Translate this page into:
Bioactivity of Syzygium aromaticum (L.) Merr. & L.M.Perry extracts as potential antimicrobial and anticancer agents
⁎Corresponding authors. mohamed.yassin.ah2016@gmail.com (Mohamed Taha Yassin), asali@ksu.edu.sa (Ashraf Abdel-Fattah Mostafa),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objectives
Cancer is the most common cause of death every year. Moreover, high incidence of bacterial resistance to the most frequently used antibiotics contributes a significant death and disability worldwide. Hence, formulation of novel antimicrobial and anticarcinogenic agents is required.
Methods
In the current study, the antibacterial efficiency of clove extracts (acetonic, dichloromethane, ethanolic, and petroleum ether) against four pathogenic bacterial strains [Escherichia coli, Salmonella typhi, Staphylococcus aureus and Methicillin-resistant Staphylococcus aureus (MRSA)] was estimated by disc diffusion method. Furthermore, the anticancer potency of S. aromaticum extracts against HCT human colon carcinoma was appraised using MTT assay. The phytochemical ingredients of the most effective extract were determined using gas chromatography–mass spectrometry (GC–MS) analysis.
Results and conclusion
The dichloromethane extract presented the highest antibacterial potency against the pathogenic isolates recording minimum inhibitory concentration (MIC) of 1 mg/disc against MRSA strain and 0.5 mg/disc against both of E. coli and S. typhi strains. Furthermore, the minimum bactericidal concentration (MBC) of clove dichloromethane extract was recoded at 2 mg/disc against MRSA stain, and 1 mg/disc against E. coli and S. typhi strains. The dichloromethane extract of clove showed the lowest cytotoxic activity against HCT cell line whereas, the ethanolic extract exerted the highest efficacy with relative IC50 of 6.71 and 2.53 µg/ml respectively. GC–MS analysis revealed that the clove dichloromethane extract was comprised of eugenol (50.65%) and eugenyl acetate (12.54%) as major active components. In conclusion, clove extracts could be utilized as potential antibacterial and anticarcinogenic agents.
Keywords
Syzygium aromaticum, Antibacterial
Minimum inhibitory concentration
Anti-cancer assay, GC–MS
1 Introduction:
Multidrug resistant bacteria causing infectious diseases were responsible for significant mortality, particularly in developing countries (Nii-Trebi, 2017). Bacterial resistance in the U.S contributed to over 2 million disorders and 23,000 deaths per year according to statistics of the CDC center (Okwu et al., 2019). High prevalence of methicillin-resistant Staphylococcus aureus strains (MRSA) was recorded in both health-care and community environments (David and Daum, 2010). Epidemiological studies reported the variation in MRSA infection rates among different countries, for example: 33%–43% in Nigeria (Okwu et al., 2014); 25%–50% in India (Arunkumar et al., 2017). Staphylococcus aureus was responsible for approximately 1064 hospitalizations and 241,188 disorders in the United States, according to statistics of CDC center (Scallan et al., 2011). Ingestion of the S. aureus enterotoxin followed by a 6–10 hrs incubation period resulted in food poisoning episodes as vomiting, diarrhea, nausea, abdominal cramps, dizziness, and general weakness (Ravensbergen et al., 2017). In contrast, Salmonella spp. belonging to Enterobacteriaceae group were reported to be majorly accountable for foodborne outbreaks in the U.S (Control and Prevention, 2012). Reportedly, a high incidence of salmonellosis outbreaks was recorded by a total of 94,625 infections in Europe for the year 2015 (Authority, Prevention et al., 2018). Furthermore, the pathogenic strains of E. coli were responsible for significant morbidity and mortality worldwide, for example, E. coli strain O157:H7 was recorded to cause 63,000 outbreaks, 2100 hospitalizations, and 20 deaths every year (Croxen et al., 2013). Cancer is considered as one of the outstanding causes of death worldwide that is characterized by uncontrolled multiplication of normal human cells (Mubeen and Kini, 2012). The main conventional therapies commonly used for cancer management were chemotherapy, radiotherapy and surgery (Ju et al., 2018). The conventional therapies target both cancer and healthy cells nonselectively so that it is necessary to elaborate new anticancer drugs using medicinal plants as a substitution of these conventional therapies avoiding incidence of cancer cell resistance and reducing the toxicity resulting from using single drug (Aumeeruddy and Mahomoodally 2019). Furthermore, medicinal plants were reported as a prospective source of antimicrobial bioactive agents because of their constituents as tannins, flavonoids, phenolic compounds and alkaloids (Djeussi et al., 2013).
