Translate this page into:
Bioactivity of natural compounds extracted from Oedogonium cilitum isolated from Qalachwalan pond
⁎Corresponding author. trifa.omar@univsul.edu.iq (Trifa Attar Omar)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Natural extracts of microalgae are widely recognized for their biologically active compound. They represent an untapped supply of pharmaceutical substances. In this study, the freshwater macroalgae (Oedogonium cilitum) was collected from Qalachwalan Pond in Sulaimani City. The sample was identified, authenticated, and investigated for their chemical composition and pharmacological properties. the GC/MS was used to identify and quantify using methanol extract of O. cilitum macroalgae. The GC/MS results of (Oedogonium cilitum) revealed the existence of various significant chemicals, such as long chain fatty acids (saturated fatty acid, unsaturated fatty acids), ester, volatile organic compounds(saturated aldehyde, polyunsaturated aldehydes(PUAs), terpene Accordingly, palmitic acid(12.71 %),9-Octadecenoic acid,(E) (9.59 %),9- Octadecenoic acid,(Z) (5.67 %), Acetic acid, propyl ester(24.93 %), Acetic 2-methylpropyl ester(13.64 %), Acetic acid, butyl ester(8.75 %), Thiosulphuric-(2-aminoethyl)ester(2.15 %), Hexanal (5.58 %), Nonanal (1.61 %),2-Decenal,(E)-(3.23 %),2-Undecenal(2.71 %),2-Pentene,4,4-dimethyl-(2.32 %), Neophytadiene (5.58 %) respectively. macroalgae extract was tested utilizing the DPPH radical scavenging assay for its antioxidant properties, with a comparatively low IC50(0.538 mg/ml), extract bioactive components demonstrated enhanced antioxidant activity, by increasing concentration (4.8 µg/ml to 1250 µg/ml) Antioxidant activity (Radical scavenging activity) increase. This suggests that antioxidant activity and concentration are related. On several microorganisms, the antimicrobial effects of (Oedogonium cilitum) extract were studied using a well diffusion method. Three organic extracts (Dioxane, chloroform, Dichloromethane) were used against three Gram-negative bacteria (Escherichia coli, Acinetobacter species, and Enterobacter species), and one Gram-positive bacteria (Staphylococcus aureus). The highest zone of inhibition was observed in Dioxane extract which showed the maximum antibacterial activity against Staphylococcus aureus (12.4 mm), Escherichia coli (10.6 mm), and Acinetobacter species(9.9 mm) respectively. The present finding revealed that freshwater macroalgae is more effective against Gram-positive bacteria than Gram-negative germs. Our findings highlight the value of bioactive compounds as potential antibacterial agents and suggest that they might be used in place of traditional antibiotics.
Keywords
Oedogonium cilitum
Freshwater macroalgae
Antioxidant activity
Antimicrobial activity
Volatile compound
Long chain fatty acid
1 Introduction
Algal flora is one of the most important organisms that are found in the aquatic ecosystems especially which is the main source of oxygen and food chain. Recently algae entered nanotechnology and micro-technology for industrial purposes, antibiotics, toxicity, productivity, water quality assessment, and water pollution indicators (Dash et al.,2021). A wide variety of chemicals from several metabolic pathways, including amino acids, fatty acids, polysaccharides, and carotenoids are produced by microalgae (Martínez-Ruiz, 2022). Numerous ecologists and scientists have studied the importance of naturally occurring algae in various ecosystems, including freshwater, because they may have biological effects on microorganisms like bacteria and fungi (Thomas et al.,2021). Among the reported pharmacological effects of bioactive substances are their anti-inflammatory, anti-bacterial antioxidant, antiviral, and anthelmintic properties. (El-Sheekh et al.,2021). Both marine and freshwater algae are a good source of pharmacologically active metabolites (Gullon, 2020) and(Pulz and Gross,2004]. The presence of bioactive secondary metabolites with antitumor activity, antibacterial (Shamsabadi et al.,2013) antioxidant, and anti-inflammatory (Zbakh et al.,2020) activities has been recognized in various macroalgae as they contain active biological phytochemicals, such as fatty acids, sterols, peptides, proteins, polysaccharides, heterocyclic carbons and terpenes, activities have been identified in several macroalgae because they include biologically active phytochemicals like fatty acids, sterols, peptides, proteins, polysaccharides, heterocyclic carbons, and terpenes (Wu et al.,2013). Recently, the