sepsis on admission to medical intensive care units. J. Crit. Care. 2008;12(2):1-10.
- [Google Scholar]
- Scenario of pathogenesis and socioeconomic burden of human brucellosis in Saudi Arabia. Saudi J. Biol. Sci.. 2021;28(1):272-279.
- [Google Scholar]
- Sero-prevalence and risk factors of brucellosis among suspected febrile patients attending a referral hospital in southern Saudi Arabia (2014–2018) BMC Infect Dis. 2020;20(1)
- [Google Scholar]
- Trends of reported human cases of brucellosis, Kingdom of Saudi Arabia, 2004–2012. J. Epidemiol. Glob. Health.. 2016;6(1):11-18.
- [Google Scholar]
- Treatment of brucellosis: a systematic review of studies in recent twenty years. Casp. J. Intern. Med.. 2013;4(2):636-641.
- [Google Scholar]
- Corbel, M.J., 2006. Brucellosis in humans and animals [Internet]. WHO. 2006. p. 1–102. Available from: http://www.who.int/csr/resources/publications/Brucellosis.pd.
- Specific antibody profile in human brucellosis. Clin. Infect. Dis.. 1992;14(1):131-140.
- [Google Scholar]
- Brucellosis: Global Status. Los Angeles, CA: GIDEON Informatics. Inc.; 2019.
- The current therapeutical strategies in human brucellosis. Infection.. 2021;49(5):823-832.
- [Google Scholar]
- Comparison of inflammatory markers between brucella and non-brucella epididymo-orchitis. Int. Braz. J. Urol.. 2018;44:771-778.
- [Google Scholar]
- Clinical manifestations of human brucellosis: a systematic review and meta-analysis. PLoS. Negl. Trop. Dis. 2012;6(12)
- [Google Scholar]
- “New parameter in diagnosis of acute appendicitis: platelet distribution width. World. J. Gastroenterol.. 2015;21(6):1821-1826.
- [Google Scholar]
- Brucellosis in sub-Saharan Africa: current challenges for management, diagnosis and control. Acta. Trop.. 2017;165:179-193.
- [Google Scholar]
- Clinical manifestations and treatment outcomes of human brucellosis at a tertiary care center in Saudi Arabia. Ann. Saudi Med.. 2021;41(2):109-114.
- [Google Scholar]
- Acute liver failure complication of brucellosis infection: a case report and review of the literature. J. Med. Case Rep.. 2018;12(1):1-5.
- [Google Scholar]
- Comparisons of brucellosis between human and veterinary medicine. Infect. Ecol. Epidemiol.. 2018;8(1):1500846.
- [Google Scholar]
- Epidemiological characteristics, clinical manifestations and laboratory findings in 850 patients with brucellosis in Heilongjiang Province. China. BMC infect. Dis.. 2019;19(1):1-6.
- [Google Scholar]
- Brucellosis in Riyadh, Saudi Arabia. A microbiological and clinical study. Trans. R. Soc. Trop. Med. Hyg.. 1983;77(6):820-824.
- [Google Scholar]
- White blood cells and bone marrow in typhoid fever. J. Pediatr. Infect. Dis.. 1987;6(6):527-529.
- [Google Scholar]
- Brucellosis control in Saudi Arabia: prospects and challenges. J. Chemother.. 2001;13(1):11-17.
- [Google Scholar]
- Systematic review of brucellosis in the Middle East: disease frequency in ruminants and humans and risk factors for human infection. Epidemiol. Infect.. 2016;144(4):671-685.
- [Google Scholar]
- Epidemiological characteristics of human brucellosis in Hamadan Province during 2009–2015: results from the National Notifiable Diseases Surveillance System. Int. J. Infect. Dis.. 2017;61:56-61.
- [Google Scholar]
- Mean platelet volume in assessment of brucellosis disease. Biomed. Res.. 2012;23(4):541-546.
- [Google Scholar]
- Caprine brucellosis: a historically neglected disease with significant impact on public health. PLoS. Negl. Trop. Dis.. 2017;11(8):e0005692.
- [Google Scholar]
- Prominent features of platelet count, plateletcrit, mean platelet volume and platelet distribution width in pulmonary tuberculosis. Multidiscip. Respir. Med.. 2012;7(1):1-7.
- [Google Scholar]
- How useful are CBC and reticulocytereports to clinicians? Am. J. Clin. Pathol.. 2002;118(5):787-793.
- [Google Scholar]
- Monocyte and lymphocyte apoptosis resistance in acute and chronic brucellosis and its possible implications in clinical management. Clin. Infect. Dise.. 2003;36(12):1533-1538.
- [Google Scholar]
- Human brucellosis sero-prevalence and associated risk factors in agro-pastoral communities of Kiboga District, Central Uganda. BMC Public Health.. 2015;15(1):1-8.
- [Google Scholar]
- An unusual presentation of brucellosis: acute hepatitis. Braz. J. Infect. Dis.. 2010;14:641-642.
- [Google Scholar]
- Estimating the prevalence of brucellosis in cattle in Zimbabwe from samples submitted to the central veterinary laboratory between 2010 and 2014. Vet. Ital.. 2018;54(1):21-27.
- [Google Scholar]
- Fact sheet N173. Geneva, Switzerland: World Health Organization; 1997.
- Salmonella enterica Serovar Typhi conceals the invasion-associated type three secretion system from the innate immune system by gene regulation. PLoS Pathogens. 2014;10(7):e1004207
- [Google Scholar]