The genus Syzygium has been reported as the largest genus of flowering plants that was comprised of approximately 1200–1800 species (Mahomoodally et al., 2020). Syzygium aromaticum belonging to Myrtaceae family was of medical importance due to its antimicrobial, antioxidant, anti-cancer, anti-inflammatory and anti-diabetic properties (Lau and Rukayadi 2015). The essential oil of clove suppressed the growth of S. aureus and E. coli strains with inhibition zone diameters of 16 and 6 mm respectively (Saikumari et al., 2016). Syzygium aromaticum extracts were also reported to prohibit the proliferation of HeLa (cervical cancer), Te-13 (esophageal cancer), DU-145 (prostate cancer) and MCF-7 (breast cancer) cell lines significantly (Dwivedi et al., 2011).
Excessive incidence of multidrug resistant bacterial strains necessitates the formulation of novel antimicrobial therapeutic agents. Furthermore, high death rate globally due to cancer and the side effects of commonly used chemotherapeutic agents necessitate the formulation of new anti-carcinogenic agents. Thus, the current study was conducted to detect the antibacterial efficiency of clove extracts against pathogenic bacterial strains. Moreover, the anticancer potency of S. aromaticum extracts was detected using the MTT assay.
2 Material and methods
2.1 Plant extracts preparation
Clove buds were collected from the local market in Riyadh, Saudi Arabia, identified by herbarium of the Botany Department, College of Science, King Saud University and deposited with voucher number of (KSU-14682). All active phytochemicals were extracted using four different organic solvents (petroleum ether, dichloromethane, acetone and ethanol) with different polarities of 0.1, 3.1, 5.1, and 5.2, respectively. The buds were washed with 0.5% sodium hypochlorite solution (NaOCl) for disinfection, washed with sterilized distilled water and dried. Grinding of the buds was performed using a mechanical mortar to attain a homogenized powder. 50 g of clove powder was drenched in 200 ml of different solvents and was incubated for 48 h at 25 °C over a magnetic stirrer. Centrifugation of different solvent extracts was carried out at a speed of 9000 rpm for 10 min, filtered using a Whatman filter paper to obtain clear filtrates and discard of the plant remainders. Eventually, the solvents were evaporated using a rotatory evaporator in order to concentrate the extracts. The extracts were preserved at 4 °C till use and the extraction yields were calculated as shown in the subsequent formula: Percentage of the extract yield = (R/S) × 100 where R referred to the extract residue weight and S referred to the weight of the raw sample.
2.2 Antimicrobial efficiency of the clove extracts
2.2.1 Preparation of bacterial inocula
Bacterial strains subjected to the study were (S. aureus, Staphylococcus-MRSA, S. typhi and E. coli) and were attained from culture collection of Botany and Microbiology Department, College of Science, King Saud University, Saudi Arabia. The microbial strains were subcultured in Mueller-Hilton agar slants and kept for 48 h at 35 °C to obtain fresh inoculums. The microbial growth was gathered using 5 ml of sterile saline water. Finally, adjustment of absorbance was done at 580 nm using a spectrophotometer to obtain a microbial count of 107/ml.