possibility of discovering medications made from natural sources rather than synthetic compounds has received attention (Blunt et al.,2017). Marine algae, often known as seaweeds, are one of the natural resources primarily used for the production of a variety of bioactive secondary metabolites with potential use as new medicinal and industrial agents (Rico et al.,2017). According to several studies, algae's capacity to create secondary metabolites with a range of pharmacological actions, including anti-inflammatory, antioxidant, antibacterial, cytotoxic, antiviral, and anticoagulant effects, macroalgae plays an important role in the pharmaceutical business (Al-Enazi et al.,2018b). Natural antioxidants found in terrestrial plants have been extensively researched, as well as their uses in nutraceuticals and food preservation. Tertiary butyl hydroquinone, propyl gallate, butylated hydroxytoluene, and butylated hydroxyanisol (BHA, BHT, and BHT) are some examples of synthetic antioxidants that have been used in food, cosmetic, and pharmaceutical products (Ahn et al.,2007). Scientists are looking for natural antioxidants that can be utilized in food and medicine without posing any safety or toxicity risks as the safety and toxicity of these synthetic antioxidants have been questioned (Wang et al.,2009). Natural antioxidants like Oedogonium algae are widely used and could take the place of synthetic antioxidants. Antioxidants found in algae and lichen extracts are particularly attractive for usage in nutraceuticals, cosmetics, and nutraceutical goods since their potential toxicity and health risks are fewer than those of synthetic antioxidants (Thomas and Kim, 2013). By delaying oxidation, the antioxidant qualities of natural algal chemicals can lengthen the shelf life of foods and cosmetics (Chintale et al.,2013). Numerous chemicals have been isolated and identified as antimicrobial agents, including chlorellin derivatives, acrylic acid, halogenated aliphatic compounds, terpenes, sulfur-containing heterocyclic compounds, phenolic inhibitors, alkaloids, etc. (Espeche et al.,1984) and (Etahiri et al., 2001; 2003 and 2007). Extract of Oedogonium capillary, a freshwater green alga, has a potent antibacterial agent against 23 different Enterobacteriaceae bacterial species., Pseudomonadaceae, Aeromonadaceae, and Vibrionaceae families; a strong association between the effectiveness of algal extract and antibiotics such as kanamycin, tetracycline, and chloramphenicol has been found (Rosell and Srivastava, 1987) and (Rosell and Srivastava, 2006). Algae are used in traditional medicine to treat a variety of human disorders, including dysentery, diarrhea, and thrush on infants' tongues, and to heal wounds and treat other skin conditions as an antiseptic (Pérez-Gutiérrez, 2016). The present study is aimed to assess the biological and chemical characteristics of the alga Oedogonium cilitum. from Sulaimani city that has not yet been described, it is hoped that this would serve as an illustration for describing the potentially beneficial of Oedogonium cilitum strain for medicinal use.
2 Material and methods:
2.1 Algal sample collection
Algal bloom samples were collected from Qalachwalan pond freshwater in Sulaimani district, Kurdistan region of Iraq. Fig. 1 during October 2021, and December 2021. After being carefully chosen from a blooming of algal biomass, samples were cleansed of epiphytes, extraneous matter, and necrotic particles. They were then put in clean plastic containers with their original water and taken to the laboratory. The sample was then dried at 40C in an oven for 2–3 days until the dry weight was constant after being rinsed with distilled water, shade-dried, chopped into small pieces, and powdered. (Elsie and DhanaRajan,2010).
Oedogonium cilitum Bloome at Qalachwalan open pond.
2.2 Morphological identification of algal
The algae sample was identified as Oedogonium cilitum according to the keys of identification (Prescott,1982). The filamentous green alga belonging to the genus Oedogonium featured cylindric cells, some of which were ring-shaped at the end of each cell had pyrenoids and partial chloroplast. Oedogonium species are often found in ponds, lakes, and rivers. Fig. 2.
Oedogonium cilitum.
2.3 Extract preparation
According to (Wong and Shahirah,2019) instructions were followed while making the organic extraction, with some modifications. Soxhlet extraction was performed using 20 g of powdered algae and 200 ml of methanol. The samples were then incubated for 96 h at 56 °C.
-
Gas Chromatography-Mass Spectrometer (GC MS) analysis:
Freshwater microalgae were analyzed using a gas chromatograph using a Carbowax capillary column-equipped quadrupole gas chromatography-mass spectrometer (GC–MS) from Shimadzu (30 m 0.25 mm ID; 0.25im film thickness) (intercut DB5MS. Japan). The capillary column was filled with a sample volume of two microliters. As a carrier gas, helium was used. Temperatures were set at 210 °C for the injector and detector. Split mode was used to inject the fluid (1:30). The column's temperature was programmed to begin at 50 °C for 1 min before rising by 3 °C each minute to 210 °C at the end. NIST Library 2008′s mass spectral database is used to compare the mass spectra, peaks in the separation of bioactive chemicals at constant pressure (100 kPa) were found. The components of the test materials' names, molecular weights, and structures were determined. (Toshihiro Obata.,2013; Saeed et al., 2020; Palaniappan, 2022).
-
Free radical-scavenger activity assessment (DPPH° assay):
A method for conducting the DPPH assay. Extreme potential for scavenging was calculated according to the recorded technique (Blois, 1958). At different concentrations, 1.5 ml of 0.25 m DPPH mixture and 1.5 ml of extract were diluted in methanol. For a constant state, this combination was vigorously shaken at ambient temperature. After thirty minutes DPPH decolorization was measured by taking a spectrophotometer (UVMini-1240 model, Shimadzu, Columbia, USA). to measure the absorbance at 517 nm. Then, the process of scavenging DPPH radicals was calculated using the following equation. By using a linear regression analysis, the half-maximal inhibitory concentration (IC50) was determined and expressed as the mean of three measurements. A positive control was ascorbic acid.
A0 denoted the control absorbance (unqualified, with no extraction).
A1 Denoted the absorbance when the excerpt or regular example is present.
2.4 Antimicrobial bioassays
Dioxane, Chloroform, and Dichloromethane were used to extract Oedogonium cilitum from three different organic solvents. These solvents were utilized to treat one Gram-positive bacteria, Staphylococcus aureus, as well as three Gram-negative bacteria, Escherichia coli, Acinetobacter species, and Enterobacter species. Muller Hinton agar was used for the bacterial culture's incubation (24 hr. at 37οC). With the help of a cork borer, four wells measuring 6 mm each were created into the medium to assess the extracts' antimicrobial activity using the Agar well diffusion method. After allowing bacteria to acclimate to medium on Petri dishes for 15 min, extracts (50 l) were added to the wells. After 24 h, the agar plates were incubated at 37 °C for the negative control well (which contained only the necessary solvent) and the positive control well (15 g) of erythromycin, and the inhibition zones were measured using a ruler. Tests were carried out in duplicate. Following incubation, the inhibition zones surrounding the wells were measured underneath and represented in millimeters as proof of antibacterial activity (Abdo et al.,2012).
3 Result and discussion
3.1 Chemical composition of algal extracts
By using GC/MS analysis, a total of fifteen bioactive chemicals were discovered in the peaks of methanolic extracts of fresh (Oedogonium cilitum). Table 1. Contains information on the bioactive chemicals in tabular form. The chromatogram of the chemicals found by GC/MS is shown in Fig. 3. The compounds' mass spectra were described and identified by comparison with the databases from the standard library. There were obviously several types of chemical compounds in (Oedogonium cilitum) such as fatty acids, esters, terpenes, hydrocarbons, and aldehydes.
Serial N.