2.2.2 Antibacterial assay
Disk diffusion method was done to detect the antibacterial efficacy of S. aromaticum extracts against some Gram-positive strains as (S. aureus and MRSA) and other Gram-negative isolates as (E. coli and S. typhi). About fifteen ml of Mueller-Hinton agar medium was poured into sterile Petri dishes as a basal layer followed by the addition of 10 ml seeded medium that was previously inoculated with bacterial suspension (1 ml of 107CFU/100 ml of medium) to obtain 105 CFU for each ml of the medium (Yassin et al., 2020a). 8 mm sterile paper disks were impregnated with clove extracts at a concentration of 10 mg/ml and put over the seeded plates. Chloramphenicol as antibacterial agent (30 µg/discs) was utilized as positive control against different bacterial strains according to CLSI (CLSI, 2000). The seeded plates were kept in a refrigerator at 4 °C for 2 h to permit the diffusion of S. aromaticum extract through the medium. Incubation of plates was achieved at 35 °C for 48 h and the diameters of suppressive zones were calculated using a Vernier caliper.
2.3 Detection of minimum inhibitory concentration (MIC)
Minimum inhibitory concentration (MIC) is specified as the least concentration of clove extract showing antibacterial potency. MIC was detected for clove dichloromethane extract as it demonstrated the highest antibacterial efficacy. Detection of MIC was achieved by disk diffusion method as mentioned above, in which 8 mm sterile filter paper disks were impregnated with clove extracts (0.25, 0.5, 1.0, 2.0, 4.0, 8.0 mg/disc). Plates were refrigerated at 4 °C for 2 h to permit the diffusion of extracts through the medium. Eventually, the plates were put in the incubator at 35 °C for 48 h and the diameters of suppressive zones were imputed using a Vernier caliper.
2.4 Determination of minimum microbicidal concentration
Minimum bactericidal concentration (MBC) was specified as the least clove concentration resulting in removal of bacterial growth. Inocula were collected from the suppressive zones of MIC concentration and the two other sequential concentrations and plated onto Mueller-Hinton agar plates. The plates were finally put in the incubator at 35 °C for 48 h.
2.5 In vitro anticancer assay
Human colon carcinoma cells (HCT) were supplemented from Zoology dept., Faculty of Science, King Saud University. Anticancer efficacy of clove (acetonic, dichloromethane, ethanolic and petroleum ether) extracts was done using MTT assay. Anti-cancer potency of S. aromaticum extracts against HCT cells was performed as described by (Yassin et al., 2020b).
2.6 GC–MS analysis of clove extracts
Chemical investigation of clove dichloromethane extract was performed as it exhibited the highest antibacterial potency. The chemical analysis was determined using the GC–MS (Trace 1300/Tsq 8000 Triple Quadrupole) with a TG 5MS column (30 m × 0.25 mm, 0.25 μm film thickness). Conditions of GC/MS analysis were optimized as: The carrier gas was Helium with a flow rate of 1 ml/min, 250 °C was the temperature of injector and detector, 1:50 was the split ratio, oven temperature was 280 °C and it was programmed to be increased at a rate of 5 °C/min, and sample injection volume was 1.0 μl. Conditions of mass spectrometry were optimized as follows: 70 eV was the ionization potential; mass range from m/z, 40–400 amu; 2000 V was the electron multiplier energy. The phytochemicals of clove dichloromethane extract were detected by comparing the GC–MS results with reference spectral mass data and retention times of the NIST database (Yassin et al., 2020c).
3 Results
3.1 Extracts yield
The highest extraction yield (7.21%) was attained using dichloromethane as a solvent followed by ethanol (5.21%), petroleum ether (4.21%), and acetone (2.96%).
3.2 Antibacterial assay
All S. aromaticum extracts exhibited antimicrobial potency against the pathogenic bacterial strains with different sensitivity patterns as demonstrated in Fig. 1. The dichloromethane extract of clove showed the highest antimicrobial efficacy against the concerned bacterial isolates (S. aureus, MRSA, S. typhi, and E. coli) with inhibition zone diameters of 18.20, 17.25, 21.15, and 24.2 mm, respectively as listed in Table 1. The Gram-negative strains (E. coli and S. typhi) were more susceptible to different clove extracts than the Gram-positive strains (S. aureus, MRSA).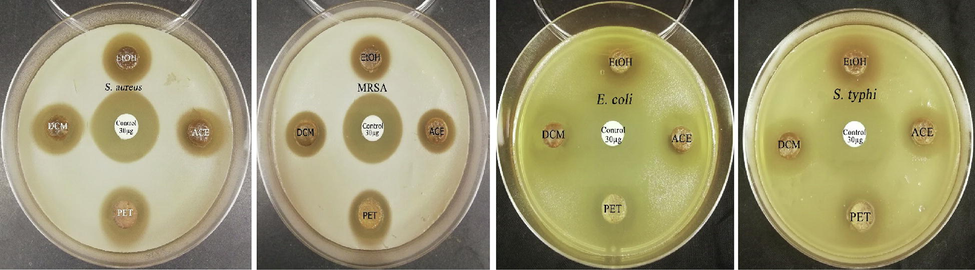
Antibacterial activity of clove extracts (10 mg/disc) against different bacterial strains. EtOH: ethanolic extract, ACE: acetonic extract, PET: petroleum ether extract, DCM: dichloromethane extract. Control: Chloramphenicol antibacterial agent (30 µg/disc).
Clove extracts (10 mg/disc)
Inhibition zone diameter (mm) of bacterial strains
Gram + ve bacteria
Gram −ve bacteria
S. aureus
MRSA
S. Typhi
E. coli
Acetonic extract
16.05 ± 0.09
14.85 ± 0.26
19.60 ± 0.28
17.80 ± 0.35
Dichloromethane extract
18.20 ± 0.63
17.25 ± 0.09
21.15 ± 0.43
24.20 ± 0.06
Ethanolic extract
18.10 ± 0.34
16.55 ± 0.12
19.45 ± 0.49
19.12 ± 0.23
Petroleum ether extract
15.90 ± 0.46
16.65 ± 0.14
18.95 ± 0.25
18.55 ± 0.14
Chloramphenicol (30 µg/disk)
26.55 ± 0.16
25.30 ± 0.29
28.05 ± 0.89
31.80 ± 0.34
3.3 Detection of minimum inhibitory concentration
Minimum inhibitory concentration (MIC) for the dichloromethane extract of clove was detected as that extract revealed the highest efficiency against the concerned pathogenic bacterial strains, for example, MIC for S. aureus and MRSA was 1 mg/disc and exhibited suppressive zones of 9.63 and 8.67 mm, respectively. In contrast, MIC of dichloromethane extract against Gram-negative strains (E. coli and S. typhi) was 0.5 mg/disc with corresponding suppressive zones of 9.23 and 10.64 mm, respectively as listed in Table 2. MIC data confirmed that the Gram-negative isolates were more sensitive to clove extracts than the Gram-positive ones.
Concentration of clove extract (mg/disc)
Inhibition zone diameter (mm) of bacterial strains
Gram + ve bacteria
Gram − ve bacteria
S. aureus
MRSA
S. Typhi
E. coli
0.25
0.00 ± 0.0
0.00 ± 0.00
0.00 ± 0.0
0.00 ± 0.0
0.50
0.00 ± 0.0
0.00 ± 0.00
9.23 ± 0.34
10.64 ± 0.12
1.00
9.63 ± 0.18
8.67 ± 0.26
12.34 ± 0.41
13.65 ± 0.56
2.00
12.35 ± 0.25
11.18 ± 0.31
15.18 ± 0.12
16.23 ± 0.17
4.00
14.23 ± 0.19
13.21 ± 0.47
17.33 ± 0.24
19.23 ± 0.32
8.00
16.45 ± 0.38
15.29 ± 0.08
19.14 ± 0.15
21.18 ± 0.29
3.4 Detection of minimum bactericidal concentration
The microbicidal activity of the effective extract was evaluated to detect the lowest concentration presenting cidal activity. Minimum bactericidal concentration (MBC) of clove dichloromethane extract was 2 mg/disc against S. aureus and MRSA strains, whereas it was 1 mg/disc against E. coli and S. typhi isolates.