Compounds
Area %
Retention time
Molecular weight
Molecular structure
1
(1S,2S)-1-fluoro-2-methyl cyclopentane
1.54
5.362
102.15
C6H11F
2
Acetic acid, propyl ester
24.93
6.099
102.13
C5H10O2
3
Acetic acid, 2-methyl propyl ester
13.64
7.408
116.16
C6H12O2
4
Hexanal
5.58
8.197
100.16
C6H12O
5
Acetic acid, butyl ester
8.75
8.540
116.16
C6H12O2
6
Nonanal
1.61
21.216
142.23
C9H18O
7
2-Pentene, 4,4-dimethyl-
2.32
28.074
98.18
C7H14
8
2-Decenal, (E)-
3.23
28.806
154.25
C10H18O
9
2-Undecenal
2.71
33.447
168.28
C11H20O
10
Neophytadiene
3.52
51.816
278.5
C20H38
11
Neophytadiene
2.06
53.290
278.5
C20H38
12
n-Hexadecanoic acid
12.71
56.148
256.4
C16H32O2
13
9-Octadecenoic acid, (E)-
9.59
61.680
282.5
C18H34O2
14
9-Octadecenoic acid (Z)-
5.67
61.823
282.5
C18H34O2
15
Thiosulphuric acid (H2S2O3), S-(2-aaminoethyl)
ester
2.15
62.372
157.22
C2H7NO3S2
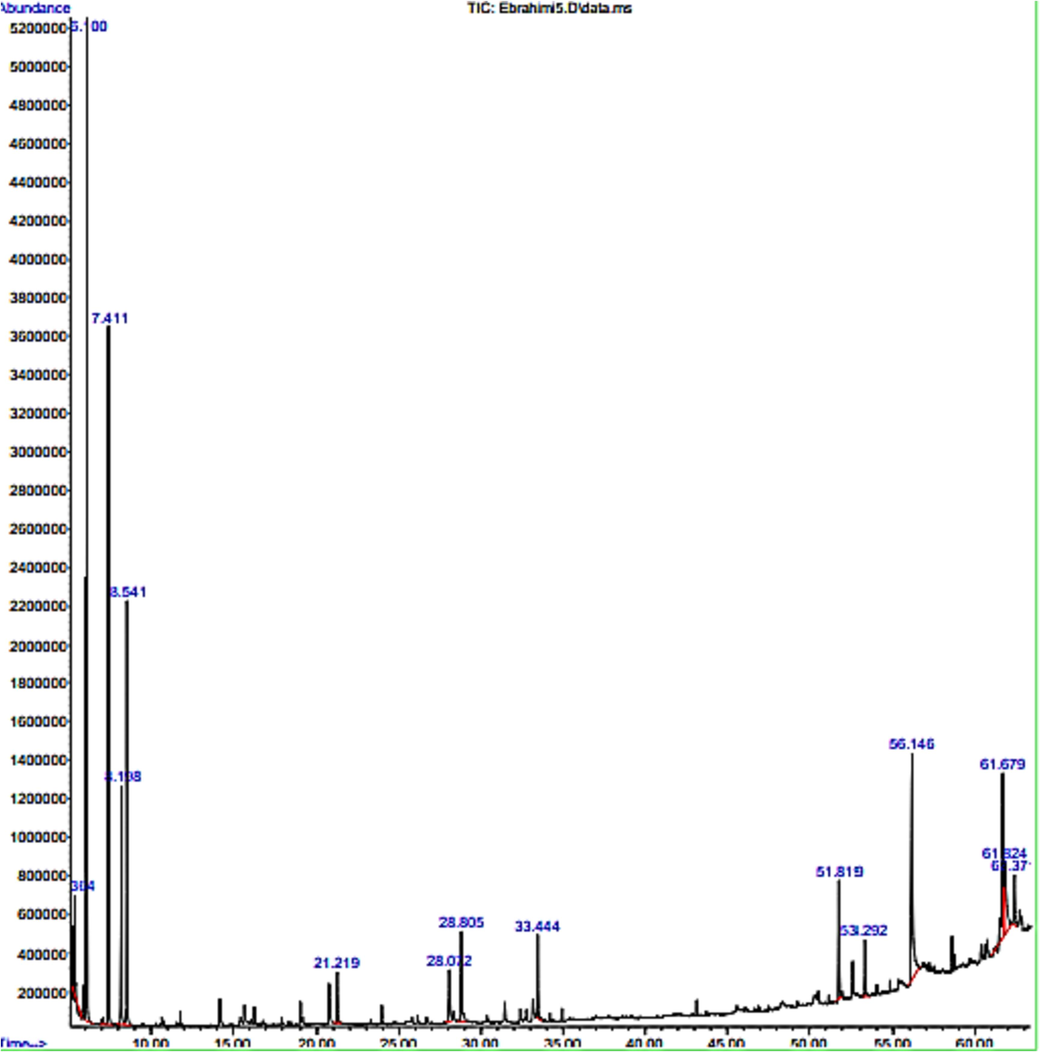
Chromatograms of GC/MS of (Oedogonium cilitum).
Fatty acids like (n-Hexadecenoic acid %12.71,9-Octadecenoic acid, (E) % 9.59,9-Octadecenoic acid, (Z) 5.67 %), another class of aroma compounds like esters was abundant in this algae as (Acetic acid propyl ester %24.93, Acetic acid, 2-methyl propyl ester %13.64, Acetic acid butyl ester %8.75, Thiosulphuric acids-(2-aminoethyl) ester %2.15). By peak percentage in all three fractions, the total quantity of ester compounds in Oedogonium cilitum was about (47.32 %). For algae to have order and flavor, volatile chemicals are essential. Consequently, it is not surprising that researchers have looked at the volatile chemicals in algae. volatile compounds classed as alkanes, alkene, aldehyde, sulfur compounds, alcohol, and ester were identified in the freshwater macroalgae (Table 1).