3.5 Cytotoxicity assay
The ethanolic extract of clove exerted the maximum anticancer potency against HCT cell line with IC50 of 2.53 µg/ml. In contrast, dichloromethane extract of clove demonstrated the least efficacy against HCT cells with relative IC50 of 6.71 µg/ml. Moreover, the petroleum ether and acetonic clove extracts showed a moderate cytotoxic efficiency against HCT cells recording IC50 of 6.48 and 2.91 µg/ml respectively as demonstrated in Fig. 2.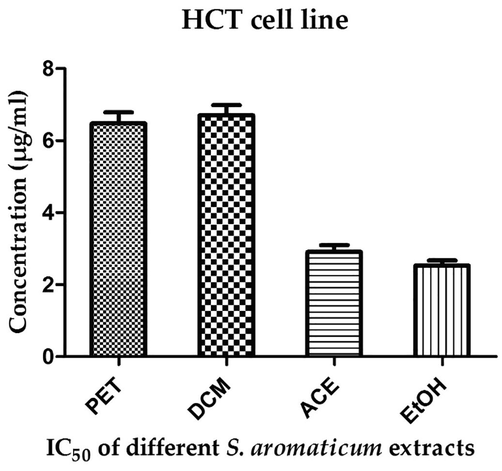
Anticancer activity of clove extracts against HCT human colon carcinoma cell line. EtOH: ethanolic extract, ACE: acetonic extract, PET: petroleum ether extract, DCM: dichloromethane extract.
3.6 GC–MS analysis of clove extracts
Chemical investigation of clove dichloromethane extract was performed as it exhibited the highest antibacterial potency. The dominant constituent of clove dichloromethane extract was found to be eugenol (50.65%), followed by eugenyl acetate (12.54%), allopurinol, dimethyl (10.98%), and isoquinoline,1-[3-methoxy-5-hydroxybenzyl]-1,2,3,4,5,8-hexahydro-6-methoxy (9.86%) (Table 3).
Compounds
Chemical formula
Mol. weight
RT
% of Total
3-[(Z)2-Phenylethenyl]cholestan-2-one
C35H52O
488.80
9.271
4.17
2H-Pyrazino[1,2-b]isoquinoline-1,3(4H,6H)-dione,2-(4-fluorophenyl)-11,11a-dihydro-8,9-dimethoxy
C20H19FN2O4
370.40
9.668
4.60
Eugenol
C10H12O2
164.20
10.773
50.65
Acetyl eugenol
C12H14O3
206.24
11.310
12.54
Allopurinol, dimethyl
C7H8N4O
164.16
11.531
10.98
Allocryptopine
C21H23NO5
369.41
11.676
7.19
Isoquinoline,1-[3-methoxy-5-hydroxybenzyl]-1,2,3,4,5,8-hexahydro-6-methoxy
C18H23NO3
301.40
11.787
9.86
4 Discussion
High incidence of multidrug resistant pathogens worldwide contributes to a significant mortality rate every year (Chan et al., 2014). The current study demonstrated that the S. aromaticum (acetonic, dichloromethane, ethanolic, petroleum ether) extracts possessed a potential anti-MRSA activity with suppressive zones of 14.85, 17.255, 16.55, and 16.65 mm, respectively. These findings were in accordance with those of Mandal et al. (2011) who confirmed the antibacterial efficacy of S. aromaticum extract against MRSA isolates with inhibitory zones ranging from 19 to 23 mm. Pandey and Singh (2011) stated that clove methanolic extract exerted antibacterial potency against S. aureus and E. coli strains recording MIC values of 0.385 and 2.31 mg/ml respectively.