Hydrocarbons, including alkane, and feasible. euro −2-methyl cyclopentene 2.71 %, Neophytadiene 2.06 %,2-pentene,4,4-dimethyl 2.32 %. The relative concentration of saturated aldehydes like hexanal has been observed to follow an opposite trend 5.58 %, Nonanal 1.61 %, the first identification of 2-Decenal (E), 3.23 %,2-Undecenal 2.71 %. Unsaturated aldehyde in Oedogonium cilitum extracts shows that parallel-focused and untargeted investigation of previously unidentified chemicals is feasible.
3.2 Antioxidant activity
In recent years, many samples have been investigated using a variety of approaches due to the existence of various bioactive components with antioxidant potential. The DPPH radical scavenging method was chosen in the current investigation to assess the antioxidant potential of the algal extracts since it provided an easy, quick, and practical way to test the antioxidants and radical scavengers (Nickavar et al.,2007). Antioxidants were considered to have a scavenging impact on DPPH radicals because of their capacity to donate hydrogen (Ilhami et al.,2004). The ability of the DPPH radical to reduce was evaluated using the reduction caused by antioxidants. Results are shown in (Table 2), and (Figs. 4 and 6). Antioxidant activity is increased when the IC50 value is reduced. In this study, the standard antioxidant ascorbic acid's IC50 was calculated to be 0.021 mg/ml, which was much lower than the IC50 of the algal extract. Ascorbic acid's scavenging power ranged from 11.44 % at a concentration of 8.25 μg/ml to 80.54 % at a concentration of 66 μg/ml as shown in (Table 3), (Figs. 5 and 7). Extract of Oedogonium cilitum exhibited high antioxidant activity with a relatively low IC50 (0.538 mg/ml). The scavenging effect of the tested extracts at 4.88 μg/ml to 1250 μg/ml increased with increasing concentration as shown in (Fig. 4) which showed higher scavenging activity (56.68 %)on DPPH free radical.
Concentration
(µg/ml)1250
625
312.5
156.25
78.125
39.0625
19.53125
9.765625
4.882813
Control
OD1
0.426
0.418
0.538
0.685
0.823
0.856
0.912
0.993
0.984
0.992
OD2
0.432
0.42
0.551
0.672
0.811
0.861
0.904
0.982
0.97
0.975
OD3
0.422
0.426
0.529
0.668
0.809
0.847
0.919
0.98
0.978
0.988
Average
0.426
0.421
0.5393
0.675
0.814
0.8546
0.911
0.985
0.977
0.985
RSA% 1
56.751
57.563
45.380
30.456
16.44
13.096
7.411
−0.812
0.101
−0.710
RSA% 2
56.142
57.360
44.060
31.776
17.664
12.588
8.223
0.304
1.522
1.015
RSA% 3
57.157
56.751
46.294
32.182
17.868
14.010
6.7005
0.507
0.710
−0.304
Average
56.683
57.225
45.245
31.472
17.326
13.231
7.445
0
0.778
0.00
STDEV
0.5109
0.4226
1.122
0.902
0.7687
0.720
0.761
0.710
0.713
0.902

The percentage of Radical Scavenging Activity of Oedogonium cilitum algae on DPPH radicals.
concentration (μg/ml)
66
33
16.5
8.25
Control
0.171
0.298
0.455
0.785
0.886
0.173
0.298
0.456
0.784
0.885
0.173
0.3
0.453
0.784
0.886
Average
0.172333
0.298667
0.454667
0.784333
0.885667
RSA1
80.69251
66.35303
48.62627
11.3662
−0.03764
RSA2
80.46669
66.35303
48.51336
11.47911
0.075273
RSA3
80.46669
66.12721
48.85209
11.47911
−0.03764
RSA%
80.54196
66.27776
48.66391
11.44148
4.18E-15
STDEV
0.130376
0.130376
0.172472
0.065188
0.065188

The percentage of Radical Scavenging Activity of Ascorbic acid.