Bacterial food spoilage constitutes the most common cause of food poisoning especially by Gram-negative (E. coli and S. typhi) and Gram-positive (S. aureus and MRSA) bacterial strains (Solomakos et al., 2008). The potent antibacterial activity of S. aromaticum extracts against food spoilage bacteria supports the utilizing of these extracts in the formulation of natural food preservatives avoiding antimicrobial resistance to chemical preservatives and the deleterious impact of these preservatives to the human health (Bialonska et al., 2010).
The clove dichloromethane extract exerted the highest antimicrobial efficacy against the pathogenic bacterial strains, which may be assigned to the high percentage of eugenol and eugenyl acetate compounds of 50.65 and 12.54% respectively as presented in GC–MS results. GC–MS results were in accordance with that of Tischer et al. (2019), who reported the effectiveness of eugenyl acetate compound against S. aureus and E. coli strains recording inhibitory zones of 33 and 37.55 mm, respectively. Moreover, the efficiency of eugenol as antimicrobial agent was confirmed by Qiu et al. (2010) who reported the antibacterial efficacy of eugenol against S. aureus with MIC value of 256 μg/ml. Nazzaro et al. (2013) attributed the efficacy of eugenol as antibacterial agent to the free hydroxyl group in eugenol molecule. The free hydroxyl groups of eugenol block the enzymatic activity of microbial cells through binding to the bacterial proteins. The antimicrobial potency of S. aromaticum extracts may be ascribed to its eugenol content which affects the permeability of cytoplasmic membrane negatively resulting in disturbance of ions, ATP transport and initiation of cell death (Devi et al., 2013). The high phenolic content of clove oil causes microbicidal action against different bacterial pathogens through disruption of the active transport, electron flow and proton motive force resulting in coagulation of bacterial cell contents (Elhoussine et al., 2010). The mode of eugenol action against S. aureus strains was confirmed by Das et al. (2016) who explained that eugenol compound induces bacterial cell toxicity through production of reactive oxygen species (ROS) resulting in disturbance of cell membrane permeability, growth inhibition of microbial cells, DNA damage and finally bacterial cell death.
Other researchers attributed the antibacterial efficacy of clove extracts to the destructive action of clove oil that acts on different bacterial cell components due to its hydrophobic feature resulting in partitioning lipids of mitochondria and cell membrane (Xu et al., 2016). Also, clove oil targets the proteins of the electrons transport system in bacterial cell membrane causing inhibition of microbial growth (Wongsawan et al., 2020).
On the other hand, medicinal plant extracts provide a powerful tool in controlling malignant nature of cancer cells avoiding the side effects of chemotherapeutic agents (Mbaveng et al., 2011). Syzygium aromaticum extracts are rich in tannins which were reported to possess potential anticancer activity (Batiha et al., 2020). The cytotoxic efficiency of S. aromaticum extracts was confirmed by Kumar et al. (2014), who demonstrated the cytotoxic impact of clove oil to the MCF-7 breast cancer cell line. The potent anticancer activity of S. aromaticum extracts may be attributed to their phytoactive constituents of eugenol which was proved to perform antiproliferative action against malignant melanoma cells, oral squamous carcinoma and androgen-insensitive prostate cancer cell lines (Carrasco et al., 2008).
5 Conclusion
The current study confirmed the potent antimicrobial potency of S. aromaticum extracts against different pathogenic bacterial strains. Clove dichloromethane extract exhibited the highest antimicrobial activity against bacterial strains. The prospective antimicrobial efficacy of clove extracts authenticated the ability of utilizing these extracts in the production of novel antimicrobial agents. Results on the antibacterial activity of clove extracts against food poisoning bacterial strains emphasize the application of these extracts in formulation of natural food preservatives. Strong anticancer activity of clove extracts against HCT cancer cell line supports the ability of utilizing these extracts in formulation of natural anticarcinogenic agents.
Acknowledgment
Author M.A.El-Sheikh extends his appreciation to the Researchers Supporting Project Number (RSP-2020/182), King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Prevalence of methicillin-resistant Staphylococcus aureus (MRSA) infections among patients admitted in critical care units in a tertiary care hospital. Int. J. Res. Med. Sci.. 2017;5:2362-2366.
- [Google Scholar]
- Combating breast cancer using combination therapy with 3 phytochemicals: Piperine, sulforaphane, and thymoquinone. Cancer. 2019;125(10):1600-1611.
- [Google Scholar]
- The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2017. EFSA J.. 2018;16(12):e05500
- [Google Scholar]
- Syzygium aromaticum L. (Myrtaceae): Traditional uses, bioactive chemical constituents, pharmacological and toxicological activities. Biomolecules. 2020;10(2):202.
- [Google Scholar]
- The influence of pomegranate by-product and punicalagins on selected groups of human intestinal microbiota. Int. J. Food Microbiol.. 2010;140(2–3):175-182.
- [Google Scholar]
- Eugenol and its synthetic analogues inhibit cell growth of human cancer cells (Part I) J. Brazil Chem. Soc.. 2008;19(3):543-548.
- [Google Scholar]
- Risk factors for healthcare-associated extensively drug-resistant Acinetobacter baumannii infections: a case-control study. PLoS One.. 2014;9(1)
- [Google Scholar]
- Clinical and Laboratory Standards Institute. “Performance standards for antimicrobial disk susceptibility tests, 5th ed.,” CLSI document M7- A5. USA: CLSI, National Committee for Clinical Laboratory Standards, Wayne, Pa, USA, 2000.
- Control, C. f. D., Prevention. 2012. Multistate outbreak of Salmonella Bareilly and Salmonella Nchanga infections associated with a raw scraped ground tuna product (final update): July.
- Recent advances in understanding enteric pathogenic Escherichia coli. Clin. Microbiol. Rev.. 2013;26(4):822-880.
- [Google Scholar]
- Das, B., Mandal, D., Dash, S. K., Chattopadhyay, S., Tripathy, S., Dolai, D. P., Dey, S. K., Roy, S., 2016. Eugenol provokes ROS-mediated membrane damage-associated antibacterial activity against clinically isolated multidrug-resistant Staphylococcus aureus strains. Infect Dis Res and Treat, 9, IDRT. S31741.
- Community-associated methicillin-resistant Staphylococcus aureus: epidemiology and clinical consequences of an emerging epidemic. Clin. Microbiol. Rev.. 2010;23(3):616-687.
- [Google Scholar]
- Eugenol alters the integrity of cell membrane and acts against the nosocomial pathogen Proteus mirabilis. Arch. Pharm. Res.. 2013;36(3):282-292.
- [Google Scholar]
- Antibacterial activities of selected edible plants extracts against multidrug-resistant Gram-negative bacteria. BMC Complement. Altern. Med.. 2013;13(1):164.
- [Google Scholar]
- Comparative anticancer potential of clove (Syzygium aromaticum)—an Indian spice—against cancer cell lines of various anatomical origin. Asian Pac. J. Cancer Prev.. 2011;12(8):1989-1993.
- [Google Scholar]
- GC/MS analysis and antibacterial activity of the essential oil of Mentha pulegium grown in Morocco. Res. J. Agric. Biol. Sci.. 2010;6(3):191-198.
- [Google Scholar]
- Progress in targeted therapy for breast cancer. Chronic Dis. Transl. Med.. 2018;4(3):164-175.
- [Google Scholar]
- Anticancer potential of Syzygium aromaticum L. in MCF-7 human breast cancer cell lines. Pharmacog. Res.. 2014;6(4):350.
- [Google Scholar]
- Screening of tropical medicinal plants for sporicidal activity. Int. Food Res. J.. 2015;22(1):421.