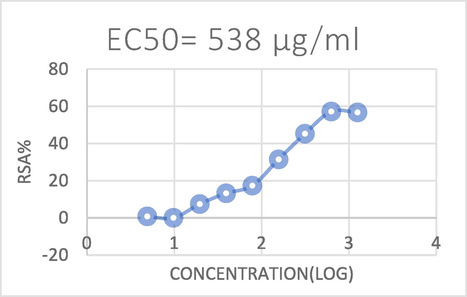
The DPPH radical scavenging activities of Oedogonium cilitum extract.
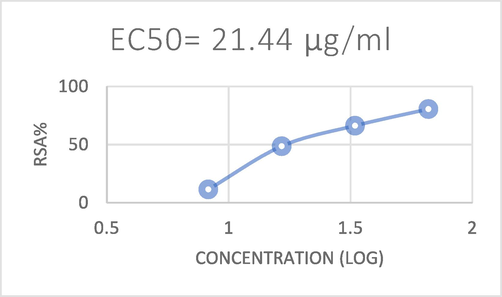
The DPPH radical scavenging of Ascorbic acid.
3.3 Antimicrobial activity
As a potential source of bioactive chemicals, freshwater Oedogonium cilitum is one of the primary topics for the development of many medicinal drugs. In this work, three agal extracts made from three organic solvents and tested against four microorganisms were found to have antibacterial properties. (Table 4). The results showed that algal extracts included many antibacterial substances. The finding demonstrated that the Dioxane extract provided stronger antimicrobial activity than the Dichloromethane and chloroform extract. The present study investigated that microalgae exhibited the strongest antibacterial activity against Staphylococcus aureus (12.4 mm) microalgae extract, and reported the lowest antibacterial activity against Enterobacteria’s(4.4 mm), The results of this study showed that the macroalgae Dioxane extracts had the greatest level and widest range of antibacterial activity against the tested microorganisms. Results show Dioxane extract exhibited strong inhibition against Staphylococcus aureus (12.44 mm) followed by E.coli bacteria (10.6 mm) Acinetobacter sp. (9.9 mm) and Enterobacter sp.(8.4 mm).In contrast, Dichloromethane extract showed a strong inhibition zone against Staphylococcus aureus (10.2), followed by Acinetobacter sp. (6.9 mm)and E.coli (4.8 mm), from the result seen that Dichloromethane extract showed no inhibition zone against Enterobacter sp., chloroform extract showed high inhibition zone (7.2 mm)against Acinetobacter species, followed by E.coli (5.6 mm) and showed lowest inhibition zone against Enterobacter species(4.4 mm).chloroform extract showed no inhibition zone against Staphylococcus aureus, also, investigated that chloroform extract showed least efficiency antimicrobial activity compared to two the extracts. These variations could be related to the different solubility behavior of bioactive compounds. The efficiency of extraction, the solvent used, and the resistance of the tested bacteria are only a few of the variables that may have affected the antibacterial potency of freshwater macroalgae and caused this variation in the results (Seenivasan et al.,2010). Gram-negative bacteria were resistant to the mild antibacterial activity displayed by all three macroalgae extracts, Dioxane extract, and Dichloromethane extract showed high activity against Gram-positive bacteria. While chloroform extract shows no activity against Gram-positive bacteria. The role of bacteria and algae as antibacterial agents, as well as their structure were dependent upon the processes by which the freshwater algae, including both macro and microalgae, were used as antimicrobial agents (Pina-Pérez et al., 2017) and (Breijyeh et al.,2020). Antibiotics were typically less successful against Gram-negative bacteria because of their stiff cell walls, which make them more complex than Gram-positive bacteria. Because of this, it was challenging for active compounds like -lactams, quinolones, and other antibiotics to penetrate bacteria and exert their antibacterial effects. (Breijyeh et al.,2020) The results demonstrated that Gram-positive bacteria were much more resistant to the antibacterial effect than Gram-negative bacteria due to their cell wall structure and components as well as the presence of short-chain fatty acids (Santoyo et al.,2009). Although it is still unclear how the fatty acids were able to block the entry of the active chemicals from algal extracts, numerous studies have found that fatty acids and lipids were the reason for cellular membrane breakdown (Leflaive 2009) and (Al-Saif at el.,2014). The study concluded by stressing the importance of naturally occurring freshwater algae as a possible antibiotic against a variety of harmful and disease-causing bacteria. However, more research is necessary to fully understand the mechanism by which they fight germs and if they could replace traditional antibiotics.
Bacterial species
Control
Dioxane
chloroform
Dichloromethane
Staphylococcus aureus
–
12.4
–
10.2
Acinetobacter species
–
9.9
7.2
6.9
Enterobacter species
–
8.4
4.4
–
E -Coli
–
10.6
5.6
4.8
4 Conclusion
The selected freshwater green macroalgae's phytochemical screening using GC/MS analysis revealed significant components such (as aldehyde, terpenes, and esters of fatty acids, fatty acid, unsaturated fatty acid, and Hydrocarbons), the chemical structure of these compounds was identified using GC/MS. The research clearly shows that Oedogonium cilitum is a rich potential source of many bioactive compounds. For this reason, we assessed the antioxidant and antibacterial activity of the extract Oedogonium cilitum, which is rich in biologically active compounds, making it a rich source of natural antioxidants and antimicrobial compounds. Important applications for these substances include medicine, nutraceuticals, cosmeceuticals, and agriculture. In the current study, the antioxidant activities of Oedogonium cilitum were evaluated. The results indicated that the microalgae possess high antioxidant activity with low IC50 (0.538 mg/ml), the strong positive significant correlation between DPPH radical scavenging and concentration of extracts, so (Oedogonium cilitum) can be used as a natural antioxidant source. This study describes the existence of antibacterial chemicals in algae gathered from ponds in Qalachwalan. Depending on the class of algae, algae have different antibacterial properties; regarding the extraction method, dioxane, dichloromethane, and chloroform were the solvents that, in most cases, increased the activity of the extracts toward the bacterial strain utilized for the antibiotic test. algae extract showed high activity against Gram-positive and Gram-negative. Despite the fact that Gram-positive bacteria were more vulnerable. As a result, it can be concluded that macroalgae from sulaimani should be researched for the isolation of natural antibiotics as they are potential sources of bioactive chemicals. From the result, we suggest that Oedogonium cilitum could be very beneficial to pharmaceutical and therapeutic applications in the future. Therefore, after confirming their safety, these species can be raised in captivity or in the wild for use as food by humans, farm animals, or as a nutritional supplement.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Phytochemical and antibacterial study of five freshwater algal species. Asian J. Plant Sci.. 2012;11(3):109-116.
- [Google Scholar]
- Antioxidant activities of phlorotannins purified from Ecklonia cava on free radical scavenging using ESR and H 2 O 2-mediated DNA damage. Eur. Food Res. Technol.. 2007;226:71-79.
- [CrossRef] [Google Scholar]
- Antimicrobial, antioxidant and anticancer activities of Laurencia catarinensis, Laurencia majuscula, and Padina pavonica extracts. Saudi Pharmaceutical Journal. 2018;26(1):44-52.
- [CrossRef] [Google Scholar]
- Antibacterial substances from marine algae isolated from the Jeddah coast of Red Sea, Saudi Arabia. Saudi Journal of Biological Sciences. 2014;21(1):57-64.
- [CrossRef] [Google Scholar]
- Antioxidant determinations by the use of a stable free radical. Nature. 1958;181(4617):1199-1200.
- [CrossRef] [Google Scholar]
- Resistance of gram-negative bacteria to current antibacterial agents and approaches to resolve it. Molecules. 2020;25(6):1340.
- [CrossRef] [Google Scholar]
- Algal Flora of Tampara Lake, Chhatrapur, Odisha, India. The Journal of Indian Botanical Society. 2021;101(1and2):1-15.
- [CrossRef] [Google Scholar]
- Biological control of soil-borne cucumber diseases using green marine macroalgae. Egyptian Journal of Biological Pest Control. 2021;31(1):1-7.
- [CrossRef] [Google Scholar]
- Evaluation of antimicrobial activity and phytochemical screening of Gelidium acerosa. J. Pharm. Sci. Res.. 2010;2(11):704.
- [Google Scholar]
- Espeche, M.E., Fraile, E.R. and Mayer, A.M., 1984. Screening of Argentine marine algae for antimicrobial activity. In Eleventh International Seaweed Symposium: Proceedings of the Eleventh International Seaweed Symposium, held in Qingdao, People’s Republic of China, June 19–25, 1983 (pp. 525-528). Springer Netherlands. https://doi.org/10.1007/978-94-009-6560-7_107.
- New bromoditerpenes from the red alga Sphaerococcus coronopifolius. J. Nat. Prod.. 2001;64(8):1024-1027.