- [Google Scholar]
- Syzgium coriaceum Bosser & J. Guého—An endemic plant potentiates conventional antibiotics, inhibits clinical enzymes and induces apoptosis in breast cancer cells. Ind. Crop Prod. 2020;143:111948
- [Google Scholar]
- In vitro antibacterial activity of three Indian spices against methicillin-resistant Staphylococcus aureus. Oman Med. J.. 2011;26(5):319.
- [Google Scholar]
- Evaluation of four Cameroonian medicinal plants for anticancer, antigonorrheal and antireverse transcriptase activities. Environ. Toxicol. Phar.. 2011;32(2):162-167.
- [Google Scholar]
- A review on the design and development of EGFR tyrosine kinase inhibitors in cancer therapy. Int. J. Ther. Appl.. 2012;5:29-37.
- [Google Scholar]
- Effect of essential oils on pathogenic bacteria. Pharmaceuticals. 2013;6(12):1451-1474.
- [Google Scholar]
- Emerging and neglected infectious diseases: insights, advances, and challenges. BioMed Res. Int.. 2017;2017
- [Google Scholar]
- Prevalence and comparison of three methods for detection of Methicillin-resistant Staphylococcus aureus (MRSA) isolates in tertiary health institutions in Nigeria. Can. Open Biol. Sci.. 2014;1:1-12.
- [Google Scholar]
- Methicillin-resistant Staphylococcus aureus (MRSA) and anti-MRSA activities of extracts of some medicinal plants: A brief review. AIMS Microbiol.. 2019;5(2):117.
- [Google Scholar]
- Antibacterial activity of Syzygium aromaticum (clove) with metal ion effect against food borne pathogens. Asian J Plant Sci Res. 2011;1(2):69-80.
- [Google Scholar]
- Eugenol reduces the expression of virulence-related exoproteins in Staphylococcus aureus. Appl. Environ. Microbiol.. 2010;76(17):5846-5851.
- [Google Scholar]
- High prevalence of MRSA and ESBL among asylum seekers in the Netherlands. PLoS One.. 2017;12(4)
- [Google Scholar]
- Antibacterial activity of Syzygium aromaticum L. (clove) Int. J. Curr. Microbiol. Appl. Sci. 2016;5(11):484-489.
- [Google Scholar]
- Scallan, E., Hoekstra, R.M., Angulo, F.J., Tauxe, R.V., Widdowson, M.-A., Roy, Jones, J. L., Griffin, P. M., 2011. Foodborne illness acquired in the United States—major pathogens. Emerg. Infect. Dis. 17(1), 7.
- The antimicrobial effect of thyme essential oil, nisin and their combination against Escherichia coli O157: H7 in minced beef during refrigerated storage. Meat Sci.. 2008;80(2):159-166.
- [Google Scholar]
- Tischer, J.S., Possan, H., Luiz, J., Malagutti, N.B., Martello, R., Valério, A., Dalmagro, J., A., de Oliveira, D., Oliveira, J., 2019. Synthesis of eugenyl acetate through heterogeneous catalysis. J. Essent. Oil Res. 31(4), 312-318.
- Bactericidal Effect of Clove Oil against Multidrug-Resistant Streptococcus suis Isolated from Human Patients and Slaughtered Pigs. Pathogens. 2020;9(1):14.
- [Google Scholar]
- Chemical composition, antibacterial properties and mechanism of action of essential oil from clove buds against Staphylococcus aureus. Molecules. 2016;21(9):1194.
- [Google Scholar]
- In vitro antifungal resistance profile of Candida strains isolated from Saudi women suffering from vulvovaginitis. Eur. J. Med. Res.. 2020;25(1):1-9.
- [Google Scholar]
- In vitro anticandidal potency of Syzygium aromaticum (clove) extracts against vaginal candidiasis. BMC Complement Med. Ther.. 2020;20(1):25.
- [Google Scholar]
- Anticandidal and anti-carcinogenic activities of Mentha longifolia (Wild Mint) extracts in vitro. J. King Saud Univ. Sci.. 2020;32(3):2046-2052.
- [Google Scholar]