- [CrossRef] [Google Scholar]
- Antibacterial activities of marine algae from the Atlantic coast of Marocco. Marine Life. 2003;13(1–2):3-10.
- [Google Scholar]
- Etahiri, S., El Kouria, A.K., Bultel-Poncé, V., Guyot, M. and Assobhei, O., 2007. Antibacterial bromophenol from the marine red alga Pterosiphonia complanata. Natural Product Communications, 2(7), p.1934578X0700200708. https://doi.org/10.1177/1934578X0700200708.
- Seaweeds as a promising resource of bioactive compounds: Overview of novel extraction strategies and design of tailored meat products. Trends Food Sci. Technol.. 2020;100:1-18.
- [CrossRef] [Google Scholar]
- Chemical interactions in diatoms: role of polyunsaturated aldehydes and precursors. New Phytol.. 2009;184(4):794-805.
- [CrossRef] [Google Scholar]
- Microalgae bioactive compounds to topical applications products—a review. Molecules. 2022;27(11):3512.
- [CrossRef] [Google Scholar]
- In vitro free radical scavenging activity of five Salvia species. Pak J Pharm Sci. 2007;20(4):291-294.
- [Google Scholar]
- Anticancer, antioxidant, and antimicrobial properties of solvent extract of Lobophora variegata through in vitro and in silico studies with major phytoconstituents. Food Biosci.. 2022;48:101822
- [CrossRef] [Google Scholar]
- Antimicrobial potential of macro and microalgae against pathogenic and spoilage microorganisms in food. Food Chem.. 2017;235:34-44.
- [CrossRef] [Google Scholar]
- Prescott, G.W., 1982. Algae of the western Great Lakes area, Otto Kaetz Science publishers. W. German.
- Valuable products from biotechnology of microalgae. Appl. Microbiol. Biotechnol.. 2004;65:635-648.
- [CrossRef] [Google Scholar]
- Production of primary and secondary metabolites using algae. Prospects and Challenges in Algal Biotechnology 2017:311-326.
- [CrossRef] [Google Scholar]
- Rosell, K.G. and Srivastava, L.M., 1987. Fatty acids as antimicrobial substances in brown algae. In Twelfth International Seaweed Symposium: Proceedings of the Twelfth International Seaweed Symposium held in Sao Paulo, Brazil, July 27–August 1, 1986 (pp. 471-475). Springer Netherlands.
- In vitro assessment of antimicrobial, antioxidant and anticancer activities of some marine macroalgae. Egypt. J. Bot.. 2020;60(1):81-96.
- [CrossRef] [Google Scholar]
- Green processes based on the extraction with pressurized fluids to obtain potent antimicrobials from Haematococcus pluvialis microalgae. LWT-Food Science and Technology. 2009;42(7):1213-1218.
- [CrossRef] [Google Scholar]
- The antibacterial activity of some marine algae from south east coast of India. J. Pharm. Res.. 2010;3(8):1907-1912.
- [Google Scholar]
- Comparison of tamoxifen with edible seaweed (Eucheuma cottonii L.) extracts in suppressing breast tumor. Nutr. Cancer. 2013;65(2):255-262.
- [CrossRef] [Google Scholar]
- Beneficial effects of marine algal compounds in cosmeceuticals. Mar. Drugs. 2013;11(1):146-164.
- [CrossRef] [Google Scholar]
- Antibacterial effects of the organic crude extracts of freshwater algae of Sulaymaniyah, Kurdistan Region. Iraq. Journal of Medicinal Plants Research. 2021;15(4):178-187.
- [CrossRef] [Google Scholar]
- Total phenolic compounds, radical scavenging and metal chelation of extracts from Icelandic seaweeds. Food Chem.. 2009;116(1):240-248.
- [CrossRef] [Google Scholar]
- Effect of different solvents and ratios towards microalgae oil production by ultrasonic-assisted Soxhlet extraction techniques. Orient. J. Chem.. 2019;35(4):1377.
- [CrossRef] [Google Scholar]
- Antibacterial and cytotoxic new napyradiomycins from the marine-derived Streptomyces sp. SCSIO 10428. Mar. Drugs. 2013;11(6):2113-2125.
- [CrossRef] [Google Scholar]
- Antibacterial activity of benthic marine algae extracts from the Mediterranean Coast of Morocco. Journal of Microbial Biotechnology Food Sc. 2020;10:219219-219228.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2023.102984.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







